Evidence exists that interferon-beta therapy can modulate inflammasome activity in multiple sclerosis. However, the role of inflammasomes in shaping the interferon-beta response is unclear. Malhotra et al. report that expression of the NLRP3 inflammasome and the related cytokine IL1B in blood cells discriminates between interferon-beta responders and non-responders.
Keywords: biomarkers, association study, multiple sclerosis, imaging
Abstract
Evidence exists for a potential modulation of inflammasome activity by interferon beta. Here, we investigated the roles of inflammasomes [absent in melanoma 2 (AIM2); NLR family, CARD domain containing 4 (NLRC4); NLR family, pyrin domain containing 1 and 3 (NLRP1 and NLRP3)] and related cytokines (IL1B, IL10, IL18) in the response to interferon beta in patients with relapsing-remitting multiple sclerosis. Ninety-seven patients treated with interferon beta were classified into responders and non-responders according to clinical criteria after 24 months and clinical-radiological criteria after 12 months of treatment. Messenger RNA expression levels of inflammasomes and cytokines were determined by real-time polymerase chain reaction in peripheral blood mononuclear cells collected before treatment with interferon beta. In a subgroup of patients, NLRP3 and IL1B expression was also determined after 3 months (n = 32) and 12 months (n = 20) of interferon beta treatment. A polymorphism located in the NLRP3 gene, rs35829419, was genotyped in 789 multiple sclerosis patients treated with interferon beta. Baseline mRNA expression levels for NLRP3 and IL1B were increased in peripheral blood mononuclear cells from non-responders compared to responders classified according to clinical criteria after 24 months (P = 0.02 and P = 0.001, respectively). No significant differences were observed for other inflammasomes and related cytokines. Differences in NLRP3 and IL1B expression remained significant following a clinical-radiological classification after 12 months (P = 0.007 and P = 0.02, respectively). After treatment with interferon beta, NLRP3 and IL1B expression was increased in responders but unchanged in non-responders. A trend for association was observed between rs35829419 and interferon beta response (pM-H = 0.08). These results point to a role of the NLRP3 inflammasome and its related cytokine IL1B in the response to interferon beta in patients with relapsing-remitting multiple sclerosis.
Introduction
Inflammasomes are multi-oligomeric subunits that regulate maturation of pro-inflammatory cytokines such as IL1B and IL18 (Schroder and Tschopp, 2010). At least four major inflammasomes have been identified: absent in melanoma 2 (AIM2), NLR family, CARD domain containing 4 (NLRC4), and NLR family, pyrin domain containing 1 and 3 (NLRP1 and NLRP3, respectively) (Schroder and Tschopp, 2010). Inflammasomes are gaining increasing attention in multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis. In experimental autoimmune encephalomyelitis, Nlrp3-deficient mice had reduced disease severity and a decrease of Th1 and Th17 cells in the peripheral lymphoid tissues and spinal cord (Gris et al., 2010; Inoue et al., 2012). Similarly, in a cuprizone-induced demyelination model Nlrp3 gene expression was significantly upregulated and Nlrp3-deficient mice showed delayed demyelination and oligodendrocyte loss (Jha et al., 2010). In another study, interferon beta (IFN-β) inhibited IL1B production in mouse bone marrow-derived macrophages through suppression of NLRP1 and NLRP3 inflammasome activity and IL10 induction (Guarda et al., 2011). Furthermore, IL1B production was significantly reduced in blood monocytes isolated from IFN-β treated patients with multiple sclerosis compared to monocytes from healthy control subjects (Guarda et al., 2011). In more recent studies, CD4+CD45RO+ memory T cells from patients with multiple sclerosis had reduced capacity to suppress NLRP3 inflammasome activation that was restored by treatment with IFN-β (Beynon et al., 2012). Also, IFN-β may indirectly suppress the activation of the NLRP3 inflammasome via induction of IL27 by dendritic cells (Molle et al., 2010; Mascanfroni et al., 2013).
