Preusser et al. use MRI-based lesion-symptom mapping to confirm the causal role of a ventral pathway in the perception of touch. This pathway originates downstream of the postcentral gyrus in the parietal operculum, passes the insula and the putamen, before terminating in white matter projections extending to inferior lateral prefrontal cortex.
Keywords: perception, touch, somatosensory cortex, lesion symptom mapping, ventral somatosensory pathway
Abstract
In humans, touching the skin is known to activate, among others, the contralateral primary somatosensory cortex on the postcentral gyrus together with the bilateral parietal operculum (i.e. the anatomical site of the secondary somatosensory cortex). But which brain regions beyond the postcentral gyrus specifically contribute to the perception of touch remains speculative. In this study we collected structural magnetic resonance imaging scans and neurological examination reports of patients with brain injuries or stroke in the left or right hemisphere, but not in the postcentral gyrus as the entry site of cortical somatosensory processing. Using voxel-based lesion-symptom mapping, we compared patients with impaired touch perception (i.e. hypoaesthesia) to patients without such touch impairments. Patients with hypoaesthesia as compared to control patients differed in one single brain cluster comprising the contralateral parietal operculum together with the anterior and posterior insular cortex, the putamen, as well as subcortical white matter connections reaching ventrally towards prefrontal structures. This finding confirms previous speculations on the ‘ventral pathway of somatosensory perception’ and causally links these brain structures to the perception of touch.
Introduction
In humans, converging evidence suggests that the perception of touch involes not only the postcentral gyrus and the parietal operculum, as the anatomical sites of primary and secondary somatosensory cortex, respectively (Auksztulewicz et al., 2012), but also brain areas further downstream such as the insular cortex (Dijkerman and de Haan, 2007). The main thalamic output targets Brodmann area (BA) 3b, which was proposed as the human homologue of the primary input area SI-proper in non-human primates (Kaas, 1983). As in monkeys, lesions affecting BA 3b and neighbouring BAs on the postcentral gyrus were shown to cause impaired spatial, shape or texture perception (Roland, 1987; Pause et al., 1989). This suggests that the primary somatosensory cortex, as the assumed main entry site of cortical somatosensory processing (Kaas, 1983; Pons et al., 1987; Kalberlah et al., 2013), is causally involved in the perception of touch. What remains less clear is to what extent such perceptual impairments occur as a consequence of the local damage or an impaired gating function to areas further downstream such as the parietal operculum and insular cortex.
Dijkerman and de Haan (2007) formulated a somatosensory model of perception and action, mimicking other sensory domains such as the visual or auditory one (Rauschecker, 1998; Belin and Zatorre, 2000). Their model conceptualizes a ventral pathway subserving recognition and perception that terminates in the insular cortex after passing the postcentral gyrus and parietal operculum. In humans there is, to the best of our knowledge, no causal proof for the implementation of perception in brain regions downstream to the postcentral gyrus. The most common causes of brain damage in humans are stroke and trauma which, because of the anatomy of parietal blood supply and the mostly more extended structural damage due to brain trauma, rarely affect exclusively the inferior parietal lobe, without affecting the postcentral gyrus for studies on lesions to postcentral gyrus and parietal opercumum see (Carey et al., 2011; Juenger et al., 2011). Furthermore, major parts of the inferior parietal lobe are largely hidden in the depth of the Sylvian fissure, making them hardly reachable for any non-invasive electrophysiological recordings or brain stimulation methods.
In the present voxel-based lesion-symptom mapping (VLSM) study, we aimed to address the open question which brain regions beyond the postcentral gyrus are causally involved in the perception of touch. From a large Max Planck Institute database containing more than 2700 structural MRI scans of patients with lesioned brains, we identified structural MRI scans and neurological examination reports of 61 patients presenting lesions in either the left or right hemispheres, but not in the postcentral gyrus as the assumed entry site of cortical somatosensory processing (Kaas, 1983; Pons et al., 1987; Kalberlah et al., 2013).
