Abstract
Introduction
Radical cystectomy in elderly patients is a controversial issue that has noticed an increase in importance overtime because of the lengthening average life span. Our objective was to determine if there were significant differences in the perioperative outcomes of patients over 70 years with bladder cancer treated with laparoscopic radical cystectomy (LRC) compared to those of younger patients.
Material and methods
We selected 180 patients who underwent LRC in our department in the period between 2005-2012. We divided them into 2 groups: 57% <70 years and 43% >70 years, and we compared the different parameters such as: comorbidities, intraoperative and post-operative complications, TNM stage and overall survival.
Results
The group <70 years had less comorbidities when compared with the group >70 years. Heterotopic urinary diversion was the diversion of choice in the elderly patients (97.4%). Paralytic ileus and the worsening of renal function were the only complications with statistical differences between the groups. Mean hospital length of stay was not significantly different between the groups. Younger and older patients had similar pathological staging : pT1 or less: 26,2 vs. 18.2%, pT2: 19.4 vs. 16.9%, pT3 38.8 vs. 37.7% and pT4 15.6 vs. 17.2%. Kaplan-Meier curves did not show significant differences in survival.
Conclusions
Laparoscopic radical cystectomy in the elderly patient has similar rates of perioperative morbidity when compared with the younger patient and may be offered as a treatment option in selected elderly patients.
Keywords: bladder cancer, laparoscopic radical cystectomy, elderly patients
INTRODUCTION
Bladder cancer is the second most common urologic malignancy; with a high incidence in the elderly patients [1]. The population has aged progressively as the average life span has increased in the last decades [2]. Therefore, is highly important to solve the issues of managing bladder cancer in the elderly patient.
Radical cystectomy with pelvic lymph node dissection and urinary diversion has been the standard treatment for muscle invasive and recurrent high-grade bladder cancer [3]. Radical cystectomy is a major procedure with the potential of serious complications. According to the literature, the incidence of such complications varies widely from 19 to 64% depending on the series [4–6]. In the elderly patient, some authors advocated for the use of less aggressive forms of treatment such as radiation therapy, repeated transurethral resection, partial cystectomy or palliative care [5]. Nevertheless, a number of other authors have shown that radical cystectomy may be performed safely in well-selected elderly patients.
Laparoscopic radical cystectomy (LRC) is a challenging technique, but it has been proposed as an alternative to open radical cystectomy (ORC), which is currently the gold standard technique. LRC is still considered an experimental procedure [3], despite being a minimally invasive technique and existing several comparative series between ORC and LRC which demonstrate some advantages such as reduced blood loss, which leads to improved recovery of patients, and shorter hospital stay after the procedure [7, 8]. LRC has been performed in our department since 2005, and nowadays is the treatment of choice for muscle invasive bladder cancer.
The aim of our work was to determine if there are significant differences in the comorbidities, complications and survival of patients over 70 years with bladder cancer treated with LRC in comparison with younger patients.
MATERIAL AND METHODS
We reviewed the hospital records of 180 patients who underwent LRC between 2005 and 2012 in our department. The main indication for surgery included muscle invasive bladder cancer and recurrent high-grade bladder cancer. Patients were divided into 2 different age groups according to the age at the time of the surgery: <70 years (n = 103) and ≥70 years (n = 77).
All patients who were considered for LRC underwent a rigorous preoperative physical examination and blood test to ensure adequate physical performance status. Patients also had routine pre operativeimaging evaluation with computer tomography of the chest-abdomen-pelvis and bone scan to diagnose distant metastases.
LRC was performed following the technique described by Cansino et al. [9]. Bilateral extended lymphadenectomy was performed in all cases. The urinary diversion type was based on patient characteristics. Urinary diversion was performed extracorporeally, except in the 28 cases that were performed intracorporeally. The pathology specimens were reviewed by a genitourinary pathologist.
