Abstract
Introduction
Inflammatory responses following tissue injury are essential for proper tissue regeneration. However, dysfunctional or repetitive inflammatory tissue assaults can lead to poor tissue regeneration and ultimate tissue failure via fibrosis. Previous attempts at urinary bladder tissue regeneration utilizing polymeric and biologic scaffolding materials tended to elicit these responses leading to poor tissue regeneration. Recent advances in bladder regeneration utilizing bone marrow derived mesenchymal stem cells (MSCs) and CD34+ hematopoietic stem/progenitor cells (HSPCs) with biocompatible citric acid based scaffolds have provided an environment that not only promotes the growth of architecturally germane and physiologically functional tissue, but also modulates aspects of the innate immune response.
Material and methods
Within this study MSCs, CD34+ HSPCs, or MSC/CD34+ HSPC seeded POC [poly (1,8-octanediol-co-citrate)] scaffolds were utilized in an established rodent bladder augmentation model to evaluate inflammation as it pertains to bladder tissue regeneration.
Results
Quantified data from post-augmentation regenerated tissue samples at the 4 week time-point demonstrated that POC/MSC and POC/MSC + CD34+ HSPC grafts markedly reduced the presence of pro-inflammatory CD68+ macrophages and MPO+ neutrophils compared to unseeded POC or POC/CD34+ HSPC-only seeded grafts. Pro-inflammatory cytokines TNFα and IL-1b were also significantly down-regulated with a concomitant increase in the anti-inflammatory cytokines IL-10 and IL-13 in the aforementioned POC/MSC and POC/MSC + CD34+ HSPC composites. Furthermore, this led to fewer instances of bladder tissue granuloma formation combined with greater muscle content and tissue angiogenic events as previous data has demonstrated.
Conclusions
Data indicates that POC/MSC and POC/MSC + CD34+ HSPC grafts attenuate the innate inflammatory response and promote bladder tissue regeneration.
Keywords: hematopoietic stem cells, mesenchymal stem cells, bone marrow, CD34+ hematopoietic progenitors, tissue regeneration
INTRODUCTION
Strategies for tissue regeneration must consider an assortment of factors that influence the proper reformation of functional tissue including inflammation [1–5]. Components of the inflammatory pathway can shape the means in which proper wound healing is attained or negatively effect overall tissue remodeling [2, 6, 7]. Specifically, cellular constituents of the innate immune system are typically the first to arrive at areas of tissue injury in an effort to provide the foundation for subsequent tissue regeneration [8–11]. However, in situations where adverse pathology or reaction to a foreign body plays a pivotal role in wound healing outcomes, alternative strategies must be employed to temper overzealous or dysfunctional innate inflammatory immune responses.
Two major factors that can affect the role of inflammation during tissue regenerative processes are the scaffold composition and choice of cells used for scaffold seeding. Studies employing synthetic and biological scaffolds for tissue regeneration have been hampered by a myriad of obstacles, including scaffolds that were non-compliant or possessed limited structural integrity and lacked a means to mediate inflammation while simultaneously promoting tissue regeneration [12–14]. POC [poly (1,8-octanediol-co-citrate)] is an elastomeric scaffold that has demonstrated the ability to mimic mechanical properties of the bladder while providing an amenable surface for cell growth [15–17]. MSCs have been shown to modulate acute inflammatory responses and ameliorate injury in vivo, while CD34+ HSPCs robustly induce angiogenesis and provide support for peripheral nerve regeneration [15, 18, 19]. Within the framework of this study, we have attempted to determine whether human bone marrow (BM) MSCs and CD34+ HSPCs combined with POC could influence the innate inflammatory response and promote bladder tissue regeneration in a rodent model of bladder augmentation.
MATERIALS AND METHODS
Bladder augmentation model
POC [poly (1,8-octanediol-co-citrate)] was synthesized and cell seeded as previously described [15]. Athymic female rats underwent bladder augmentation as previously described in detail [15]. The cystectomized defect was augmented with 1) unseeded POC (uPOC); 2) POC/CD34+ HSPCs; 3) POC/MSCs; or 4) POC/MSCs + CD34+ HSPCs. n = 3 animals/group at 4 and 10 week time-points. Animal studies were approved by the Institutional Animal Care and Use Committee at the Stanley Mann Research Center, Chicago.
