Abstract
Background
Asymptomatic pancreatic necrosis should be managed conservatively, regardless of its extent. However, late sequelae and safety of non-interventional management in patients with asymptomatic walled-off necrosis remain unclear.
Aims
The purpose of this study was to report the clinical outcome of outpatient expectant management in a cohort of patients with walled-off necrosis who were discharged asymptomatic after an episode of acute pancreatitis.
Methods
Sixteen patients with walled-off necrosis asymptomatic at discharge were identified retrospectively from a single institution. Data were analyzed for the type of complications, their incidence and treatment.
Results
Seven of 16 patients (44 %) did not experience any complications during a median follow-up of 17 months. Nine of 16 patients (56 %) became symptomatic or developed complications within a median follow-up of 49 days after discharge. The most common complication was infection of pancreatic necrosis which occurred in 7 of 9 patients. Six of these patients were successfully treated with minimally invasive techniques. In 5 of 7 patients, infection of necrosis was due to oral commensal bacteria. Acute intracavitary hemorrhage and intractable abdominal pain developed in one patient each. There was no mortality in this series.
Conclusions
Outpatient watchful waiting can be used safely in patients with asymptomatic walled-off necrosis, although nearly half of them eventually develop complications which require interventional treatment. Most late infections of pancreatic necrosis are probably due to a blood-borne transmission of oral commensal bacteria.
Keywords: Acute pancreatitis, Pancreatic necrosis, Walled-off necrosis, Infected necrosis, Pancreatic necrosectomy, Percutaneous catheter drainage
Introduction
The revised version of the Atlanta Classification has been introduced into clinical practice recently [1]. This classification includes a newly defined entity “walled-off necrosis” (WON). Walled-off necrosis is formed from acute necrotic collections after encapsulation with an inflammatory wall within 4–6 weeks from the onset of acute pancreatitis [1]. WON represents a heterogeneous spectrum of solid and liquid collections which are composed of a variable proportion of the necrotic debris and fluid. The current international guidelines for the management of acute pancreatitis recommend conservative treatment of asymptomatic pancreatic necrosis, regardless of its size and extension of the collections [2, 3]. The popularity and success of non-interventional management has resulted in a raising number of survivors with retroperitoneal necrotic collections. Having survived the acute phase of necrotizing pancreatitis, patients with walled-off necrosis might be in good clinical condition, but a large peripancreatic necrotic collection persists in imaging studies and an abdominal mass is often palpable on physical examination. Although interventional treatment in these patients seems to have little clinical basis, the treating physicians might feel uncomfortable to discharge patients with so extensive necrotic collections, even if they remain asymptomatic. Our impression was that this subset of patients might be at an increased risk of late complications. Potential life-threatening complications of a longstanding pancreatic necrosis include infection and intracavitary hemorrhage. To date, little attention has been given to the natural course of walled-off necrosis in the literature, and there is paucity of data on the risks and consequences of a long-term expectant management of patients with asymptomatic walled-off necrosis.
The aim of this study was to review the clinical outcomes in patients with walled-off necrosis who were discharged asymptomatic and referred for outpatient follow-up.
Methods
Patients
Data from the hospital database were searched to identify patients with acute pancreatitis. The medical records and imaging scans were reviewed retrospectively for each patient in order to select patients with walled-off necrosis which did not require interventional treatment at the initial hospitalization and who were discharged asymptomatic. As asymptomatic were regarded patients who survived the acute phase of pancreatitis and were in good clinical condition, tolerated oral nutrition and suffered only occasional and mild abdominal pain or discomfort. Follow-up data were obtained from the outpatient clinic and hospital records, or the patients were contacted by phone. The clinical, laboratory, imaging, and management parameters at the initial and subsequent hospitalizations or outpatient visits were reviewed. The study group was evaluated for the type of complications, their incidence and treatment. The study was approved by our institutional Ethics Committee.