We compared different parameters such as comorbidities, intraoperative data, early complications (defined as occurring within 90 days of the LRC) and late complications (defined as occurring more than 90 days after the LRC). Complications were graded using the modified Clavien system [10]. Minor complications were defined as Clavien Grade 1-2 and major complications as Clavien Grade 3 or greater. Also we analyzed the TNM classification, using the seventh edition of the TNM classification of malignant tumors [11], and overall survival.
Contingency tables and the Pearson chi-square test were used to evaluate the association between the pairs of categorical variables. p <0.05 was considered statistically significant. The one-way ANOVA test was used to examine the differences of the length of hospital stay after LRC between groups. The Kaplan-Meier estimator was used to assess the overall survival of the patients.
RESULTS
A total of 180 patients were included for this study. The median age for patients <70 years and ≥70 years was 61 years (range 33-69) and 75 years (range 70-85), respectively. In the group of patients ≥70 years just 11% of them were 80 years or older. There were 81 males and 22 females in the younger group and 71 males and 6 females in the older group.
Intraoperative data were not significantly different between the younger and older group, including renal function (0.9 DE 0.8 vs. 1.1 DE 0.9), previous intra-abdominal surgery (39.8% vs. 46.7%), neoadjuvant chemotherapy (2 patients vs. 0 patients) and ASA scoring (I 23.3%, II 44.7%, III 28.1%, IV 3.9% vs. I 11.7%, II 50.6%, III 33.7%, IV 4%).
The comorbidities of the 2 groups are summarized in Table 1. The group of patients < 70 years had less comorbidities when compared with the group of patients ≥70 years. In the group of patients ≥70 years there was a higher percentage of hypertension, lung and heart disease. However, in this group there were a fewer number of diabetics and smokers.
Table 1.
Comorbidities
| <70 (N=103) | >70 (N=77) | P value | |
|---|---|---|---|
| High blood pressure | 26 (25.2%) | 36 (46.8%) | 0.020 |
| Diabetes Mellitus I | 7 (6.9%) | 1 (1.3%) | 0.740 |
| Diabetes Mellitus II | 9 (8.7%) | 13 (16.9%) | 0.780 |
| Smoker | 52 (50.2%) | 15 (19.5%) | 0.001 |
| Former smoker | 22 (21.4%) | 35 (45.5%) | 0.001 |
| Chronic obstructive pulmonary disease | 8 (7.8%) | 14 (18.2%) | 0.031 |
| Ischemic heart disease | 13 (12.6%) | 13 (16.9%) | 0.275 |
| Others | 53 (51.5%) | 49 (63.6%) | 0.069 |
Concerning urinary diversion in younger and older patients, 34,6% vs. 2,6% of patients, respectively, received an orthotopic neobladder and the rest of the patients received a heterotopic urinary diversion (52.5% vs. 79% for ileal conduit or 12.9% vs. 18.4% for cutaneous ureterostomy). There was a statistical significance in the difference between the groups (p = 0.001).
Intraoperative data was not significantly different between the younger and older group, including mean estimated blood loss (432 ml vs. 376 ml) and mean operating time (355 min vs. 365 min).
Intraoperative complication rates were similar in both groups, 16,5% and 13%, respectively (p = 0.43). The major intraoperative complication was blood loss requiring a transfusion of 2 or more red cell concentrates during the intervention (Table 2). There has not been any case of intraoperative death.
Table 2.