In view of this relationship between inflammasomes and experimental autoimmune encephalomyelitis disease course, and particularly the potential regulation of inflammasome activity by IFN-β, one of the most widely prescribed disease modifying therapies for relapsing-remitting multiple sclerosis, in the present study we aimed to address the roles of inflammasomes (NLRP3, NLRP1, NLRC4, and AIM2) and related cytokines (IL1B, IL10, IL18) in the response to IFN-β in patients with multiple sclerosis.
Materials and methods
Study design
The main, prospective part of the study included patients with relapsing-remitting multiple sclerosis treated with IFN-β at the outpatient clinic of the Centre d’Esclerosi Multiple de Catalunya (Cemcat). Patients were included in a follow-up protocol collecting basal and longitudinal clinical and radiological data, as previously described (Río et al., 2006, 2009). The study was approved by the local ethics committee, and all patients gave their informed consent.
Clinical criteria of response to IFN-β therapy
Clinical criteria of response to IFN-β were applied after 2 years of treatment. Responders were patients having no relapses and no increase in the Expanded Disability Status Scale (EDSS) score over the follow-up period. Non-responders were patients having one or more relapses and an increase of at least 1 point in the EDSS score confirmed at 6 months during the follow-up period. Intermediate response phenotypes, i.e. presence of relapses with an increase of <1 point in the EDSS score, or absence of relapses with an increase in the EDSS score of 1 point or higher, were considered intermediate responders.
Ninety-seven patients with relapsing-remitting multiple sclerosis were included in the study. Of these, 48 (49.5%) were responders, 22 (22.7%) non-responders, and 27 (27.8%) were intermediate responders to IFN-β. None of these patients had ever received treatment with IFN-β or other immunosuppressive therapy before study entry. No patient had clinical exacerbations or received corticosteroid treatment during the month before initiation of IFN-β.
A control group of 14 individuals was also included in the study, for comparison purposes. Table 1 summarizes demographic and main clinical characteristics of patients and controls included in the study.
Table 1.
Demographic and baseline clinical and radiological characteristics of multiple sclerosis patients and healthy controls included in the study
| Baseline characteristics | Healthy controls | Responders | Non- responders | intermediate responders | P-valuesd |
|---|---|---|---|---|---|
| n | 14 | 48 | 22 | 27 | – |
| Age (years) | 33.9 (6.2) | 36.0 (8.2) | 35.9 (8.9) | 34.0 (7.5) | 0.521 |
| Female/male (% female) | 8/6 (57.1) | 35/13 (72.9) | 15/7 (68.2) | 15/12 (55.5) | 0.414 |
| Duration of disease (years) | – | 4.1 (4.9) | 4.4 (4.7) | 6.3 (5.6) | 0.573 |
| EDSSa | – | 1.8 (1.0–2.4) | 2.3 (1.5–3.0) | 2.1 (1.0–3.0) | 0.197 |
| Number of relapsesb | – | 1.8 (0.7) | 1.8 (0.7) | 1.6 (0.7) | 0.431 |
| Number of Gd-enhancing lesionsc | – | 2.4 (4.2) | 3.7 (3.8) | 5.1 (7.3) | 0.197 |
| Type of IFN-β [n (%)] | |||||
| IFN-β 1a IM | – | 13 (27.1) | 7 (31.8) | 3 (11.1) | |
| IFN-β 1b SC | – | 17 (35.4) | 8 (36.4) | 13 (48.1) | 0.450 |
| IFN-β 1a SC | – | 18 (37.5) | 7 (31.8) | 11 (40.7) | |
Data are expressed as mean (SD) unless otherwise stated.
aData are expressed as mean (interquartile range).
bNumber of relapses in the two previous years.
cNumber of gadolinium enhancing lesions at baseline.
dP-values obtained following comparisons between responders, intermediate responders, and non-responders by means of an ANOVA test (age, disease duration, EDSS, number of relapses, and number of Gd-enhancing lesions) and chi-square test (gender and type of IFN-β).
IM = intramuscular; SC = subcutaneous.