Statistical Analysis
Statistical analysis was performed using STATISTICA 10 software (StatSoft Poland). Descriptive statistics were presented as medians and range. The Mann–Whitney U test was used for continuous data, and the Fisher exact test was used for categorical data analysis. Two-tailed p value of ≤0.05 was regarded statistically significant.
Results
Between January 2007 and December 2012, 321 patients were admitted to our department with the diagnosis of acute pancreatitis. A total of 116 patients (36 %) had necrotizing pancreatitis. Sixteen patients progressed to walled-off necrosis which did not require intervention at the initial hospitalization. The patient group consisted of 14 males and 2 females. The median age of patients was 52.5 years (range 22–71). Acute pancreatitis was most commonly due to alcohol abuse. The median computed tomography severity index of acute pancreatitis (CTSI) was 10 points (range 6–10 points). All the patients were managed conservatively including bowel rest, parenteral fluids and nutritional support. Most patients received prophylactic antibiotics, usually carbapenems. The median interval from the onset of acute pancreatitis to the date of discharge was 26.5 days (range 13–56 days). Patients were discharged if they did not suffer intractable abdominal pain and tolerated oral nutrition. The inflammatory parameters had decreased substantially by the time of discharge. The median levels of the C-reactive protein and white cell count measured before discharge were 38.4 mg/L and 7.2 × 109/L, respectively. The median size of the necrotic collection (largest dimension on cross section) was 130 mm (range 80–200 mm). In 6 of 16 patients, the necrotic collection was confined to the lesser sac, and in the remaining patients, the collections extended along the paracolic gutters. In 11 of 16 patients, necrosis involved more than 50 % of the pancreatic parenchyma.
Overall, 7 of 16 patients (44 %) did not experience complications throughout the median follow-up of 17 months (range 7–53.5 months). The median CRP at discharge in this group was significantly lower compared with the patients who eventually developed complications (16.8 vs. 56.8 mg/L, p = 0.04). Other demographic and clinical variables were comparable in both groups (Table 1). Three patients in this group, despite of being asymptomatic, underwent endoscopic or open drainage procedures because of a persistent collection interpreted as a pseudocyst. However, these collections still contained necrotic debris. In two patients, the necrotic collections resolved. In one patient, the necrotic collection evacuated spontaneously into the gastrointestinal lumen after 3 years from the onset of acute pancreatitis what was evidenced by gas bubbles in the residual collection on follow-up CT scans (Fig. 1a, b). The remaining two patients did not develop any symptoms or complications, but the necrotic collections persisted on serial CT or ultrasound examinations.
Table 1.
The demographic and clinical characteristics of patients with asymptomatic WON at discharge after index acute pancreatitis
| Variable | Patients with complications | Patients asymptomatic during follow-up | p value |
|---|---|---|---|
| n | 9 | 7 | |
| Male/female | 7:2 | 7:0 | 0.48 |
| Patient age (years) | 51 (36–65) | 54 (22–71) | 0.76 |
| Etiology: alcohol/other | 6:3 | 4:3 | 1.0 |
| CTSI (points) | 10 (6–10) | 9 (8–10) | 0.92 |
| Necrosis of pancreas: 0–50 %/above 50 % | 6:3 | 5:2 | 1.0 |
| Size of WON (mm) | 130 (85–200) | 140 (80–190) | 0.92 |
| Extension of WON: LS/R | 2:7 | 4:3 | 0.30 |
| WBC (×109/L) | 7.1 (4.0–16.2) | 7.4 (5.5–10.9) | 0.84 |
| CRP (mg/L) | 56.8 (8.7–245.2) | 16.8 (5.0–59.9) | 0.04 |
| Length of stay (days) | 34 (20–56) | 26 (13–54) | 0.30 |
Bold value indicates statistically significant
CTSI computed tomography severity index of acute pancreatitis, WON walled-off necrosis, LS limited to lesser sac, R extension to the pararenal regions, WBC white blood cell count, CRP C-reactive protein
Fig. 1.