Complications
| <70 (N=103) | >70 (N=77) | P value | |
|---|---|---|---|
| Intraoperatives complications | 17 (16.5%) | 10 (13%) | 0.433 |
| Anemia requiring transfusion | 15 (14.5%) | 10 (13%) | |
| Others | 2 (2%) | 0 (0%) | |
| Postoperative complications | 53 (51.5%) | 44 (57.1%) | 0.272 |
| Early | |||
| Paralytic ileus | 17 (16.5%) | 22 (28.6%) | 0.040 |
| Anemia requiring transfusion | 17 (16.5%) | 12 (15.6%) | 0.518 |
| Infections | |||
| Sepsis | 2 (1.9%) | 3 (3.9%) | 0.365 |
| Intra abdominal abscess | 4 (3.9%) | 1 (1.3%) | 0.287 |
| Wound infection | 3 (2.9%) | 2 (2.6%) | 0.182 |
| Central line infection | 8 (7.8%) | 12 (15.6%) | 0.080 |
| Acute respiratory distress | 4 (3.9%) | 2 (2.6) | 0.486 |
| Myocardial infarction | 2 (1.9%) | 1 (1.3%) | 0.608 |
| Anastomotic complications Dehiscence | 0 (0%) | 2 (2.6%) | 0.182 |
| Fistula | 10 (9.7%) | 3 (3.9%) | 0.326 |
| Thrombosis | 1 (1%) | 0 (0%) | 0.572 |
| Renal failure | 3 (2.9%) | 8 (10.4%) | 0.040 |
| Others | 22 (21.4%) | 15 (19.5%) | 0.454 |
| Late | |||
| Anastomotic stricture | 16 (15.5%) | 10 (12.4%) | 0.261 |
| Parastomal hernia | 11 (14.2%) | 9 (11.6%) | 0.334 |
The early and late complications of the 2 groups are summarized in Table 2. During the postoperative period, both groups had similar complications rate, 51.5% and 57.1% respectively (p = 0.27). The most common complication observed was paralytic ileus (16.5% vs. 28.6%) followed by anemia requiring transfusion (16.5% vs. 15.5%). There was no statistical significance difference between the younger and the older patients. The only complications with the statistical difference between the two groups were paralytic ileus (p = 0.04) and the worsening of the renal function (p = 0.04) for the elderly.
In the younger group 86.8% of the complications were minor and 13.2% major. In the older group 75% of the complications were minor and 25% were major (Table 3). There were no statistical significant differences between the groups.
Table 3.
Clavien system
| < 70 | > 70 | |
|---|---|---|
| Grade I | 37.7% | 43.2% |
| Grade II | 49.1% | 31.8% |
| Grade III | ||
| Grade IIIa | 7.5% | 6.8% |
| Grade IIIb | 5.7% | 18.2% |
| Grade IV | ||
| Grade IVa | – | – |
| Grade IVb | – | – |
| Grade V | – | – |
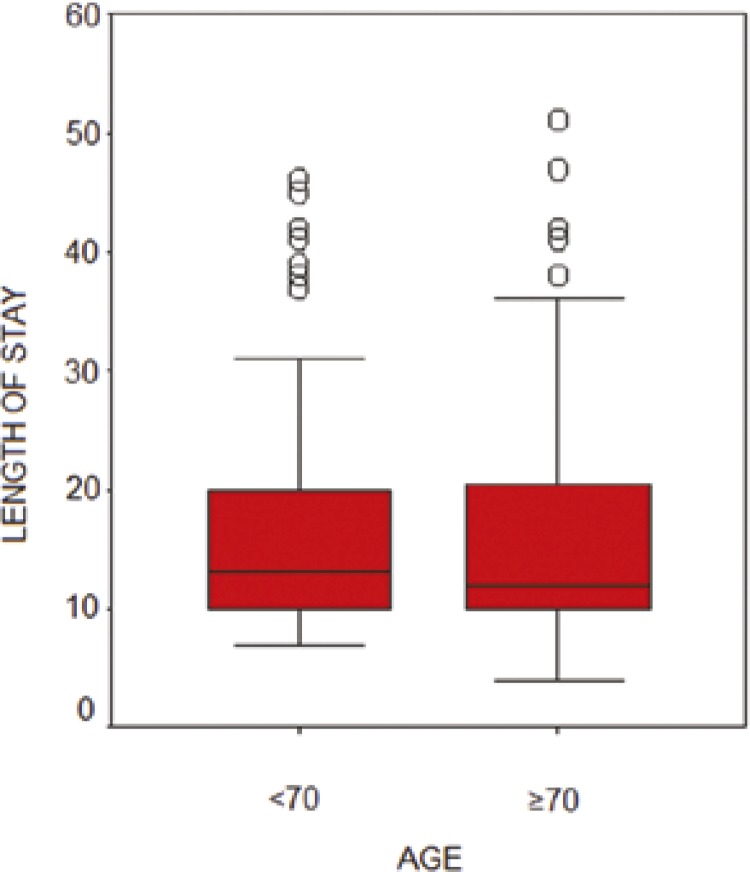
The median length of postoperative hospital stay was 13 days for the group of patients <70 years and 12 days for the group of patients ≥70 years (p = 0.61) (Figure 1).