Clinical-radiological criteria of response to IFN-β therapy
Clinical and radiological criteria of response to IFN-β were also applied to the same cohort of patients with relapsing-remitting multiple sclerosis after 1 year of treatment, as previously described (Río et al., 2009). Non-responders were patients satisfying two or three of the following criteria: (i) presence of one or more relapses; (ii) increase of one or more points in the EDSS score; and (iii) presence of three or more active lesions (new or enlarging T2 lesions or gadolinium enhancing lesions) on the 1-year brain MRI. The remaining patients were considered IFN-β responders. Sixty-nine patients (71.1%) were classified as responders and 28 patients (28.9%) as non-responders.
Messenger RNA expression levels of inflammasomes and related cytokines in peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) from patients with relapsing-remitting multiple sclerosis and healthy controls were isolated by Ficoll-Isopaque density gradient centrifugation (Gibco BRL, Life Technologies) and stored in liquid nitrogen until used. To perform an initial selection of the inflammasomes and cytokines associated with the response to IFN-β, mRNA expression levels for AIM2, NLRC4, NLRP1, NLRP3, IL10, IL1B and IL18 were first determined in a subgroup of 53 patients with relapsing-remitting multiple sclerosis (26 responders, 15 non-responders, 12 intermediate responders) and 14 healthy control subjects. In a second phase of the study, gene expression levels for NLRP3 and IL1B were interrogated in the whole cohort of 97 patients with relapsing-remitting multiple sclerosis.
Determinations were performed in PBMC collected at baseline before treatment with IFN-β. In a subgroup of relapsing-remitting multiple sclerosis patients with PBMC samples available at later time points of IFN-β treatment, NLRP3 and IL1B gene expression levels were also investigated after 3 months (n = 32; 21 responders and 11 non-responders) and 12 months (n = 20; 12 responders and eight non-responders) of treatment. As shown in Supplementary Table 1, these subcohorts were representative of the whole cohort of 97 patients with relapsing-remitting multiple sclerosis.
Messenger RNA expression levels for AIM2, NLRC4, NLRP1, NLRP3, IL10, IL1B and IL18 were determined by real-time PCR using TaqMan® probes specific for each gene (Applied Biosystems). Briefly, total RNA was extracted from PBMC using an RNeasy® kit (Qiagen) and cDNA synthesized using the High Capacity cDNA Archive kit (Applied Biosystems). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control (Applied Biosystems). Assays were run on the ABI PRISM® 7900HT system (Applied Biosystems) and data were analysed with the 2−ΔΔCT method (Livak and Schmittgen, 2001). Results were expressed as fold-change in gene expression in non-responders, intermediate responders, and healthy control subjects relative to responders (calibrators).
Quantification of IL1B levels in serum samples by ELISA
Levels of IL1B were measured in serum samples from a subgroup of 64 patients with relapsing-remitting multiple sclerosis, 28 responders, 20 intermediate responders, and 16 non-responders that were collected at the same time as the PBMC used in the expression study. Peripheral blood was collected by standard venipuncture and allowed to clot spontaneously for 30 min. Serum was isolated by centrifugation and stored frozen at −80°C until used. IL1B levels were measured in serum samples by means of a commercially available ELISA (Human IL1B ELISA; R&D Systems). Samples were measured in duplicate in undiluted serum samples. The intra-assay and inter-assay coefficients of variation were 6.0% and 9.3%, respectively.
Genotyping of NLRP3 polymorphism
Genomic DNA from peripheral blood samples was obtained using standard methods from 789 multiple sclerosis patients classified according to the same clinical criteria of response to IFN-β treatment as described above. A total of 403 patients (51.1%) were labelled as responders and 386 patients (49.9%) as non-responders. Genotyping of rs35829419 was performed by means of the 5’ nuclease assay technology for allelic discrimination using fluorogenic TaqMan® probes on a 7900 Applied Biosystems machine. Rs35829419 was commercially available from Applied Biosystems through the Assay-on-Demand service. All cohorts used in the study were genotyped in one single centre (Cemcat). Supplementary Table 2 summarizes demographic and baseline clinical details of the patient cohorts included in the study.