Materials and methods
Patients
We identified 48 patients (35 male, 13 female; average age = 45.47 ± 15.21 years) with brain lesions in the left hemisphere and another 13 patients (12 male, one female; average age = 45.77 ± 9.64 years) with lesions in the right hemisphere, meeting the inclusion criterion, namely brain lesions in the inferior parietal lobe and any other areas across the entire hemisphere, but not in the postcentral gyrus and ascending afferent pathways (see Tables 1 and 2 for patients’ age, gender, origin of lesion, location of lesion, and deficits in touch perception, and Supplementary Fig. 1A–C for the corresponding individual lesion maps). Patients with additional lesions or malformations in the contralateral hemisphere were excluded. All patients were right-handed according to the Edinburgh Handedness Inventory and tested in the chronic lesion phase (time from onset to MRI acquisition for left brain-lesioned patients: 16.3 ± 37.4 months, and for right brain-lesioned patients: 11.8 ± 7.7 months). Aphasia, as tested with the Aachener Aphasia Test (i.e. standard clincal test battery for chronic aphasia in German) and spatial neglect, as diagnosed by our physiotherapists, were exclusion criteria. MRI and clinical data were collected by the Max Planck Institute (MPI)-associated Clinic for Cognitive Neurology at the University Hospital Leipzig, which is specialized in young stroke and brain trauma patients (note the young age of our patient cohort). All patients gave written informed consent. The study was approved by the ethics committee of the University Clinic Leipzig and conducted according to the ethical guidelines of the Declaration of Helsinki.
Table 1.
Overview of left-sided brain lesioned patient’s age, gender, origin of lesion, location of lesion and deficits in touch perception
| Patient | Age (years) | Gender | Origin of lesion | Location of lesion (BA) |
Hypoaesthesia | Location of impaired touch perception | |||
|---|---|---|---|---|---|---|---|---|---|
| Frontal | Parietal | Temporal | Occipital | 0 = no | |||||
| 1 = yes | |||||||||
| P1 | 44 | M | Partial MCA stroke | 6, 8, 45 | 7, 39, 40 | 21, 22, 38, 41, 42, 48 | 19 | 0 | n/a |
| P2 | 63 | M | Partial MCA stroke with haemorrhagic transformation | 45, 47 | 40 | 21, 22, 34, 38, 48 | n/a | 0 | n/a |
| P3 | 47 | F | Ischaemia in the territories of the insula-arteries and the arteria frontobasalis lateralis of the MCA | 45, 47 | n/a | 48 | n/a | 0 | n/a |
| P4 | 50 | M | Partial MCA stroke | n/a | 7, 39, 40 | 21, 22, 34, 37, 41, 42, 48 | 17, 18, 19 | 0 | n/a |
| P5 | 70 | M | Small ventral MCA stroke | 6, 8, 9, 44, 45, 46, 47 | n/a | 48 | n/a | 0 | n/a |
| P6 | 71 | F | intracerebral haemorrhage | 6, 44, 45, 47, | n/a | 48 | n/a | 0 | n/a |
| P7 | 17 | M | Partial MCA stroke | 6, 8, 9, 44, 45, 46, 47 | n/a | 38, 48 | n/a | 1 | Right limbs |
| P8 | 18 | M | Traumatic brain injury | 6, 44, 46 | n/a | 38, 48 | n/a | 1 | Vertex |
| P9 | 69 | M | Partial MCA stroke | 4, 6, 8, 9, 44, 45, 46 | n/a | 42 | n/a | 0 | n/a |
| P10 | 26 | M | Embolic occlusion of the MCA | 4, 6, 44, 45, 47 | 43 | n/a | n/a | 0 | n/a |
| P11 | 28 | M | Traumatic brain injury and parietal subarachnoidal haemorrhage | 6 | n/a | 20, 21, 22, 37, 38, 41, 42, 48 | n/a | 0 | n/a |
| P12 | n/a | F | Partial MCA stroke | 6, 11, 44, 47 | n/a | 20, 34, 38, 48 | n/a | 1 | Right side of the body |
| P13 | 39 | M | Partial MCA stroke | 6, 11, 25, 44, 45, 46, 47 | 43 | 20, 21, 22, 28, 34, 36, 38, 42, 48 | n/a | 1 | Right side of the body |
| P14 | 36 | M | Partial MCA stroke | n/a | 7, 39, 40 | 21, 22, 37, 41, 42, 48 | 19, 18 | 0 | n/a |