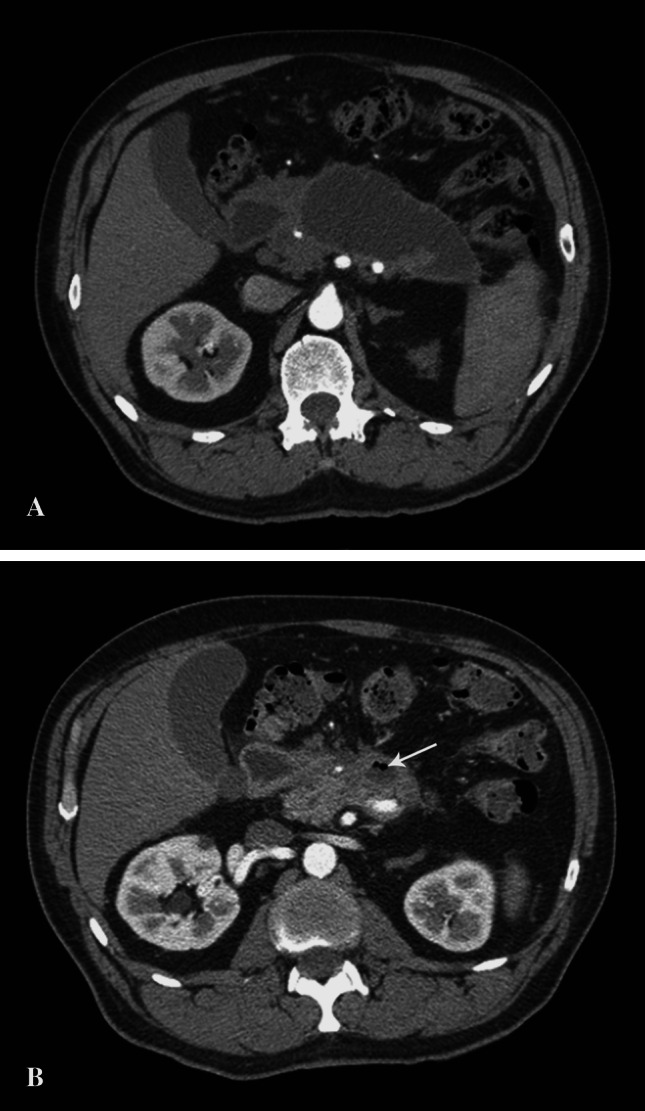
a CT scan shows an area of walled-off necrosis in the lesser sac 32 months after the episode of acute pancreatitis. b Follow-up CT scan performed 9 months later revealed almost complete resolution of the necrotic collection. Gas bubbles (arrow) within the residual collection suggest its spontaneous decompression into the adjacent duodenum. This patient (case no 13) remained asymptomatic during the whole follow-up period
Nine of 16 patients (56 %) suffered complications, and all of them required rehospitalization and interventional management within a median follow-up of 49 days (range 8–180 days). The most common complication was infection of pancreatic necrosis which occurred in 7 of 16 patients (44 %). One patient (6 %) experienced acute intracavitary hemorrhage originating from the left gastric artery. Another patient (6 %) suffered from intractable abdominal pain. The management and outcomes in patients with walled-off necrosis are shown in Table 2.
Table 2.