Immunofluorescence analysis and quantification of augmented bladder tissues
Immunofluorescence (IF) staining of explanted bladder tissues was completed as previously described [15]. CD68 (macrophage marker), MPO (myeloperoxidase; neutrophil marker), IL-1β, TNFα, IL-10, and IL-13 were used for IF. Antibodies were obtained from Abcam or Santa Cruz Biotechnology and used as described [15, 16]. Tissue samples were also quantified for respective marker/cytokine expression as previously described [15, 16].
Statistical analysis
Differences between groups were determined using ANOVA, with the Tukey–Kramer method for multiple comparisons (SAS 9.4). P values <0.05 were considered statistically significant.
RESULTS
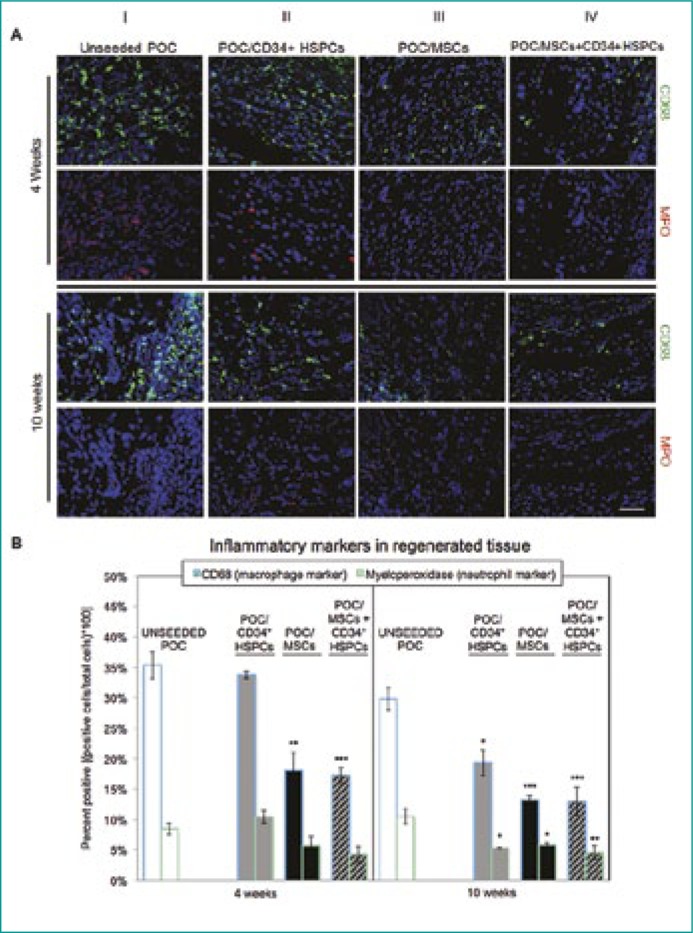
IF staining subjectively demonstrated elevated levels of CD68+ and MPO+ cells within uPOC or POC/CD34+ HSPC grafts at 4 and 10 week time-points (Figure 1A, columns I and II, respectively) compared to other graft conditions. POC/MSC and POC/MSC + CD34+ HSPC grafts demonstrated a marked decrease in both CD68+ and MPO+ cells within regenerated tissues at both time-points (Figure 1A, columns III and IV, respectively). Quantified data corroborated visual findings (Figure 1B).
Figure 1.
Macrophage and neutrophil distribution in unseeded or cell seeded bladder augmented tissue samples.
(A) CD68+ macrophage (green) expression was highly abundant in uPOC and POC/CD34+ HSPCs grafts. The infiltrating macrophage population encompassed the entirety of the graft and was also evident at contact points where the suture traversed native bladder tissue into the POC. Similarly, MPO+ neutrophils (red) were also found to be in greater numbers in the aforementioned treatment groups. In contrast, POC/MSC and POC/MSC-CD34+ HSPC grafts displayed considerably lesser levels of both CD68+ macrophage and MPO+ neutrophils at both 4 and 10 weeks post-surgery. n = 10 images/animal. Blue = DAPI. Magnification, 400x. Scale bar, 50 µm.