Figure 1.
Median length of postoperative hospital stay.
Concerning postoperative mortality (in the first ninety days), there were 2 patients in the younger group and 3 patients in the older group.
Final pathologic outcomes are summarized in Table 4. The older group had a higher rate of non-organ confined disease (≥ pT3) than the younger group on final pathology; however, the difference was not significant. Lymph node metastasis was confirmed in 33% of the younger group and 34% of the older group of patients on final pathology. Metastatic disease was confirmed in less than 5% of the patients in both groups.
Table 4.
TNM
| <70 (N=103) | >70 (N=77) | P value | |
|---|---|---|---|
| Tumor stage | 0.256 | ||
| T1 or less | 27 (26.2%) | 14 (18.2%) | |
| T2 | 20 (19.4%) | 13 (16.9%) | |
| T3 | 40 (38.8%) | 29 (37.7%) | |
| T4 | 16 (15.6%) | 21 (27.2%) | |
| Lymph node metastases | 0.527 | ||
| N0 | 69 (67%) | 51 (66%) | |
| N1 or greater | 34 (33%) | 26 (34%) | |
| Metastatic disease | 0.513 | ||
| Mx | 7 (6.8%) | 4 (5.2%) | |
| M0 | 93 (90.3%) | 69 (89.6%) | |
| M1 | 3 (2.9%) | 4 (5.2%) |
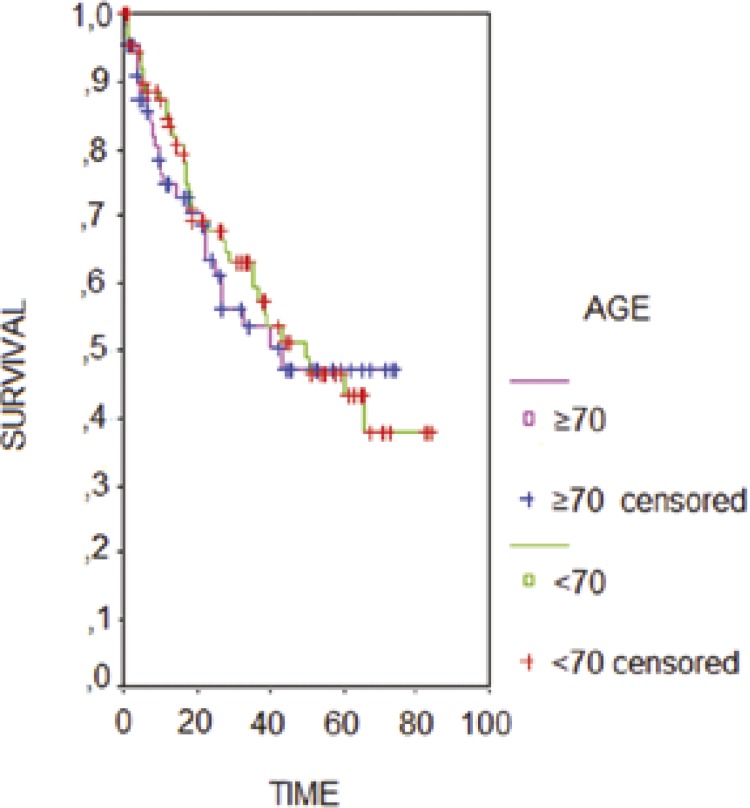
In the younger group 40% of the patients received adjuvant chemotherapy, and in the older group 36%. Kaplan-Meier curves comparing groups did not show significant differences. Overall, survival at 5 years was 50% for both groups (Figure 2).
Figure 2.