| P15 | 18 | F | Partial MCA stroke | 4, 6, 9, 44, 45, 46, 47 | 39, 40, 43 | 20, 21, 22, 38, 41, 42, 48 | n/a | 1 | Right upper limb |
| P16 | 39 | F | Intracerebral haemorrhage | n/a | 7, 39, 40 | 22, 41, 42, 48 | n/a | 0 | n/a |
| P17 | 60 | M | Partial MCA stroke | 4, 6, 8, 9, 10, 11, 32, 44, 45, 46, 47 | 40, 43 | 34, 38, 41, 42, 48 | n/a | 1 | Right upper limb |
| P18 | 48 | F | Partial MCA stroke | 4, 6, 11, 44, 45, 46, 47 | 39, 40, 43 | 20, 21, 22, 28, 34, 35, 36, 37, 38, 41, 42, 48 | 19 | 1 | Right side of the body |
| P19 | 35 | M | Meningeoma | 9, 10, 32, 46 | n/a | n/a | n/a | 0 | n/a |
| P20 | 19 | M | Partial MCA stroke | 4, 6, 45, 47 | 40, 43 | 21, 22, 38, 41, 42, 48 | n/a | 1 | Right side of the body |
| P21 | 49 | F | Partial MCA stroke | 6, 44, 45 | 40 | 22, 38, 41, 42, 48 | n/a | 0 | n/a |
| P22 | 47 | M | Partial MCA stroke | 11, 47 | n/a | 21, 22, 34, 38, 41, 48 | n/a | 0 | n/a |
| P23 | 52 | M | Traumatic brain injury | n/a | 39, 40 | 21, 22, 37, 38, 41, 42, 48 | 18, 19 | 0 | n/a |
| P24 | 67 | M | Partial MCA stroke | 6, 9, 44, 45, 46, 47 | n/a | 38, 48 | n/a | 0 | n/a |
| P25 | 58 | M | Partial MCA stroke | n/a | 7, 39, 40 | 21, 22, 37, 41, 42, 48 | 19 | 0 | n/a |
| P26 | 59 | M | Partial MCA stroke | 6, 44, 45, 47 | n/a | 21, 34, 38, 48 | n/a | 0 | n/a |
| P27 | 31 | F | Partial MCA stroke | 6, 44, 45, 47 | n/a | 38, 48 | n/a | 0 | n/a |
| P28 | 45 | F | Partial MCA stroke and ACA stroke | 6, 8, 9, 10, 12, 32, 44 | n/a | n/a | n/a | 0 | n/a |
| P29 | 21 | M | Traumatic brain injury | 9, 10, 11, 32, 44, 45, 46, 47 | n/a | 38 | n/a | 0 | n/a |
| P30 | 59 | M | Partial MCA stroke | 45, 47 | n/a | 20, 21, 22, 34, 37, 38, 41, 42, 48 | 19 | 0 | n/a |
| P31 | 59 | M | Partial MCA stroke | 4, 6, 8, 9, 44, 45, 46, 47 | 43 | 38, 48 | n/a | 0 | n/a |
| P32 | 59 | M | Partial MCA stroke | 4, 6, 8, 9, 44, 45, 46, 47 | 43 | 38, 48 | n/a | 0 | n/a |
| P33 | 38 | F | Partial MCA stroke | n/a | 7, 40 | 22, 41, 42, 48 | n/a | 1 | Right side of the body |
| P34 | 52 | F | Partial MCA stroke | 6 | 43 | 22, 41, 42, 48 | n/a | 0 | n/a |
| P35 | 60 | M | Partial MCA stroke | n/a | 39, 40 | 20, 21, 22, 41, 42, 48 | n/a | 0 | n/a |
| P36 | 53 | M | Intracerebral haemorrhage | 4, 6, 10, 32, 44, 45, 46, 47 | 40, 43 | n/a | n/a | 1 | Right side of the body w/o face |
| P37 | 49 | M | Partial MCA stroke | n/a | 40 | 22, 41, 42, 48 | n/a | 1 | Right side of the body |
| P38 | 58 | M | Partial MCA and ACA stroke | 6, 10, 11, 24, 32, 44, 45, 46, 47 | 07 | 38 | n/a | 1 | Right side of the body |
| P39 | 46 | M | Partial MCA stroke | 4, 6, 44, 45, 47 | 40, 43 | 22, 48 | n/a | 1 | Right hand |
| P40 | 54 | M | Partial MCA stroke | 4,6, 9, 44, 45, 46 | 40, 43 | 20, 21, 22, 36, 38, 41 | n/a | 1 | Right limbs and face |
| P41 | 51 | M | Partial MCA stroke | 4, 6, 44, 45, 46, 47 | 07, 40, 43 | 21, 22, 34, 41, 42, 48 | n/a | 1 | Right hand |
| P42 | 26 | F | Partial MCA stroke | 4, 6, 44, 45, 47 | 43 | 20, 21, 22, 34, 38, 41, 52 | n/a | 1 | Right limbs and face |
| P43 | 21 | F | AVM - haemorrhage | 4, 6 | 5, 7, 23, 31, 39, 40, 41, 43 | 21, 22, 26, 27, 29, 30, 37, 48 | 17, 18, 19 | 1 | Distal phalanges of the right fingers and the right lower leg |
| P44 | 48 | M | Partial MCA stroke | 4, 6, 9, 33, 44, 45, 46, 47 | 7, 39, 40 | n/a | n/a | 0 | n/a |
| P45 | 35 | M | Ischaemic stroke | 4 | 5, 7, 23, 39, 40 | 21, 22, 37, 41, 42 | 17, 18, 19 | 0 | n/a |
| P46 | 37 | M | Basal skull fracture | 4, 6 | n/a | 20, 21, 22, 28, 36, 38 | n/a | 0 | n/a |
| P47 | 44 | M | Partial MCA stroke | n/a | n/a | 21, 22, 41, 42 | n/a | 0 | n/a |
| P48 | 62 | M | Partial MCA stroke | n/a | 07, 39, 40 | 22, 41, 42 | 19 | 0 | n/a |
The corresponding T1-weighted MRI images of each patient are shown in Supplementary Fig. 1A and B.