(B) Quantification of CD68+ (left bars) and MPO+ (right bars) IF staining at 4 weeks (left panel) and 10 weeks (right panel). At 4 and 10 weeks post-augmentation, CD68+ cells comprised 35.4 ±2.2% and 29.8 ±1.9% of the regenerated tissue from unseeded grafts, with MPO+ levels (CD68+ and CD68-) of 8.5 ±1.0% and 10.5 ±1.2%. POC/MSC grafts, with or without CD34+ HSPCs, resulted in a significant decrease in the macrophage infiltrate (4 and 10 weeks) and in the neutrophil population (10 weeks) (4W: POC/MSC 18.1 ±2.9% CD68+, 5.7 ±1.5% MPO+ and POC/MSC + CD34+ HSPC 17.3 ±1.2% CD68+, 4.3 ±1.3% MPO +; 10W POC/MSC 13.2 ±0.7% CD68+, 5.8 ±0.3% MPO+ and POC/MSC+ CD34+ HSPCs 13.0 ±2.3% CD68+, 4.6 ±1.2% MPO+). POC/CD34+ HSPCs grafts showed a delayed effect, with reduced macrophage and neutrophil populations observed at 10 weeks (4W: 33.8 ±0.6% CD68+, 10.4 ±1.0% MPO+; 10W: 19.3 ±2.1% CD68+, 5.3 ±0.1% MPO+). Data shown as means ±SE. Significance shown for comparison of cell-seeded groups to uPOC; *P <0.05, **P <0.01, ***P ≤0.001, ****P <0.0001.
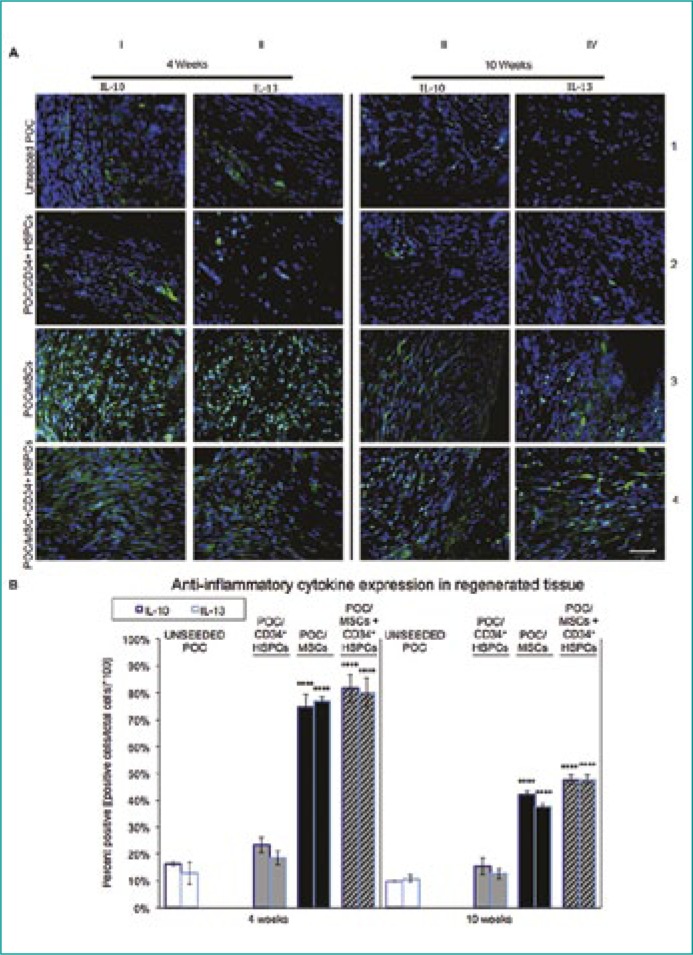
Levels of anti-inflammatory cytokines IL-10 and IL-13 were most prominent in POC/MSC and POC/MSC + CD34+ HSPC grafts at the 4 week time-point and gradually decreased at 10 weeks post-surgery (Figure 2A, rows 3 and 4, respectively). POC/CD34+ HSPCs and uPOC grafts exhibited significantly lower levels of IL-10 and IL-13 production at both time-points as quantified in Figure 2B.
