Abstract
This scientific commentary refers to ‘Independent information from cerebrospinal fluid amyloid-b and florbetapir imaging in Alzheimer's disease’ by Mattsson et al. (10.1093/brain/awu367).
This scientific commentary refers to ‘Independent information from cerebrospinal fluid amyloid-β and florbetapir imaging in Alzheimer’s disease’ by Mattsson et al. (10.1093/brain/awu367).
Studies of fluid and imaging biomarkers of Alzheimer’s disease have contributed greatly to our understanding of disease pathobiology, and in so doing have fuelled a paradigm shift in the conceptualization of Alzheimer’s disease as a chronic condition characterized by a long (∼10–20 year) preclinical phase during which hallmark pathologies develop, before the appearance of cognitive symptoms (dementia) clinically defined as Alzheimer’s disease. Revisions in diagnostic criteria to incorporate biomarker results have recently been proposed (Dubois et al., 2010; Albert et al., 2011; McKhann et al., 2011; Sperling et al., 2011) in order to increase confidence in identifying Alzheimer’s disease as the underlying aetiology of a clinical impairment and to permit a diagnosis across the disease continuum, eventually perhaps in the asymptomatic period. Biomarkers are currently being used in clinical trials for participant enrolment, evaluation of target engagement and as outcome measures (Hampel et al., 2011). Thus, validation of Alzheimer’s disease biomarkers is of critical importance. In this issue of Brain, Mattsson and colleagues (2015) evaluate and compare two amyloid-related biomarkers—CSF levels of amyloid-β42 (the primary component of amyloid plaques) versus amyloid imaging via PET with florbetapir (AMYViD™), a radiolabelled tracer approved by the US Federal Drug Administration in 2012 for the detection of brain amyloid—in their ability to predict clinical diagnosis and other measures and features of Alzheimer’s disease (Mattsson et al., 2015). Importantly, while the study confirms the previously reported diagnostic and prognostic utility of these two markers, the data expand upon previous results by exploring the discordance of these two measures in a large research cohort that spans the range of Alzheimer’s disease stages, from asymptomatic to mildly symptomatic to dementia. In addition to providing insight into processes associated with amyloid metabolism and aggregation into plaques, the results of Mattson and colleagues will likely have an impact on the use of these two markers in ongoing and future clinical trials.
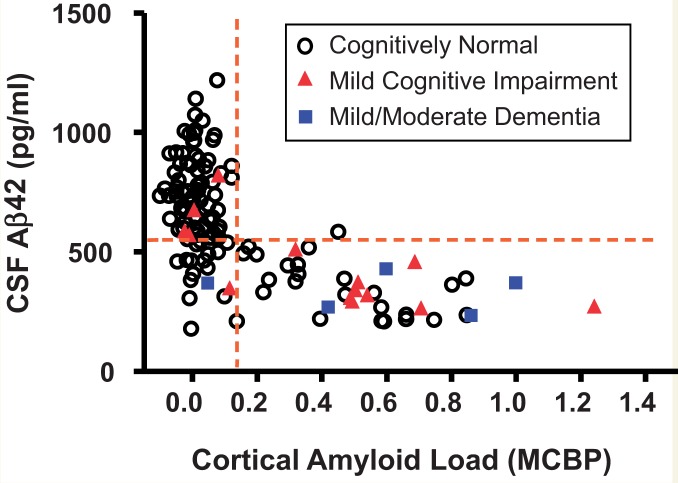
Low levels of CSF amyloid-β42 have long been associated with symptomatic Alzheimer’s disease (Motter et al., 1995), and are hypothesized to reflect the sequestration of soluble brain amyloid-β into insoluble plaques with a resultant reduction in the amount of amyloid-β42 that is cleared into the CSF. However, it was not until the advent of amyloid PET imaging with Pittsburgh Compound B (PIB) (Klunk et al., 2004) that this relationship between brain amyloid deposition and CSF amyloid-β42 could be demonstrated in living individuals (Fagan et al., 2006). This finding has subsequently been confirmed by many groups in many cohorts, leading to the use of CSF amyloid-β42 and amyloid-PET as often interchangeable metrics in defining ‘amyloid-positivity’. However, as is invariably the case with human biology, the story is not that simple. Whereas amyloid-positivity by PET is almost always associated with low CSF amyloid-β42 in individuals with symptomatic Alzheimer’s disease, there exist persons (typically cognitively normal) with similarly low levels of CSF amyloid-β42 but who are amyloid-negative by PET (Fig. 1, lower left quadrant). Although many researchers tend to view these subjects as outliers, with the discrepancy considered to reflect analytical limitations of the biomarker assays and/or scans (thereby dampening enthusiasm for the clinical utility of these measures), Mattsson and colleagues have chosen to investigate this discordance, evaluating whether these two measures might in fact provide partly independent disease-related information. Specifically, using robust statistical approaches, they characterized and compared the frequency of such discordance in the various clinical disease stages and the extent to which low CSF amyloid-β42 and amyloid-PET positivity were able to predict disease-related metrics including APOE ϵ4 carriage (the strongest genetic risk factor for Alzheimer’s disease), a clinical diagnosis of Alzheimer’s disease, cognitive deficits, brain hypometabolism and hypoperfusion, hippocampal atrophy, and increased CSF markers of tau-associated neuronal injury (tau and ptau181). Although previous studies, including those from their own group, have evaluated such relationships, the current study is the first to directly and comprehensively compare the performance of the two amyloid-related measures in the same (large) cohort.
Figure 1.