ACA = anterior cerebral artery; AVM = arteriovenous malformations; MCA = middle cerebral artery; n/a = not affected.
Table 2.
Overview of right-sided brain lesioned patient’s age, gender, origin of lesion, location of lesion and deficits in touch perception
| Patient | Age (years) | Gender | Origin of lesion | Location of lesion (BA) |
Hypoaesthesia | Location of impaired touch perception | |||
|---|---|---|---|---|---|---|---|---|---|
| Frontal | Parietal | Temporal | Occipital | 0 = no | |||||
| 1 = yes | |||||||||
| P49 | 37 | M | Partial MCA stroke | 4, 6, 44 | 39, 40, 43 | 21, 22, 37, 48 | 19 | 1 | Left upper limb |
| P50 | 36 | M | Traumatic brain injury | 11, 44, 45, 46, 47 | n/a | 20, 21, 38, 48 | n/a | 0 | n/a |
| P51 | 29 | M | Traumatic brain injury | n/a | n/a | 21, 22, 38, 48 | n/a | 0 | n/a |
| P52 | 42 | M | Intracerebral haemorrhage | 32 | 7, 39, 40, 43 | 20, 21, 22, 41, 42, 48 | n/a | 0 | n/a |
| P53 | 44 | M | Partial MCA stroke | 6, 9, 45, 47 | 40, 43 | 20, 21, 22, 38, 41, 42, 48 | n/a | 1 | Left upper limb |
| P54 | 53 | M | Partial MCA stroke | 6, 44, 45, 46, 47 | n/a | 20, 21, 22, 38, 42, 48 | n/a | 1 | Left side of the body |
| P55 | 58 | M | Basal ganglia infarction | 4, 6, 11, 44, 45, 47 | n/a | 34, 38, 48 | n/a | 1 | Left side of the body |
| P56 | 48 | M | Partial MCA stroke | n/a | 40 | 20, 21, 22, 37, 38, 41, 42, 48 | n/a | 0 | n/a |
| P57 | 45 | M | Partial MCA stroke | 6, 11, 44, 45, 46, 47 | n/a | 38, 48 | n/a | 0 | n/a |
| P58 | 37 | M | Traumatic brain injury | 11, 45, 46, 47 | n/a | 48 | n/a | 0 | n/a |
| P59 | 49 | F | Intracerebral haemorrhage | 6, 9, 44, 45, 46 | 40, 43 | 21, 22, 38, 48 | n/a | 1 | Left side of the body |
| P60 | 62 | M | Partial MCA stroke | 4, 6, 9, 10, 11, 44, 45, 46, 47 | 39, 40, 43 | 20, 21, 22, 34, 37, 38, 42, 48 | n/a | 1 | Left limbs |
| P61 | 55 | M | Partial MCA stroke | n/a | 39, 40 | 21, 22, 41, 42, 48 | n/a | 0 | n/a |
The corresponding T1-weighted MRI images of each patient are shown in Supplementary Fig. 1C.
MCA = middle cerebral artery; n/a = not affected.
For each patient, the MPI database provided high-resolution whole-brain 3D standard T1-weighted anatomical images and T2-weighted fluid-attenuated inversion-recovery (FLAIR) images. The FLAIR images are specifically sensitive for the assessment of stroke lesions and were used in parallel with the high-resolution T1 images to better delineate lesion borders. The images were acquired with a 3 T Bruker MedSpec 100 System (Bruker), a 3 T Tim TRIO, or a 3 T VERIO MRI scanner (Siemens).
Examination of touch impairments
The examination is part of our standard clinical routine work and is performed by our trained physiotherapists. It was established 16 years ago, when the Clinic for Cognitive Neurology at the University Hospital Leipzig was founded, thus the present MRI and clinical data have been collected over the last 16 years.