Figure 2.
Anti-inflammatory IL-10 and IL-13 cytokine expression in regenerating bladder tissue.
(A) The cytokines IL-10 and IL-13 are known to possess anti-inflammatory properties and facilitate tissue regeneration throughout the remodeling phases of inflammation. At 4 weeks post-augmentation, both IL-10 (green) and IL-13 (green) expression is low in uPOC and POC/CD34+ HSPC grafts. This trend continued as seen at the 10 week time-point where the levels of expression decrease even further. Antithetical results are observed with POC/MSC and POC/MSC + CD34+ HSPC grafts. There is an approximate 4-fold increase in IL-10 and IL-13 cytokine expression at 4 weeks post-augmentation compared to the uPOC control. Although the levels of the anti-inflammatory cytokines decreased at the 10 week time-point, there were still significant levels of expression of both cytokines, again approximately 4-fold greater than uPOC control grafts. n = 10 images/animal. Blue = DAPI. Magnification, 400x. Scale bar, 50µm.
(B) Quantification of anti-inflammatory cytokine expression, shown as percentage of cells staining positive for IL-10 (left bars) and IL-13 (right bars) at 4 weeks (left panel) and 10 weeks (right panel). IL-10 and IL-13 expression levels indicated a strongly anti-inflammatory profile for POC/MSCs or POC/MSC + CD34+ HSPCs. Notably, at 4 weeks the presence of MSCs increased mean expression of IL-10 and IL-13 from <20% of the cell population in the unseeded group to >70% in MSC-seeded groups (4W: uPOC 16.3 ±0.8% IL-10+ and 12.8 ±4.1% IL-13+ vs. POC/MSC 74.6 ±5.1% IL-10+ and 77.0 ±1.5% IL-13+, POC/MSC + CD34+ HSPCs 81.7 ±5.2% IL-10+ and 80.1 ±5.3% IL-13+; 10W: uPOC 9.9 ±0.3% IL-10+ and 10.7 ±1.3% IL-13+ vs. POC/MSC 16.0 ±1.6% 42.0 ±1.4% IL-10+ and 37.6 ±1.2% IL-13+, POC/MSC + CD34+ HSPCs 47.6 ±1.8% IL-10+ and 47.7 ±1.6% IL-13+). Significant effects were not observed for POC/CD34+ HSPCs grafts (4W: 23.3 ±2.9% IL-10+ and 18.6 ±2.6% IL-13+; 10W: 15.4 ±3.2% IL-10+ and 12.6 ±1.8% IL-13+). Data shown as means ±SE. Significance shown for comparison of cell-seeded groups to uPOC; *P <0.05, **P <0.01, ***P ≤0.001, ****P <0.0001.