Kaplan-Meier curves for overall survival.
DISCUSSION
LRC has been shown to improve perioperative morbidity in selected cases, and as a result may be the preferred approach for elderly patients [5, 12, 13]. LRC is a minimally invasive technique that has demonstrated many advantages such as: 1) the reduction of intraoperative blood loss [14], 2) the good visualization of the pelvis in the Trendelenburg position, allowing for a better dissection [9], 3) the capacity of performing a good hemostasis with bi-polar grasping instruments 4) the reduction of blood loss due to the intra-abdominal pressure of the pneumoperitoneum and 5) shorter hospital stays after the procedure by achieving earlier patient recovery [15]. The group of patients over 70 years, who have more comorbidities, as shown in our study, would benefit even more than the rest of the population with the LRC.
According to the EAU guidelines, the controversy still remains about age, radical cystectomy and the type of urinary diversion. It is not clearly established which is the attitude to be taken towards the elderly. The intraoperative complication rates in the young and elderly patients in our study were 16,5% vs. 13% respectively. Blood loss and the number of patients requiring transfusion were similar to those reported in the laparoscopic/robotic series, but significantly lower than those reported in the open series [14–18]. Some studies suggest some association between older age and increased blood loss and transfusion rates [19], this association was not seen in our group of patients, probably because of the advantages described previously. Blood transfusion may have had a negative impact in oncological outcomes of bladder cancer [8, 20, 21], so this low transfusion rate seen with LRC may be traduced in a better oncological outcome. These findings have to be validated in medium – long term studies.
The postoperative complication rates in our study were 51.5% (<70 years) vs. 57.1% (>70 years). These rates are in the range reported in other series [19]. Following Clavien – Dindo classification, most of them were minor. Paralytic ileus was the most common complication and the second one was the worsening of the renal function. There is some variability in the definition of ileus including time to peristalsis or the time to flatus. Both are frequently used indicators of bowel function despite its limitations, such as subjectivity and observer dependency. Paralytic ileus was defined in our study as a non-mechanical obstruction of the intestine due to paralysis of the intestinal muscles during more than 48 hrs. Regarding the worsening of the renal function, it was defined as an increase of creatinine levels if the preoperative value was altered or creatinine levels greater than 1.2 ml/dl if the preoperative value was normal.
There are different points of view in the literature about these findings, Froehner et al reviewed complications following radical cystectomy in the elderly population and he found that most studies showed no relationship between the age at radical cystectomy and perioperative complications rates. However, others like Lowrance et al found significantly increased perioperative mortality rates in the elderly [19, 22].
Although the overall rates of complications were similar in both groups, the rate of major complications was higher in the older group (25% vs. 13.3%) meaning that older patients had greater risk of being subjected to additional surgical procedures.
As reported, the heterotopic urinary was the diversion of choice in the young and the elderly population. Following the 2013 EAU guidelines on muscle-invasive bladder cancer (3), the choice of diversion depends on performing status, preexisting comorbidities, and (to a lesser extent) age. There are many facts in our LRC series that lead us to perform a heterotopic urinary diversion: 1) patients comorbidities such as renal failure, 2) more than 50% of tumors in our series were pT3 and pT4 and N +, and some of these procedures were palliative due to recurrent hematuria, pain or obstruction of the upper urinary tract. Quality of life after a neobladder or conduit seems comparable [23, 24] and experienced centers reported similar complication rates in the elderly patients, regardless of the type of urinary diversion [19].
Mean hospital stay after laparoscopic radical cystectomy were similar in both groups and similar to previous laparoscopic or robotic series, but it was short when compared with the open series [11, 12, 14, 18]. Laparoscopic approach significantly reduces the length of hospital stay by reducing postoperative opiate use, having more rapid resumption of oral intake and early recovery of bowel functions [13, 25].
In the oncological aspect, most of the series of LRC published nowadays use different selection bias, this is reason why EAU guidelines still consider this procedure as experimental [3]. In our department all patients with muscle invasive and recurrent high-grade bladder cancer undergo LRC, regardless of the tumor stage, as seen in our results; this fact is supported because we have not seen differences in different oncological outcomes such as local or distant recurrence, positive margins and survival in comparison with series performed with the open approach [14, 26].