Statistical analysis
Statistical analysis was performed by using the SPSS 17.0 package (SPSS Inc) for MS-Windows. Comparisons of mRNA expression levels for inflammasomes and related cytokines between responders and non-responders at baseline and between baseline and the IFN-β treated time points were analysed using unpaired and paired Student’s t-tests, respectively. P-values < 0.05 were considered statistically significant. Allele frequencies of the NLRP3 polymorphism were compared between IFN-β responders and non-responders taking into account possible stratification due to different population origin using the Cochran-Mantel-Haenszel test performed with standard software (Review Manager RevMan v.5.0). For the Mantel-Haenszel analysis, odds ratios (OR) and 95% confidence intervals (CI) were calculated by using raw data for each cohort and for the pooled population. The Der Simonian and Laird random effects model was used according to the results of the tests of heterogeneity. The combined effect for heterogeneity was calculated by estimating the inverse variance, P-value < 0.10 and the I2 statistic with a cut-off point of 25%, which defined a significant degree of heterogeneity. The effect of each cohort was weighted for the total number of patients included. A sensitivity analysis was performed to test the relative influence of each cohort on the results. Cohorts were sequentially dropped, and the effect on the change in the overall degree of heterogeneity was then determined.
Results
Messenger RNA expression levels of NLRP3 and IL1B are increased in PBMC from non-responders at baseline
First, we aimed to identify a potential relationship between inflammasomes and related cytokines and the response to IFN-β. To this end, AIM2, NLRC4, NLRP1, NLRP3, IL10, IL1B and IL18 expression levels were determined before treatment with IFN-β in PBMC from a subgroup of 53 patients with relapsing-remitting multiple sclerosis and 14 healthy control subjects.
As shown in Fig. 1A, significant differences were only observed for NLRP3, and mRNA expression levels for this gene were increased in PBMC from non-responders compared to responders (P = 0.038). Further comparisons of NLRP3 expression levels between non-responders and intermediate responders or healthy control subjects were not statistically significant.
Figure 1.

Baseline expression levels of inflammasomes and related cytokines in patients stratified according to clinical criteria of IFN-β response. Messenger RNA expression levels for AIM2, NLRC4, NLRP1, and NLRP3 (A), and for IL10, IL18, and IL1B (B) were determined in PBMCs from patients with multiple sclerosis and healthy control subjects by real-time PCR relative quantification. Significant P-values are shown in bold. Results are expressed as fold-change in gene expression in non-responders (NR), intermediate responders (IR), and healthy controls (HC) relative to responders. Responders to IFN-β (n = 26); non-responders to IFN-β (n = 15); intermediate responders to IFN-β (n = 12); HC = healthy controls (n = 14).
When inflammasome-related cytokines were compared among groups, significant differences were only observed for IL1B, and mRNA expression levels for this gene were increased in non-responders compared with responders (P = 0.006) and healthy controls (P = 0.007) (Fig. 1B). Comparisons of mRNA expression levels for IL10 and IL18 did not reveal statistically significant differences between responders and non-responders (Fig. 1B).
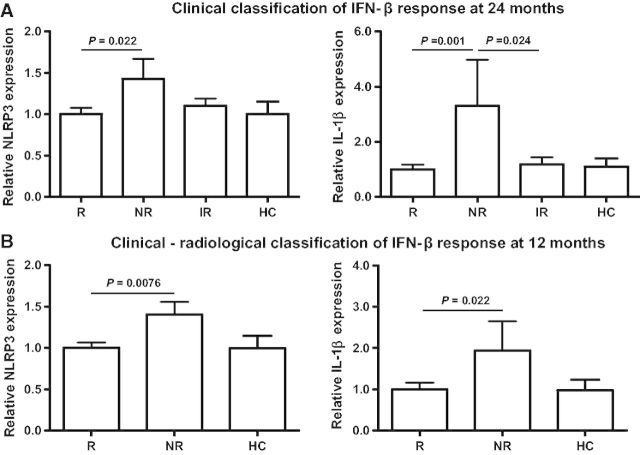
Based on these results, in a second phase of the study, NLRP3 and IL1B mRNA expression levels were measured at baseline in the whole cohort of 97 patients with relapsing-remitting multiple sclerosis. As shown in Fig. 2A, NLRP3 and IL1B expression levels remained significantly higher in PBMC from non-responders compared to responders (P = 0.022 and P = 0.001, respectively). Expression levels for IL1B were also higher in PBMC from non-responders compared with the intermediate responders group (P = 0.024; Fig. 2A).