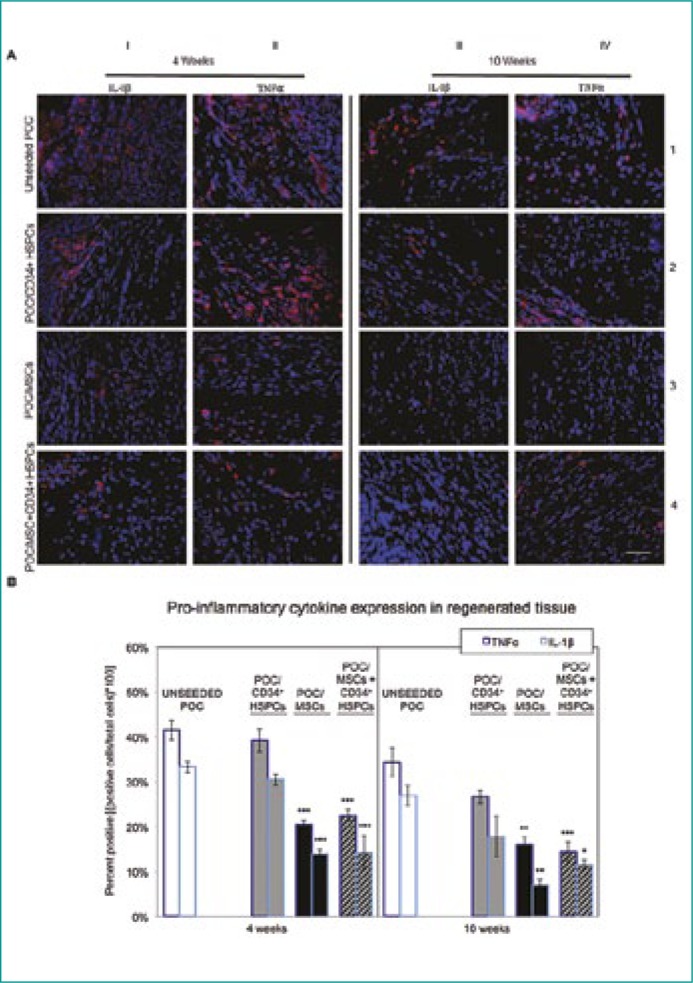
Pro-inflammatory cytokines IL-1β and TNFα exhibited their highest levels of expression at the 4 week time-point in POC/CD34+ HSPCs and uPOC grafts (Figure 3A, columns I, II and rows 1, 2) exhibited a modest decline in expression of each cytokine in uPOC grafts, while a greater decline was seen in the POC/CD34+ HSPCs grafts at 10 weeks post-surgery. IF data were verified as described in Figure 3B.
Figure 3.
Pro-inflammatory IL-1β and TNFα cytokine expression in regenerating bladder tissue.
(A) Detrimental to proper tissue regeneration is the presence of confounding pro-inflammatory cytokines including IL-1β and TNFα. IL-1β (red) and TNFα (red) levels were highly abundant in CD34+ HSPC and uPOC grafts at the 4 week time-point. TNFα levels remained elevated 10 weeks post-surgery while IL-1β decreased only moderately in the unseeded samples. IL-1β and TNFα levels in POC/MSC and POC/MSC + CD34+ HSPC grafts were approximately half the value found in POC/CD34+ HSPC and uPOC grafts at the 4 week time-point. There was a general decrease in pro-inflammatory cytokine expression at the 10 week time-point for all samples; however, TNFα expression was relatively elevated with the POC/CD34+ HSPC seeded construct. n = 10 images/animal. Blue = DAPI. Magnification, 400x. Scale bar, 50 µm.
(B) Quantification of pro-inflammatory cytokine expression, shown as percentage of cells staining positive for TNFα (left bars) and IL-1β (right bars) at 4 weeks (left panel) and 10 weeks (right panel). The cytokine expression profile for uPOC graft tissue was predominantly pro-inflammatory, with mean percentages of 41.5 ±2.2% TNFα+ and 33.3 ±1.2% IL-1β+ at 4 weeks and 34.3 ±3.1% TNFα+ and 27.0 ±2.3% IL-1β+ at 10 weeks. Significant reduction in both TNFα and IL-1β expression was observed for grafts seeded with MSCs or MSCs/CD34+ HSPCs (4W: POC/MSC 20.5 ±0.8% TNFα+ and 13.9 ±0.9% IL-1β+, POC/MSC + CD34+ HSPCs 22.5 ±1.3% TNFα+ and 14.2 ±3.7% IL-1β+; 10W: POC/MSC 16.0 ±1.6% TNFα+ and 7.0 ±1.2% IL-1β+, POC/MSC + CD34+ HSPCs 14.4 ±2.2% TNFα+ and 11.5 ±1.1% IL-1β+); grafts seeded only with CD34+ HSPCs did not show this effect (4W: 39.2 ±2.6% TNFα+ and 30.5 ±1.3% IL-1β+; 10W: 26.6 ±1.5% TNFα+ and 17.8 ±4.6% IL-1β+). Data shown as means ±SE. Significance shown for comparison of cell-seeded groups to uPOC; *P <0.05, **P <0.01, ***P ≤0.001, ****P <0.0001.