The decision for choosing the age of 70 as a cutoff point was because of the limited number of cases we have in our center. There were just 9 cases of patients over 80 years. It would have been interesting to know the results in those over 80 years, but additional trials should be conducted in a multicenter study due to the low number of patients in this age group who underwent LRC. In this group of patients there are many factors involved besides the age, such as the comorbidity index and quality of life.
There are limited studies for the long term oncological outcomes in LRC or robotic radical cystectomy and our findings have some limitations: the limitations associated with a retrospective study and a small cohort of patients. However, it is the largest single center study regarding the minimal invasive approach in bladder cancer and elderly patients. Our study suggests that there are no differences in the overall survival at 5 years between patients <70 years and > 70 years. Our medium term follow up results in overall survival (5 years) are one of the few described in literature. This is an important finding that should help to advocate for LRC in elderly patients instead of less aggressive forms of treatment such as radiation therapy, repeated transurethral resection, partial cystectomy or palliative care [5].
CONCLUSIONS
Laparoscopic radical cystectomy in the elderly patient has similar rates of perioperative morbidity when compared with younger patients and may be offered as a treatment option in selected elderly patients. Further research and comparative multicentric studies in this field are needed to make clear recommendations.
References
- 1.Novotny V, Hakenberg OW, Wiessner D, Heberling U, Litz RJ, Oehlschlaeger S, Wirth MP. Perioperative complications of radical cystectomy in a contemporary series. Eur Urol. 2007;52:397–402. doi: 10.1016/j.eururo.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Clark PE, Stein JP, Groshen SG, Cai J, Miranda G, Lieskovsky G, Skinner DG. Radical cystectomy in the elderly: comparison of clinical outcomes between younger and older patients. Cancer. 2005;104:36–43. doi: 10.1002/cncr.21126. [DOI] [PubMed] [Google Scholar]
- 3.Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, et al. EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2013 Guidelines. Eur Urol. 2014;4:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Liedberg F. Early complications and morbidity of radical cystectomy. Eur Urol. 2010;9:25–30. [Google Scholar]
- 5.Zebic N, Weinknecht S, Kroepfl D, et al. Radical cystectomy in patients aged > or = 75 years: an updated review of patients treated with curative and palliative intent. BJU Int. 2005;95:1211–1214. doi: 10.1111/j.1464-410X.2005.05507.x. [DOI] [PubMed] [Google Scholar]
- 6.Novara G, De Marco V, Aragona M, Boscolo-Berto R, Cavalleri S, Artibani W, Ficarra V. Complications and Mortality after radical cystectomy for bladder transitional cell cancer. J Urol. 2009;182:914–921. doi: 10.1016/j.juro.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Lawrentschuk N, Colombo R, Hakenberg OW, Lerner SP, Månsson W, Sagalowsky A, Wirth MP. Prevention and management of complications following radical cystectomy for bladder cancer. Eur Urol. 2010;57:983–1001. doi: 10.1016/j.eururo.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Gómez Rivas J, Alonso y Gregorio S, Cisneros Ledo J, Tabernero Gómez A, Díez Sebastián J, de la Peña Barthel J.J. The role of perioperative blood transfusion on postoperative outcomes and overall survival in patients after laparoscopic radical cystectomy. J Cancer Res Ther. [Epub ahead of print] [cited 2015 Feb 20]. Available from: http://www.cancerjournal.net/preprintarticle.asp?id=146125. [DOI] [PubMed]
- 9.Cansino JR, Cisneros J, Alonso S, Martinez-Piñeiro L, Aguilera A, Tabernero A, et al. Laparoscopic radical cystectomy: initial series and analysis of results. Eur Urol Suppl. 2006;5:956–961. [Google Scholar]