Throughout the sensory examination, patients lay supine on an examination bed in a darkened room. They are told to close their eyes. To assess touch perception, a regular paintbrush with a tip width of 6 mm was used so that minor impairments could be detected. The stimuli were first applied to either the fingers or toes, before continuously proceeding to the further proximal parts of the extremities. During the examination, the patient was continuously asked, with each contact of the paintbrush, whether the touch was perceived as normal or reduced (i.e. hypoaesthesia). After examining the arms and the legs, the trunk and the face were tested. Touch perception was then compared at distal and proximal sites of the extremities, and the left side was compared to the right side.
To better identify the exact location of hypoaesthesia, a paintbrush with a larger tip width (i.e. 14 mm) was used. For the body part presenting hypoaesthesia in the previous step (either leg, arm, face, or trunk), stimuli were applied from this part towards neighbouring body parts to identify whether the impairment was restricted to the foot or hand, to the lower or upper extremity, as well as to circumscribed areas of the face or trunk. After assessing touch perception on the ventral site of the body, the described procedures were repeated on the dorsal site.
Lesion mapping
All lesions were mapped on the T1-weighted anatomical images because of their superior resolution as compared to the FLAIR images. Lesions were manually delineated on every single transversal slice using MRIcroN (http://www.sph.sc.edu/comd/rorden/mricron). The two researchers (S.P. and B.P.) who mapped the lesions were blinded for the associated clinical deficits.
Next, the lesion maps together with the T1-weighted anatomical images including lesion masked cost function (Brett et al., 2001) were normalized to the T1-template [i.e. Montreal Neurological Institute (MNI) standard space] using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/).
For statistical inference we used the voxel-by-voxel lesion-symptom mapping (VLSM) software as implemented in MRIcron, which is a standard tool to examine the relationship between behavioural or clinical measures and brain lesions with a fairly high spatial precision (Bates et al., 2003).
We applied the non-parametric Liebermeister measure to assess between-patient group effects. We compared those patients with contralateral impairment in touch perception to those showing no such deficit. All voxels in which <10% of the patients presented a lesion were discarded from further analyses. Voxels surviving false detection rate (FDR) corrected alpha level of P < 0.05 were considered significant.
Next, we questioned whether a larger extent of touch impairment was also associated with larger lesions in the contralateral hemisphere. To this end we used the data only of left-sided brain-lesioned patients and compared those patients in whom the entire right body site was affected (n = 7, see Tables 1 and 2 and Supplementary Fig. 1: Patients P12, P13, P18, P20, P33, P37 and P38) to those showing a deficit in touch perception only in the right upper limb (n = 4; Patients P15, P17, P39 and P41). Because of the small sample sizes in both subgroups (n = 7 versus n = 4), the Liebermeister test did not reveal any significant differences. Therefore we compared both groups only descriptively by an overlay plot of the mean z-scores, separately computed for each of the two subgroups. Note that for the 13 patients with right-sided brain lesions this approach was not applicable because of the even smaller sample size as compared to the group of patients with left-sided lesions.
To assign each patient’s lesioned brain areas to their associated Brodmann areas, we superimposed the individual normalized scan to the Brodmann template as implemented in MRIcroN (Supplementary Table 1A–C). To assign the effect of touch impairment to the underlying anatomy, we superimposed the statistical lesion maps onto the maps of the parietal operculum and its subdivisions as implemented in the Anatomy toolbox for SPM (Eickhoff et al., 2005; Fig. 1), as well as the insula and the putamen as implemented in the WFU_PickAtlas by Joseph Maldjian from the Functional MRI Laboratory at the Wake Forest School of Medicine (see http://www.nitrc.org/projects/wfu_pickatlas).