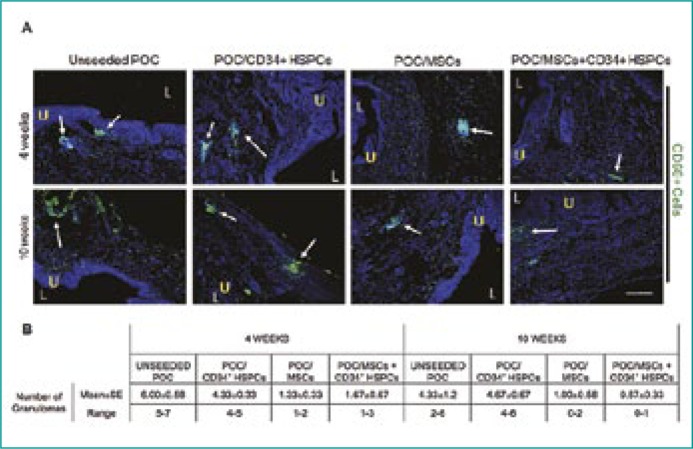
Granulomas (CD68+ macrophages clustering) in regenerating bladder tissue were highly abundant in uPOC and POC/CD34+ HSPC grafts while lesser numbers of granulomas were present in POC/MSC and POC/MSC + CD34+ HSPC grafts (Figure 4A), which remained fairly constant throughout both time-points. Quantified data supported in vivo findings (Figure 4B).
Figure 4.
Incidence of granuloma formation in uPOC or cell/scaffold composites.
(A) The accumulation of CD68+ macrophage clusters (green) was most prominent in 4- and 10-week uPOC and POC/CD34+ HSPC grafts. (Figure 4A, columns I and II). The numbers of granulomas was greatly reduced in POC/MSC and POC/MSC + CD34+ HSPC grafts (Figure 4A, columns III and IV) and both time-points. U = urothelium; L = bladder lumen; white arrows depict granulomas. n = 10 images/animal. Blue = DAPI. Magnification, 100x. Scale bar, 200 µm.
(B) Frequency of granulomas in bladder tissue decreased with the addition of MSCs, but not CD34+ HSPCs, to grafts. At 4 weeks, the mean number of granulomas for MSC-seeded groups was <2; by 10 weeks further reduction resulted in instances of no detectable granulomas (POC/MSC range 0-2; POC/MSC + CD34+ HSPCs range 0-1).
DISCUSSION
The interplay between inflammation-based response mechanisms to tissue insult and endogenous regeneration programs are pivotal for proper tissue reformation [5, 20, 21]. Aberrant expression of inflammatory pathway components can lead to suboptimal tissue regeneration, which is reflected in poor architectural remodeling and ineffective organ function. Since the initial response to tissue injury is typically a cascade of innate immune events that include macrophage and granulocyte localization, it is essential to modulate, but not abolish, the catabolic effects of pro-inflammatory cytokines in this setting [15, 16, 22]. The application of BM MSCs and the differentiated progeny of CD34+ HSPCs to attenuate highly reactive innate immune inflammation responses is potentially a feasible option for bladder regeneration.