- 10.Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobin LH, Gospodarowicz M, Wittekind C. 7th edn. Wiley-Blackwell; 2009. TNM classification of malignant tumors. UICC International Union Against Cancer; pp. 262–265. [Google Scholar]
- 12.Guillotreau J, Miocinovic R, Gamé X, Forest S, Malavaud B, Kaouk J, et al. Outcomes of laparoscopic and robotic radical cystectomy in the elderly patients. Urology. 2012;79:585–590. doi: 10.1016/j.urology.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 13.Haber G, Crouzet S, Gill I. Laparoscopic and robotic assisted radical cystectomy for bladder cancer: A critical analysis. Eur Urol. 2008;54:54–62. doi: 10.1016/j.eururo.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 14.Guillotreau J, Gamé X, Mouzin M, Doumerc N, Mallet R, Sallusto F, et al. Radical cystectomy for bladder cancer: morbidity of laparoscopic versus open surgery. J Urol. 2009;181:554–559. doi: 10.1016/j.juro.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Hemal AK, Kolla SB. Comparison of laparoscopic and open radical cystoprostatectomy or localized bladder cancer with 3-year oncological follow up: a single surgeon experience. J Urol. 2007;178:2340–2343. doi: 10.1016/j.juro.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Porpiglia F, Renard J, Billia M, Scoffone C, Cracco C, Terrone C, Scarpa RM. Open versus laparoscopy-assisted radical cystectomy: results of a prospective study. J Endourol. 2007;21:325–329. doi: 10.1089/end.2006.0224. [DOI] [PubMed] [Google Scholar]
- 17.Ng CK, Kauffman EC, Lee MM, Otto BJ, Portnoff A, Ehrlich JR, et al. A Comparison of Postoperative Complications in Open versus Robotic Cystectomy. Eur Urol. 2010;57:274–282. doi: 10.1016/j.eururo.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Styn NR, Montgomery JS, Wood DP, Hafez KS, Lee CT, Tallman C, et al. Matched comparison of robotic-assisted and open radical cystectomy. Urology. 2012;78:1303–1309. doi: 10.1016/j.urology.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 19.Froehner M, Brausi MA, Herr HW, Muto G, Studer UE. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol. 2009;56:443–454. doi: 10.1016/j.eururo.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Linder BJ, Frank I, Cheville JC, Tollefson MK, Thompson RH, Tarrell RF, et al. The impact of perioperative blood transfusion on cancer recurrence and survival following radical cystectomy. Eur Urol. 2013;63:839–845. doi: 10.1016/j.eururo.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Morgan TM, Barocas DA, Chang SS, Phillips SE, Salem S, Clark PE, et al. The relationship between perioperative blood transfusión and overall mortality in patients undergoing radical cystectomy for bladder cancer. Urol Oncol. 2013;31:871–877. doi: 10.1016/j.urolonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrentschuk N, Colombo R, Hakenberg OW, Lerner SP, Månsson W, Sagalowsky A, Wirth MP. Prevention and management of complications following radical cystectomy for bladder cancer. Eur Urol. 2010;57:983–1001. doi: 10.1016/j.eururo.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Porter MP, Penson DF. Health related quality of life after radical cystectomy and urinary diversion for bladder cancer: a systematic review and critical analysis of the literature. J Urol. 2005;173:1318–1322. doi: 10.1097/01.ju.0000149080.82697.65. [DOI] [PubMed] [Google Scholar]
- 24.Aboumarzouk OM, Drewa T, Olejniczak P, Chlosta PL. Laparoscopic radical cystectomy: neobladder or ileal conduit, debate still goes on. Cent European J Urol. 2014;67:9–15. doi: 10.5173/ceju.2014.01.art2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gómez Rivas J. Fast recovery after radical cystectomy. A multidisciplinary challenge. Cent European J Urol. 2014;67:342–343. doi: 10.5173/ceju.2014.04.art5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gómez Rivas J, Alonso y Gregorio S, Sánchez Molina S, et al. Cistectomia radical laparoscópica: 7 años de experiencia. Actas Urológicas Españolas. 2013;37:185. [Google Scholar]