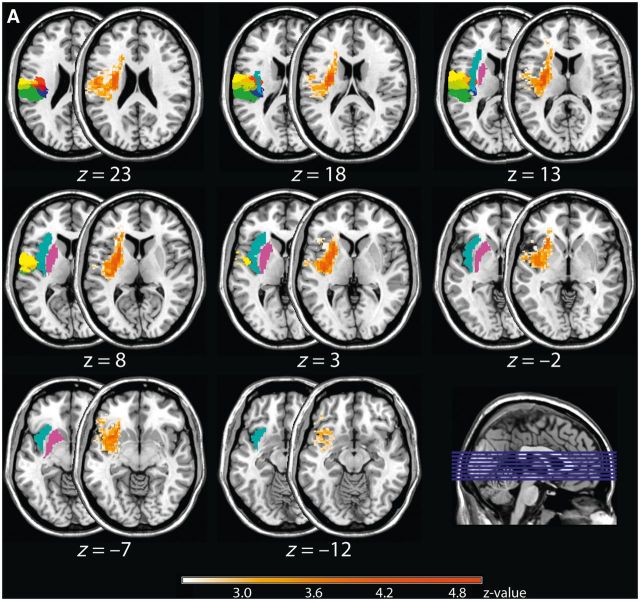
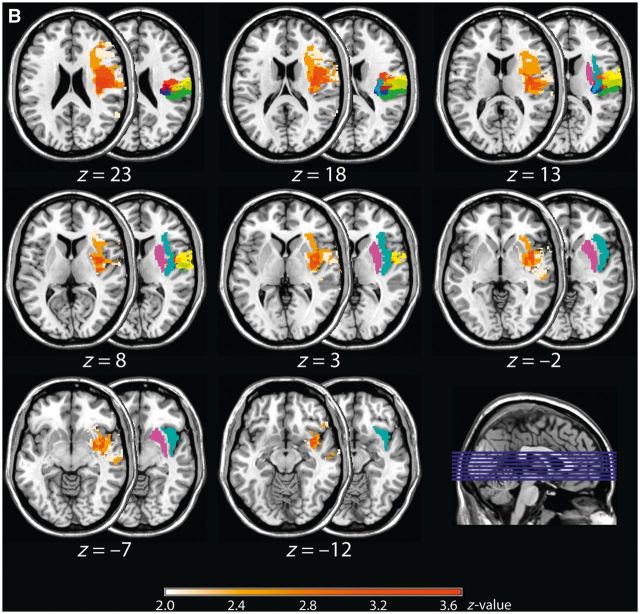
Figure 1.
Results of the non-parametric Liebermeister test for the group of left and right brain-lesioned patients. Shown are pairs of axial brain slices with the half-covered slice indicating the anatomical site of the parietal opercular regions (OP1 = green, OP2 = blue, OP3 = red, and OP4 = yellow), together with the putamen (purple) and insular cortex (cyan), and the half-overlapping slice showing the z-values of the Liebermeister test. For both left (A) and right brain-lesioned patients (B), the comparison between patients who presented an impaired touch perception and patients without such impairments revealed OP 1–4, but predominately the anterior subdivisions OP 4 and 3, the anterior and posterior insular cortex, as well as the subcortical putamen together with white matter fibre bundles reaching to the prefrontal cortex as being causally linked to the perception of touch (FDR-corrected: left brain-lesioned group: P < 0.05, Z = 2.25; right brain-lesioned group: P < 0.05, Z = 1.97). Numbers below each pair of axial brain slices indicate MNI coordinates.
Results
We compared patients with touch impairments to patients not presenting such impairments (see Fig. 1A for left-sided lesions and Fig. 1B for right-sided lesions). The analyses revealed for both brain hemispheres a significant effect of touch impairment on the respective (contralateral) side that overlapped with the parietal opercular subdivisions OP 1–4, but predominantly involving the further anterior subdivisions OP 4 and 3. Furthermore, the analyses identified the anterior and posterior insular cortex, and the putamen together with white matter connections reaching to prefrontal structures (left-sided lesions: P < 0.05, Z = 2.25, right-sided lesions: P < 0.05, Z = 1.97, FDR-corrected).
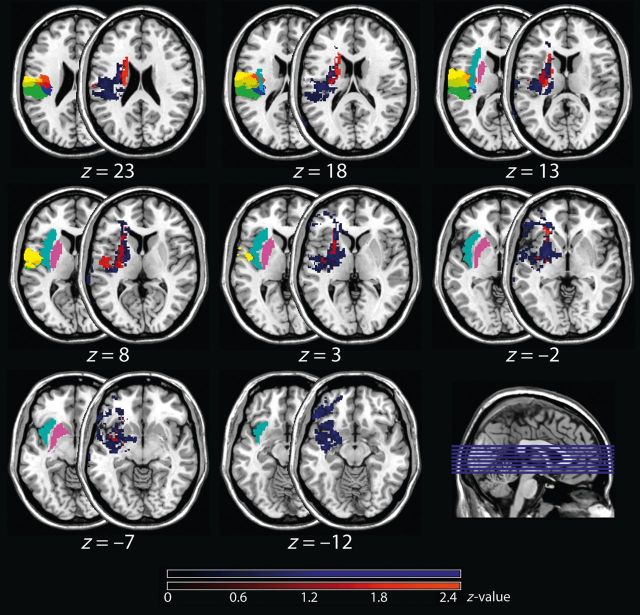
The overlay plot provided in Fig. 2 shows the mean z-score across patients with left-sided brain lesions in whom only the contralateral upper limb was affected (coloured in red) together with the mean z-score of patients in whom the entire contralateral body site was affected (coloured in blue). Although this overlay is only descriptive and does not replace a valid statistical comparison of both subgroups (which was not applicable due to the small sample sizes, see also ‘Lesion mapping’ in the ‘Materials and methods’ section for further details), it nevertheless suggests that patients, in whom the entire body site was affected, presented larger lesions in the cluster identified with the previous step (i.e. OP 1–4, insular cortex, putamen, and subcortical prefrontal connections) than patients presenting only impairments of the upper limb.