The late sequelae in patients with asymptomatic walled-off necrosis who underwent outpatient expectant management
| Case no. | Sex/age (years) | WON size (mm) | Time to discharge from the onset of AP (days) | Time to a complication after discharge (days) | Type of complication | Treatment of complication | Follow-up timea (months) | Residual necrotic collection |
|---|---|---|---|---|---|---|---|---|
| 1 | M/41 | 162 | 34 | 8 | IPN | PCD | – | – |
| 2 | M/36 | 127 | 20 | 180 | IPN | PCD | – | – |
| 3 | M/37 | 130 | 44 | 105 | IPN | PCD | – | – |
| 4 | F/51 | 200 | 41 | 74 | IPN | PCD, MARPN | – | – |
| 5 | M/57 | 130 | 21 | 47 | IPN | PCD | – | – |
| 6 | M/65 | 170 | 27 | 49 | IPN | Endoscopic cystogastrostomy | – | – |
| 7 | M/37 | 107 | 21 | 33 | IPN | PCD, Open necrosectomy | – | – |
| 8 | M/55 | 85 | 56 | 48 | Intracavitary hemorrhage | PCD, MARPN, open necrosectomy | – | – |
| 9 | F/64 | 124 | 34 | 105 | Pain, subileus | Open necrosectomy | – | – |
| 10 | M/22 | 141 | 43 | – | None | – | 9 | 2 collections: 80 and 70 mm |
| 11 | M/30 | 140 | 26 | – | None | – | 7 | Resolved |
| 12 | M/31 | 80 | 20 | – | None | – | 53.5 | 55 mm |
| 13 | M/54 | 190 | 54 | – | None | – | 41.5 | Resolved |
| 14 | M/61 | 110 | 13 | – | None | Open cystogastrostomy | 15.5 | – |
| 15 | M/61 | 124 | 19 | – | None | Open cystojejunostomy | 22 | – |
| 16 | M/71 | 180 | 26 | – | None | Endoscopic cystogastrostomy | 17 | – |
AP acute pancreatitis, IPN infected pancreatic necrosis, PCD percutaneous catheter drainage, MARPN minimal access retroperitoneal pancreatic necrosectomy
aRefers to patients who remained asymptomatic during follow-up
All the patients with infected pancreatic necrosis were initially managed by minimally invasive techniques. Six patients underwent percutaneous catheter drainage (PCD), and one patient was treated by endoscopic drainage. In five of these patients, minimally invasive drainage was definitive and no further interventional management was required. Infection was controlled and necrosis resolved gradually. One patient suffered intraperitoneal leakage of the necrotic material following PCD which required urgent laparotomy and open necrosectomy. In another patient, PCD was unsuccessful and she underwent minimal access retroperitoneal pancreatic necrosectomy (MARPN) because of persistent sepsis. Only one session of debridement was necessary in this patient.
Culture of the samples collected at the initial drainage of infected pancreatic necrosis grew the following spectrum of the pathogens: oral commensal bacteria in 5 of 7 patients, enteric bacteria in 1 of 7 patients, and single flora in 3 of 7 patients. In one patient, culture was negative. The bacterial flora isolated from infected pancreatic necrosis is shown in Table 3.
Table 3.
The spectrum of causative pathogens isolated from late infected pancreatic necrosis
| Case no. | Isolated pathogen from IPN | Characteristics of the pathogen | Antibiotic susceptibility of the pathogen |
|---|---|---|---|
| 1 | Staphylococcus aureus | G+, facultative anaerobic, skin commensal, oral commensal (±) | Methicillin-sensitive |
| Fusobacterium nucleatum | G−, anaerobic, oral commensal | Amoxicillin, clindamycin, metronidazole | |
| 2 | Propionibacterium acnes | G+, anaerobic, oral commensal | Amoxicillin, clindamycin |
| 3 | Actinomyces naeslundii | G+, anaerobic, oral commensal | Amoxicillin, clindamycin |
| 4 | Escherichia coli | G−, facultative anaerobic, enteric bacillus | Ampicillin, ciprofloxacin, gentamicin, cephazolin |
| Bacteroides fragilis | G−, anaerobic, enteric bacillus | Amoxicillin/clavulanate, clindamycin, metronidazole | |
| Peptostreptococcus spp. | G+, anaerobic, oral, skin and GI commensal | Penicillin, clindamycin, metronidazole | |
| 5 | Streptococcus intermedius | G+, microaerobic, oral commensal | Amoxicillin, clindamycin |
| 6 | Negative | – | – |
| 7 | Prevotella melaninogenica | G−, anaerobic, oral commensal | Amoxicillin/clavulanate, metronidazole (amoxicillin, clindamycin–resistant) |
| Streptococcus constellatus | G+, microaerobic, oral commensal | Amoxicillin, clindamycin, erythromycin |
IPN infected pancreatic necrosis, G gram staining, GI gastrointestinal
The patient with intracavitary hemorrhage presented with acute abdominal pain, but the initial CT scans did not reveal any signs of bleeding. He underwent open necrosectomy after a failed minimally invasive treatment. At laparotomy, there was no active bleeding. However, the postoperative course was complicated by recurrent intracavitary hemorrhage from the left gastric artery which was successfully embolized by interventional radiologists.