Illustration of the association between concentrations of CSF amyloid-β42 and cortical amyloid load as revealed by PET. Research participants in the Knight Alzheimer’s Disease Research Centre underwent clinical assessment, CSF collection by lumbar puncture and amyloid imaging by PIB PET within a 12-month period. Cortical amyloid load is presented as the mean cortical binding potential (MCBP) calculated from the prefrontal cortex, precuneus, lateral temporal cortex and gyrus rectus, with cerebellum (very low PIB binding) as the reference region. CSF amyloid-β42 (Aβ42) values were obtained with the INNOTEST® ELISA kit (Fujirebio, formerly Innogenetics). Dashed lines illustrate potential cut-offs for PIB (right of the vertical line) and CSF amyloid-β42 (below the horizontal line) positivity. The cohort (age ≥ 65 years) included 113 cognitively normal participants, 14 with mild cognitive impairment/very mild dementia, and five with mild/moderate dementia. The majority of PIB+ individuals had low CSF Aβ42 whereas the majority of PIB− individuals had high levels of CSF Aβ42. All but one of the ‘discordant’ values are to be found in the lower left quadrant (low CSF Aβ42/low PIB). Concordance is observed in symptomatic and asymptomatic (presumed pre-symptomatic) individuals. Symptomatic amyloid-negative individuals may have non-Alzheimer aetiologies. Reprinted from Advances in Medical Sciences, Biomarkers of Alzheimer's disease and mild cognitive impairment: A current perspective, available online 9 December 2014, with permission from Elsevier.
As expected, CSF amyloid-β42 and amyloid PET were both associated with the various Alzheimer’s disease-related processes and metrics; however, several differences in the strength of the associations were observed for the two amyloid-related measures. For example, amyloid PET positivity was a better predictor of a clinical diagnosis than was CSF amyloid-β42, whereas the CSF measure was more closely associated with APOE ϵ4 carriage. In addition, amyloid-PET was more closely related to CSF tau-related measures of neuronal injury and cognitive deficits compared to CSF amyloid-β42. While elucidation of such differences could be interpreted as promoting an ‘us versus them’ (CSF versus imaging) mentality in assessing biomarker utility, the greatest impact of the results is in terms of what they can tell us about underlying disease pathogenesis. Such information, in turn, may be useful in informing clinical applications.
So, what do these findings tell us about the disease process? While some may view the closer correspondence of amyloid-PET (compared to CSF amyloid-β42) with Alzheimer’s disease diagnosis as an endorsement for the use of PET, the frequency of discordance (almost exclusively CSF amyloid-β42+/PET−) was highest in cognitively normal individuals, and decreased with increasing symptom severity. This observation is consistent with a scenario in which amyloid-β42 builds up and begins to aggregate in the brain early in the pre-symptomatic phase as evidenced by decreased amyloid-β42 in the CSF. The closer correspondence of CSF amyloid-β42 levels with APOE genotype is consistent with the known influence of APOE genotype on the aggregation and clearance of soluble amyloid-β (Castellano et al., 2011). Once a certain threshold is reached, the amyloid then becomes detectable by PET, again during the presymptomatic phase. In the presence of fibrillar amyloid (detectable by PET), disease pathology progresses to involve tau-associated neuronal injury in vulnerable brain regions (as evidenced by increased levels of CSF tau and ptau), regional atrophy and cognitive decline that eventually culminates in end-stage dementia. Mattsson and colleagues astutely discuss other possible contributors to the early CSF amyloid-β42/PET discordance, including potential methodological variability, differences in overall amyloid-β production in certain individuals, and the impact of diffuse (non-fibrillar) amyloid-β deposits on the two biomarker patterns. It is also important to note that this model is based on cross-sectional data. The ultimate test of this hypothesis will require comparison of within-person longitudinal CSF, imaging (PET and volumetric) and cognitive/clinical data, as is currently being performed in several ongoing studies.
For researchers in the biomarker trenches, the data reported by Mattsson and colleagues add an important piece(s) to the ever-growing puzzle of Alzheimer’s disease pathogenesis and biomarker development (Jack et al., 2010). But how might they help clinicians who are eager to provide better care to their patients? Although CSF analysis and amyloid imaging are currently used in clinical settings, they are not universal practices. If amyloid-related biomarkers are to be used to confirm the underlying aetiology of a clinically expressed syndrome, then PET and CSF amyloid-β42 will likely both be informative. If biomarkers are to be used to provide pathological disease staging for use in clinical prognosis in asymptomatic or early symptomatic individuals (i.e. will I develop dementia, when and how fast will I progress?), the results of Mattsson et al. suggest that CSF amyloid-β42 may be more appropriate in the very earliest (presymptomatic) stages, whereas PET may be a more sensitive marker of subsequent disease progression (along with increases in tau-related markers such as CSF tau/ptau and/or tau imaging, which is currently in its infancy). Both CSF amyloid-β42 and amyloid PET will likely be useful in determining eligibility for enrolment into clinical trials, be they early clinical stage or secondary prevention trials; however, the choice of biomarker will depend on the disease stage to be enrolled, the length of the trial, and the defined outcome measure(s). Once disease-modifying therapies become available, these biomarkers could conceivably be used to monitor drug efficacy, including target engagement and effect on downstream pathological processes. It is only through elegant and comprehensive studies such as the one by Mattsson and colleagues that we will be able to refine our understanding of the disease process so as to enable the proper and most efficient use of biomarkers in clinical settings.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–9. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Hampel H, Wilcock G, Andrieu S, Aisen P, Blennow K, Broich K, et al. Biomarkers for Alzheimer's disease therapeutic trials. Prog Neurobiol. 2011;95:579–93. doi: 10.1016/j.pneurobio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Insel P, Donohue M, Landau S, Jagust W, Trojanowki J, et al. Independent information from cerebrospinal fluid β-amyloid and florbetapir imaging in Alzheimer’s disease. Brain. 2015;138:729–40. doi: 10.1093/brain/awu367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995;38:643–8. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]