Figure 2.
NLRP3 and IL1B baseline expression levels in patients stratified according to different IFN-β response criteria. (A) mRNA expression levels for NLRP3 and IL1B in PBMCs from multiple sclerosis classified based on clinical criteria applied after 24 months of treatment with IFN-β. Responders (R) to IFN-β (n = 48). Non-responders (NR) to IFN-β (n = 22); intermediate responders (IR) to IFN-β (n = 27); healthy controls (HC; n = 14). (B) mRNA expression levels for NLRP3 and IL1B in PBMC from multiple sclerosis classified according to clinical-radiological criteria applied after 12 months of IFN-β treatment. Responders to IFN-β (n = 69); non-responders to IFN-β (n = 28); healthy controls (n = 14). Expression levels for NLRP3 and IL1B were determined by real-time PCR relative quantification. Significant P-values are shown in bold. Results are expressed as fold-change in gene expression in non-responders, intermediate responders, and healthy control relative to responders.
Interestingly, these differences in baseline gene expression levels between responders and non-responders were maintained regardless of the IFN-β response criteria used to classify patients, and NLRP3 and IL1B were found to be significantly higher in PBMC from non-responders compared with responders following a clinical-radiological classification of patients with relapsing-remitting multiple sclerosis after 12 months of IFN-β treatment (P = 0.0076 and P = 0.022, respectively; Fig. 2B).
To investigate whether IL1B mRNA expression findings in PBMC were also observed at the protein level, IL1B was measured in serum samples from a subgroup of 64 patients with relapsing-remitting multiple sclerosis by ELISA. IL1B levels were detected in serum samples of six non-responders [38%; mean levels (standard deviation): 25.8 ng/ml (30.7)], four intermediate responders [20%; mean levels: 16.9 ng/ml (12.3)], but in none of the 28 serum samples from responders (data not shown).
NLRP3 and IL1B expression is increased in responders but unchanged in non-responders after IFN-β treatment
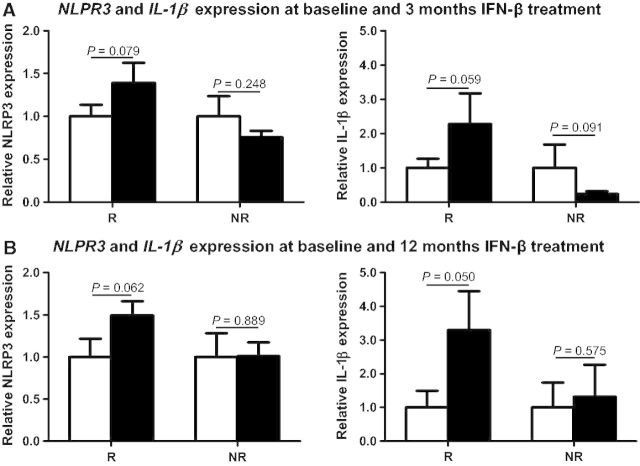
As shown in Fig. 3, after 3 and 12 months of IFN-β treatment, trends towards increased mRNA expression levels for NLRP3 and IL1B were observed in PBMC from responders when compared with the baseline time points (for NLRP3: P = 0.079 and P = 0.062 after 3 and 12 months of treatment, respectively; for IL1B: P = 0.059 and P = 0.050 after 3 and 12 months of treatment, respectively). In contrast, in non-responders, investigation of NLRP3 and IL1B expression levels after treatment revealed either no changes in NLRP3 expression (P = 0.248 and P = 0.889 after 3 and 12 months, respectively) and IL1B expression (P = 0.575 after 12 months), or a trend towards decreased IL1B levels at 3 months compared with baseline (P = 0.091) (Fig. 3).
Figure 3.