Postoperative pancreatic fistula developed in 4 of the 7 patients who underwent external drainage, minimally invasive or open. In two of these patients, the fistula resolved spontaneously, and the remaining patients required endoscopic placement of a stent into the main pancreatic duct.
Overall, two-thirds of the patients who suffered late complications of the walled-off necrosis recovered with minimally invasive techniques. There were no mortalities during the follow-up period or inpatient treatment.
Discussion
Walled-off necrosis was referred in the past as organized pancreatic necrosis, subacute pancreatic necrosis or necroma [4, 5]. The term “organized pancreatic necrosis” was coined by Baron et al. [4] to emphasize only partially liquefied nature of these collections which contained a variable amounts of the necrotic debris. These authors regarded organized pancreatic necrosis as an entity in transition from acute necrosis to pancreatic pseudocyst. The notion “walled-off necrosis” was popularized by the Working Group of the Pancreas Club and further included in the recent revision of the Atlanta classification [1]. The well-defined indications for intervention in walled-off necrosis include infection or pressure symptoms. Currently, the pancreatic and gastroenterological societies recommend that asymptomatic pancreatic necrosis should be managed conservatively, regardless of its size and extension of the necrotic collection [2, 3]. Most of these recommendations focus on the early management and assume that necrosis may resolve over time. However, long-term sequelae of the pancreatic necrosis are unclear. With time, pancreatic necrosis is believed to undergo gradual liquefaction and reabsorption. In our series, some patients who were intervened later than a year from the onset of acute pancreatitis still had solid debris within the necrotic collections. This finding suggests that the process of liquefaction might be incomplete even months or years after the episode of necrotizing pancreatitis.
A burden of the necrotic collection in the retroperitoneum might cause the so-called persistent unwellness characterized by abdominal discomfort or occasional pain and poor oral intake. In a series reported by Fernandez-del Castillo et al. [6, 7], 39 % of the patients were operated on for persistent pancreatitis/unwellness, and most of these operations were performed later than 7 weeks from the beginning of the disease. Nevertheless, there is no clear-cut definition of this condition, and the indications for intervention are disputable, and to some extent, also subjective. The patients in our series, in fact, were not “purely” asymptomatic because most of them experienced occasional episodes of abdominal discomfort or pain which were transient, well tolerated and did not influence the nutritional status of the patients what would otherwise justify interventional treatment.
In our series, nearly half of the patients with walled-off necrosis who were referred for expectant management became symptomatic within a median period of 49 days after discharge. None of the complications occurred later than 7 months from the onset of acute pancreatitis. This observation emphasizes the necessity of a close surveillance in this subset of patients surviving acute pancreatitis for the first several months after discharge to avoid a delay in appropriate treatment if complications occur. Afterward, complications are uncommon. All the patients who experienced complications required readmission and interventional management. Fortunately, none of these complications proved fatal, especially in the period prior to admission.
In view of a high rate of complications in patients with walled-off necrosis over time, it might be tempting to intervene earlier on these patients to anticipate the potential complications. However, the rationale and timing of such management are questionable. This series confirms that patients even with extensive walled-off necrosis might be followed up safely and subsequent complications usually allow for a timely intervention without negative impact on morbidity and mortality. In addition, the risk of complications seems to decrease with time. On the other hand, a long interval between the onset of acute pancreatitis and intervention results in better liquefaction and separation of the necrosis which increases the efficacy of minimally invasive techniques. Consequently, the mortality rate is low at this stage of disease, regardless of the technique used—open or minimally invasive [8–10]. In this series, 86 % of the patients with infected necrosis were treated successfully with minimally invasive techniques without mortality. Occasionally, the necrotic collection might evacuate spontaneously into the gastrointestinal lumen as occurred in one of our patients.