MSCs known to possess potent anti-inflammatory properties and the ability to alter macrophage phenotype [22–24] were not only able to modulate aspects of macrophage and neutrophil migration, but were also able to influence pro- and anti-inflammatory cytokine secretion in vivo. Specifically, MSC/POC grafts decreased the presence of macrophage and neutrophils ∼2- and 1.5-fold, respectively, compared to uPOC grafts at 4 weeks post-augmentation (Figure 1). This correlated to a substantial up-regulation in anti-inflammatory cytokine secretion of IL-10 and IL-13 (Figure 2), which was ∼4-fold greater than control samples combined with a significant reduction of pro-inflammatory cytokines TNFα and IL-β (Figure 3). Decreased levels of TNFα and IL-β expression mediated by a reduction in macrophage activity or by agonist supplementation have been shown to be conducive to tissue repair and regeneration in a number of settings including nerve regeneration [25–28]. Although POC/MSC + CD34+ HSPC grafts did not appear to greatly alter the levels of macrophage and neutrophil tissue content and cytokine expression compared to POC/MSC grafts, it had a profound effect on other aspects of bladder tissue regeneration. We have previously demonstrated that POC/MSC + CD34+ HSPC grafts greatly increased bladder muscle growth and promoted peripheral nerve regeneration and tissue vascularization when compared to uPOC or POC/MSC grafts at the 4 week time-point [15]. As TNFα maintains a pleiotropic role with regards to angiogenesis, markedly reduced levels of TNFα did not appear to effect levels of angiogenesis in our model [15, 29, 30]. Compensatory attributes provided by the CD34+ HSPCs may have accounted for the robust angiogenic response. The data presented here supports data derived from a previous study by Pokrywczynska and colleagues, which also demonstrated the pro-regenerative and anti-inflammatory power of bone marrow cells [31]. Within the context of that study, it was observed that there was an increased expression of anti-inflammatory cytokines along with corresponding matrix metalloproteinases (MMPs) that provided the impetus for rigorous bladder detrusor muscle regeneration in an in vivo model of bladder augmentation.
The onset of granuloma formation is initiated with the localized accumulation of inflammatory mononuclear cells including macrophages [32]. Granulomas can eventually transition to fibrosis and compromise bladder elasticity and function with the potential to negatively impact the upper urinary tract. In the presence of POC/MSC grafts, augmented bladders had a diminished capacity to form granulomas (Figure 4B) in part due to a reduction in macrophage numbers. Thus, an augmentation scheme utilizing POC/MSCs and POC/CD34+ HSPCs can help alleviate the formation of granulomas and promote healthy bladder tissue regeneration.
This study provided a limited but poignant examination of the anatomical and histological features in which inflammatory factors that may affect bladder tissue regeneration in the presence of MSCs and CD34+ HSPCs. As only specific aspects of the innate immune system were studied, other arms of the immune system must also be scrutinized to delineate the roles between inflammation and regeneration.
CONCLUSIONS
Compared to controls, POC/MSCs (± CD34+ HSPCs) decreased numbers of macrophages and neutrophils along with levels of pro-inflammatory cytokines while promoting anti-inflammatory cytokine expression during regeneration with a concomitant reduction in the number of tissue granulomas. However, the addition of CD34+ HSPCs to the grafts was necessary for multifaceted bladder regeneration. The partial modulation of inflammation likely produced an environment advantageous to overall bladder tissue regeneration.
References
- 1.Lilja HE, Morrison WA, Han XL, Palmer J, Taylor C, Tee R, Möller A, et al. An adipoinductive role of inflammation in adipose tissue engineering: key factors in the early development of engineered soft tissues. Stem Cells Dev. 2013;22:1602–1613. doi: 10.1089/scd.2012.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bury MI, Fuller NJ, Meisner JW, Hofer MD, Webber MJ, Chow LW, et al. The promotion of functional urinary bladder regeneration using anti-inflammatory nanofibers. Biomaterials. 2014;35:9311–9321. doi: 10.1016/j.biomaterials.2014.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujihara Y, Takato T, Hoshi K. Macrophage-inducing FasL on chondrocytes forms immune privilege in cartilage tissue engineering, enhancing in vivo regeneration. Stem Cells. 2014;32:1208–1219. doi: 10.1002/stem.1636. [DOI] [PubMed] [Google Scholar]