Figure 2.
Mean z-scores. Shown are the mean z-scores of left brain-lesioned patients in whom the entire right body site was affected (n = 7, coloured in blue) and in whom only the upper limb was affected (n = 4; coloured in red). Note that due to a smaller sample size, this approach was not applicable to right brain-lesioned patients. The mean z-scores are depicted on the half-overlapping right-sided slices, while the left-sided slices indicate the anatomical sites of the parietal opercular regions (OP1 = green, OP2 = blue, OP3 = red, and OP4 = yellow), the putamen (purple) and the insular cortex (cyan). Numbers below each pair of axial brain slices indicate MNI coordinates.
Discussion
In the present human voxel-based lesion-symptom mapping study we asked which brain regions downstream of primary somatosensory cortex are causally involved in the perception of touch and identified a specific cluster within the parietal operculum, comprising the OP 1 to 4, but predominantly the more anteriorly localized subdivisions, together with the insular cortex, putamen, and subcortical connections reaching towards the prefrontal cortex.
In search of the secondary somatosensory cortex’s specific anatomical site in the human brain, Eickhoff and colleagues (2006a, b, 2007) classified the parietal operculum according to its cytoarchitectonic organization and identified four subdivisions, termed OP 1–4. Next, they matched meta-analytic assessments and functional imaging studies tothese four regions and proposed that the areas OP 1, OP 4 and OP 3 represent the human homologues of the areas SII, parietal ventral, and ventral somatosensory areas in non-human primates, respectively (Eickhoff et al., 2006a, b, 2007). The present findings generally underscore these assumptions, but highlight the more anteriorly located subdivisions closer to the prefrontal cortex, such as OP 4 and 3, to be predominantly involved in the perception of touch.
But the parietal operculum does not only contribute to touch perception, as right-sided brain lesions comprising this region were shown to cause spatial neglect (Karnath et al., 2001). This causal link to spatial neglect limited the number of suitable patients with right-sided brain lesions for the present study, resulting in a larger group of patients with lesions in the left brain hemisphere. Nevertheless, statistical analysis performed on right-sided brain lesions revealed a pattern comparable to that of the left hemisphere, thus providing further support for the validity of our results.
A series of studies agreed on the notion that the human parietal operculum represents a crucial hub connecting the somatosensory system to several other brain systems (Eickhoff et al., 2010), such as the prefrontal cortex (Spitzer et al., 2010; Spitzer and Blankenburg, 2011). Based on similarities to higher visual and also auditory processing, Dijkerman and de Haan (2007) proposed a model of somatosensory processing that assumes a perception-related ventral pathway originating in the postcentral gyrus, passing the parietal operculum before terminating in the insular cortex (Murray and Mishkin, 1984; Friedman et al., 1986; Pons et al., 1987; Burton and Sinclair, 2000).
Regarding the insular cortex and its functional implementation, recent studies suggest that the anterior insula plays a major role in interoceptive processing (for a review see Craig, 2009), whereas the posterior insula is thought to contain perceptual representations for bodily awareness (Karnath et al., 2005). The insula furthermore connects to the prefrontal cortex, via the subcortically located myelinated fibre bundles that we also identified here in the context of perceiving touch, reaching from the insula cortex towards the inferior lateral prefrontal structures, presumably involved in working memory and other attention-related processes (Burton and Sinclair, 2000). Based on this anatomical interconnectedness, the insula cortex is at the crossroads for integrating both the cognitive and sensory aspects of perception of cutaneous stimuli.
Besides the insula and parietal operculum, we also identified the involvement of a subcortical structure, namely the contralateral putamen. The putamen is one of the major sites of cortical input into basal ganglia loops and is assumed to play a key role in multimodal sensory integration processes (Starr et al., 2011; von Saldern and Noppeney, 2013), also, as indicated by the present findings, for the perception of tactile events.
Taken together, our findings suggest that the perception of touch does not only require the information exchange between the postcentral gyrus and parietal operculum (Jones et al., 2007; Auksztulewicz et al., 2012), but also involves the insular cortex and putamen together with prefrontal structures. Hence, we here confirm previous speculations on a ‘ventral pathway of somatosensory processing’ underpinning the perception of touch and hence further cognitive implementation of tactile information (Dijkerman and de Haan, 2007).