The most common complication in this series was infection of the walled-off necrosis. This complication required uniformly interventional treatment. Essential finding from this study is that the spectrum of bacteria isolated from infected pancreatic necrosis in this series was distinct to that reported in the literature. The offending pathogens in 5 of 7 patients with infected pancreatic necrosis in this series were oral commensal bacteria which show low-pathogenic potential. In comparison, bacteria cultured during the first several weeks of necrotizing pancreatitis are most often highly virulent enteric bacteria such as Escherichia coli, Pseudomonas aeruginosa, Klebsiella or Enterococcus spp. [11, 12]. In our series, such bacteria were isolated in only one patient. The pathogenesis of late infections of walled-off necrosis is difficult to elucidate, but bacterial translocation from the intestines seems to play a less important role. A possible explanation for such bacterial spectrum in this series might be a blood-borne transmission of oral commensal bacteria. Dental procedures, but also daily oral activities such as toothbrushing or dental flossing, have been shown to cause a transient bacteremia with oral commensals [13]. Interestingly, the bacterial species isolated from blood after toothbrushing are similar to that causing infection of pancreatic necrosis in our series. This phenomenon might be responsible for late infections of pancreatic necrosis in a similar way as it occurs in infective endocarditis. Moreover, the solid debris within the walled-off necrosis constitute a friendly environment for the growth of these anaerobic or microaerophilic bacteria which might seed the necrosis and subsequently cause clinically significant infection. Infection of encapsulated retroperitoneal collections often has an insidious course. Rodriguez et al. [14] reported that 42 % of patients operated on for persistent unwellness unexpectedly proved to be infected, although they had not manifested overt symptoms of infection. Conversely, all the patients with infected necrosis in our series presented acutely with a septic syndrome. However, some of them might have had an indolent infection which became fully symptomatic over time. Although most of these patients had increased CRP levels at discharge, it seems unlikely that this infection could have persisted for weeks or months after the initial hospitalization. In view of the aforementioned spectrum of the causative bacteria, amoxicillin or clindamycin should be selected for empiric antibiotic therapy of late infections of pancreatic necrosis.
A longstanding peripancreatic necrotic collection might lead to the most feared complication—an erosion of the adjacent blood vessels with subsequent intracavitary bleeding. Fortunately, hemorrhagic complications occurred in only one patient in our series. Although hemorrhage originated from a large splanchnic artery, it stopped spontaneously. Encapsulation with firm and rigid inflammatory tissues in walled-off necrosis seems to be sufficiently resistant to additional intracavitary pressure forces.
Walled-off necrosis often communicates with the pancreatic ductal system. This communication has some practical implications. The pancreatic ductal leakage feeds the liquid part of the walled-off necrosis which sometimes is more extensive than the true necrosis. Differentiation between the necrotic and liquid components of WON is often difficult in CT and might require MRI or ultrasonography [15]. Moreover, percutaneous drainage or necrosectomy carries a high risk of pancreatic fistula. In this series, half of the patients who underwent external drainage developed pancreatic fistula postoperatively.
This study has some limitations. Firstly, this is a retrospective study with a limited number of patients. However, some patients were followed up for fairly long periods exceeding 3 years. Secondly, most patients in this cohort were in fact mildly symptomatic what some clinicians could have considered as a sufficient indication to interventional or surgical treatment.
In summary, this series provides some new insights into the natural progression of the walled-off necrosis. Asymptomatic patients with walled-off necrosis can be safely managed by watchful waiting in an outpatient setting, but a high rate of late complications, especially septic, might be expected. Most complications occur within the first several months after acute pancreatitis and usually require interventional treatment. However, liquefaction of pancreatic necrosis and favorable bacteriological spectrum render minimally invasive techniques highly successful for the treatment of late infections. Blood-borne transmission of oral commensal bacteria seems to play a more important role in the pathogenesis of late infections of pancreatic necrosis rather than bacterial translocation from the intestines. Nevertheless, the findings from this series should be validated in larger longitudinal studies.