- 4.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 6.Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 8.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi ME, Manfredi AA. How macrophages ring the inflammation alarm. Proc Natl Acad Sci USA. 2014;111:2866–2867. doi: 10.1073/pnas.1324285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu B, Antoine DJ, Kwan K, Lundbäck P, Wähämaa H, Schierbeck H, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci USA. 2014;111:3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 12.Ashley RA, Roth CC, Palmer BW, Kibar Y, Routh JC, Fung KM, et al. Regional variations in small intestinal submucosa evoke differences in inflammation with subsequent impact on tissue regeneration in the rat bladder augmentation model. BJU Int. 2010;105:1462–1468. doi: 10.1111/j.1464-410X.2009.08965.x. [DOI] [PubMed] [Google Scholar]
- 13.Ashley RA, Palmer BW, Schultz AD, Woodson BW, Roth CC, Routh JC, et al. Leukocyte inflammatory response in a rat urinary bladder regeneration model using porcine small intestinal submucosa scaffold. Tissue Eng Part A. 2009;15:3241–3246. doi: 10.1089/ten.TEA.2008.0699. [DOI] [PubMed] [Google Scholar]
- 14.Adamowicz J, Kowalczyk T, Drewa T. Tissue engineering of urinary bladder - current state of art and future perspectives. Cent European J Urol. 2013;66:202–206. doi: 10.5173/ceju.2013.02.art23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma AK, Bury MI, Fuller NJ, Marks AJ, Kollhoff DM, Rao MV, et al. Cotransplantation with specific populations of spina bifida bone marrow stem/progenitor cells enhances urinary bladder regeneration. Proc Natl Acad Sci USA. 2013;110:4003–4008. doi: 10.1073/pnas.1220764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma AK, Hota PV, Matoka DJ, Fuller NJ, Jandali D, Thaker H, et al. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials. 2010;31:6207–6217. doi: 10.1016/j.biomaterials.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 17.Chung EJ, Kodali P, Laskin W, Koh JL, Ameer GA. Long-term in vivo response to citric acid-based nanocomposites for orthopaedic tissue engineering. J Mater Sci Mater Med. 2011;22:2131–2138. doi: 10.1007/s10856-011-4393-5. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 20.Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Gourevitch D, Kossenkov AV, Zhang Y, Clark L, Chang C, Showe LC, Heber-Katz E. Inflammation and Its Correlates in Regenerative Wound Healing: An Alternate Perspective. Adv Wound Care (New Rochelle) 2014;3:592–603. doi: 10.1089/wound.2014.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichim TE, Alexandrescu DT, Solano F, Lara F, Campion Rde N, Paris E, et al. Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol. 2010;260:75–82. doi: 10.1016/j.cellimm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 24.Wise AF, Williams TM, Kiewiet MB, Payne NL, Siatskas C, Samuel CS, Ricardo SD. Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306:F1222–F1235. doi: 10.1152/ajprenal.00675.2013. [DOI] [PubMed] [Google Scholar]
- 25.Roth CC, Mondalek FG, Kibar Y, Ashley RA, Bell CH, Califano JA, et al. Bladder regeneration in a canine model using hyaluronic acid-poly(lactic-co-glycolic-acid) nanoparticle modified porcine small intestinal submucosa. BJU Int. 2011;108:148–155. doi: 10.1111/j.1464-410X.2010.09757.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Wei FX, Cen JS, Ping SN, Li ZQ, Chen NN, et al. Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. Brain Res. 2014;1575:87–100. doi: 10.1016/j.brainres.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 27.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, et al. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain. J Neurosci. 2011;31:12533–12542. doi: 10.1523/JNEUROSCI.2840-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutton DL, Kondragunta R, Moore EM, Hung BP, Jia X, Grayson WL. Tumor Necrosis Factor Improves Vascularization in Osteogenic Grafts Engineered with Human Adipose-Derived Stem/Stromal Cells. PLoS One. 2014;9:e107199. doi: 10.1371/journal.pone.0107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pokrywczynska M, Jundzill A, Bodnar M, Adamowicz J, Tworkiewicz J, Szylberg L, et al. Do mesenchymal stem cells modulate the milieu of reconstructed bladder wall? Arch Immunol Ther Exp (Warsz) 2013;61:483–493. doi: 10.1007/s00005-013-0249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sneller MC. Granuloma formation, implications for the pathogenesis of vasculitis. Cleve Clin J Med. 2002;69(suppl 2):SII40–SII43. doi: 10.3949/ccjm.69.suppl_2.sii40. [DOI] [PubMed] [Google Scholar]