Acknowledgements
We are thankful to Elizabeth Kelly, who proofread the manuscript.
Funding
This work was supported by the German Federal Ministry of Education and Research (BMBF) (Bernstein Focus, State Dependencies of Learning 01GQ0975; Project 18GL4DW4 to B.P.).
Supplementary material
Supplementary material is available at Brain online.
References
- Auksztulewicz R, Spitzer B, Blankenburg F. Recurrent neural processing and somatosensory awareness. J Neurosci. 2012;32:799–805. doi: 10.1523/JNEUROSCI.3974-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–50. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ. “What”, “where” and “how” in auditory cortex. Nat Neurosci. 2000;3:965–6. doi: 10.1038/79890. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff a P, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ. Attending to and remembering tactile stimuli: a review of brain imaging data and single-neuron responses. J Clin Neurophysiol. 2000;17:575–91. doi: 10.1097/00004691-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Carey LM, Abbott DF, Harvey MR, Puce A, Seitz RJ, Donnan GA. Relationship between touch impairment and brain activation after lesions of subcortical and cortical somatosensory regions. Neurorehabil Neural Repair. 2011;25:443–57. doi: 10.1177/1545968310395777. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dijkerman HC, de Haan EHF. Somatosensory processes subserving perception and action. Behav Brain Sci. 2007;30:189–201. doi: 10.1017/S0140525X07001392. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006a;16:268–79. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex. 2007;17:1800–11. doi: 10.1093/cercor/bhl090. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, et al. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010;30:6409–21. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006b;16:254–67. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Murray E a, O’Neill JB, Mishkin M. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J Comp Neurol. 1986;252:323–47. doi: 10.1002/cne.902520304. [DOI] [PubMed] [Google Scholar]
- Jones SR, Pritchett DL, Stufflebeam SM, Hämäläinen M, Moore CI. Neural correlates of tactile detection: a combined magnetoencephalography and biophysically based computational modeling study. J Neurosci. 2007;27:10751–64. doi: 10.1523/JNEUROSCI.0482-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juenger H, de Haan B, Krägeloh-Mann I, Staudt M, Karnath HO. Early determination of somatosensory cortex in the human brain. Cereb Cortex. 2011;21:1827–31. doi: 10.1093/cercor/bhq258. [DOI] [PubMed] [Google Scholar]
- Kaas JH. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev. 1983;63:206–31. doi: 10.1152/physrev.1983.63.1.206. [DOI] [PubMed] [Google Scholar]
- Kalberlah C, Villringer A, Pleger B. Dynamic causal modeling suggests serial processing of tactile vibratory stimuli in the human somatosensory cortex-an fMRI study. Neuroimage. 2013;74:164–71. doi: 10.1016/j.neuroimage.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nägele T. Awareness of the functioning of one’s own limbs mediated by the insular cortex? J Neurosci. 2005;25:7134–8. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411:950–3. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Relative contributions of SII and area 5 to tactile discrimination in monkeys. Behav Brain Res. 1984;11:67–83. doi: 10.1016/0166-4328(84)90009-3. [DOI] [PubMed] [Google Scholar]
- Pause M, Kunesch E, Binkofski F, Freund HJ. Sensorimotor disturbances in patients with lesions of the parietal cortex. Brain. 1989;112-:1599–625. doi: 10.1093/brain/112.6.1599. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Friedman DP, Mishkin M. Physiological evidence for serial processing in somatosensory cortex. Science. 1987;237:417–20. doi: 10.1126/science.3603028. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical processing of complex sounds. Curr Opin Neurobiol. 1998;8:516–21. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- Roland PE. Somatosensory detection in patients with circumscribed lesions of the brain. Exp Brain Res. 1987;66:303–17. doi: 10.1007/BF00243307. [DOI] [PubMed] [Google Scholar]
- Spitzer B, Blankenburg F. Stimulus-dependent EEG activity reflects internal updating of tactile working memory in humans. Proc Natl Acad Sci USA. 2011;108:8444–9. doi: 10.1073/pnas.1104189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer B, Wacker E, Blankenburg F. Oscillatory correlates of vibrotactile frequency processing in human working memory. J Neurosci. 2010;30:4496–502. doi: 10.1523/JNEUROSCI.6041-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, et al. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134:1987–2004. doi: 10.1093/brain/awr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Saldern S, Noppeney U. Sensory and striatal areas integrate auditory and visual signals into behavioral benefits during motion discrimination. J Neurosci. 2013;33:8841–9. doi: 10.1523/JNEUROSCI.3020-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]