Conflict of interest
None.
Contributor Information
Marek Wroński, Phone: 0048 22 599 24 82, Email: mwronski@vp.pl.
Włodzimierz Cebulski, Email: wlocebu@wp.pl.
Waldemar Pawłowski, Email: wpeacock@wp.pl.
Ireneusz W. Krasnodębski, Email: ireneusz.krasnodebski@wum.edu.pl
Maciej Słodkowski, Email: maciejslodkowski@wp.pl.
References
- 1.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 2.Freeman ML, Werner J, van Santvoort HC, et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41:1176–1194. doi: 10.1097/MPA.0b013e318269c660. [DOI] [PubMed] [Google Scholar]
- 3.Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 4.Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–764. doi: 10.1053/gast.1996.v111.pm8780582. [DOI] [PubMed] [Google Scholar]
- 5.Petrakis I, Vrachassotakis N, Kogerakis N, Koutsoumpas V, Chalkiadakis G. Subacute pancreatic necrosis. Panminerva Med. 2000;42:279–286. [PubMed] [Google Scholar]
- 6.Fernandez-del Castillo C, Rattner DW, Makary MA, Mostafavi A, McGrath D, Warshaw AL. Debridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998;228:676–684. doi: 10.1097/00000658-199811000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warshaw AL. Pancreatic necrosis: to debride or not to debride-that is the question. Ann Surg. 2000;232:627–629. doi: 10.1097/00000658-200011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voermans RP, Veldkamp MC, Rauws EA, Bruno MJ, Fockens P. Endoscopic transmural debridement of symptomatic organized pancreatic necrosis (with videos) Gastrointest Endosc. 2007;66:909–916. doi: 10.1016/j.gie.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Lakshmanan R, Iyer SG, Lee VT, Chang SK, Madhavan K. Minimally invasive retroperitoneal pancreatic necrosectomy in the management of infected pancreatitis. Surg Laparosc Endosc Percutan Tech. 2010;20:e11–e15. doi: 10.1097/SLE.0b013e3181c8f340. [DOI] [PubMed] [Google Scholar]
- 10.Beck WC, Bhutani MS, Raju GS, Nealon WH. Surgical management of late sequelae in survivors of an episode of acute necrotizing pancreatitis. J Am Coll Surg. 2012;214:682–688. doi: 10.1016/j.jamcollsurg.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Luiten EJ, Hop WC, Lange JF, Bruining HA. Differential prognosis of gram-negative versus gram-positive infected and sterile pancreatic necrosis: results of a randomized trial in patients with severe acute pancreatitis treated with adjuvant selective decontamination. Clin Infect Dis. 1997;25:811–816. doi: 10.1086/515545. [DOI] [PubMed] [Google Scholar]
- 12.Tsui NC, Zhao E, Li Z, et al. Microbiological findings in secondary infection of severe acute pancreatitis: a retrospective clinical study. Pancreas. 2009;38:499–502. doi: 10.1097/MPA.0b013e3181a16d12. [DOI] [PubMed] [Google Scholar]
- 13.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez JR, Razo AO, Targarona J, et al. Debridement and closed packing for sterile or infected necrotizing pancreatitis: insights into indications and outcomes in 167 patients. Ann Surg. 2008;247:294–299. doi: 10.1097/SLA.0b013e31815b6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi N, Papachristou GI, Schmit GD, et al. CT findings of walled-off pancreatic necrosis (WOPN): differentiation from pseudocyst and prediction of outcome after endoscopic therapy. Eur Radiol. 2008;18:2522–2529. doi: 10.1007/s00330-008-1039-1. [DOI] [PubMed] [Google Scholar]