NLRP3 and IL1B expression levels between baseline and 3 and 12 months of IFN-β treatment. Messenger RNA expression levels for NLRP3 and IL1B were determined in PBMCs from a subgroup of patients after 3 months (n = 32; 21 responders and 11 non-responders) and 12 months (n = 20; 12 responders and 8 non-responders) of treatment with IFN-β. Clear bars indicate baseline untreated samples and black bars represent the corresponding IFN-β treated time points. NLRP3 and IL1B expression levels were determined by real-time PCR relative quantification. Results are expressed as fold-change in gene expression in the treated time point relative to the untreated (baseline) condition.
NLRP3 rs35829419 polymorphism showed borderline association with the response to IFN-β
The NLRP3 rs35829419 in exon 3 (Q705K) was genotyped in 789 patients with relapsing-remitting multiple sclerosis, 402 responders and 387 non-responders, from 11 independent relapsing-remitting multiple sclerosis cohorts. No deviations from Hardy-Weinberg equilibrium were observed for this polymorphism across cases (P > 0.05). As summarized in the forest plot in Fig. 4, none of the tested cohorts showed independent association of the rs35829419*A allele with the response to IFN-β therapy, although apparent heterogeneity was evidenced (Fig. 4 and Table 2; I2 = 33%). After a sensitivity analysis, heterogeneity was eliminated by excluding the Málaga cohort (Fig. 4 and Table 2; I2 = 0%) and the overall meta-analysis by a Mantel-Haenszel test revealed a trend for association between the NLRP3 rs35829419*A allele and the response to IFN-β [Fig. 4 and Table 2: pM-H = 0.08; ORM-H (95% CI) = 1.7 (0.93–3.11)].
Figure 4.
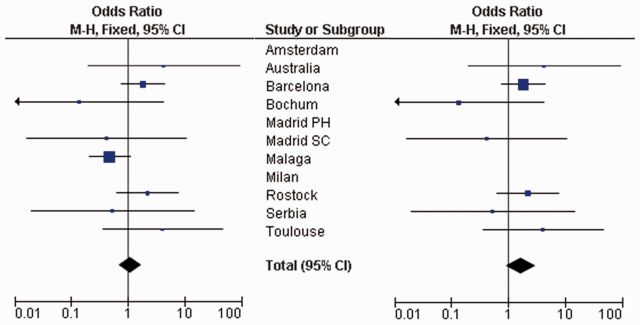
Meta-analysis showing the association between NLRP3 rs35829419 and IFN-β response in the different study cohorts. Forest plots showing the overall cohorts (left) and after eliminating heterogeneity by removing the Malaga cohort (right). M-H = Mantel-Haenszel; Madrid SC = Hospital Clínico San Carlos, Madrid; Madrid PH = Hospital Universitario Puerta de Hierro, Madrid.
Table 2.
Summary of NLRP3 rs35829419 genotyping in responders and non-responders to IFN-β in the different study cohorts
| Study or Subgroup | R Events | Total | NR Events | Total | Weight | Weight (excluding Malaga cohort) | Odds Ratio M-H, Fixed, (95% CI) |
|---|---|---|---|---|---|---|---|
| Amsterdam | 0 | 58 | 0 | 40 | Not able to estimate | ||
| Australia | 2 | 28 | 0 | 22 | 1.5% | 3.0% | 4.25 (0.19, 93.10) |
| Barcelona | 16 | 246 | 8 | 220 | 23.3% | 47.1% | 1.84 (0.77, 4.40) |
| Bochum | 0 | 8 | 1 | 4 | 5.4% | 10.9% | 0.14 (0.00, 4.26) |
| Madrid PH | 0 | 3 | 0 | 2 | Not able to estimate | ||
| Madrid SC | 0 | 23 | 1 | 30 | 3.8% | 7.7% | 0.42 (0.02, 10.75) |
| Malaga | 9 | 292 | 17 | 268 | 50.6% | 0.0% | 0.47 (0.21 1.07) |
| Milan | 0 | 10 | 0 | 0 | Not able to estimate | ||
| Rostock | 8 | 84 | 4 | 88 | 10.4% | 21.1% | 2.21 (0.64, 7.64) |
| Serbia | 0 | 8 | 1 | 14 | 3.1% | 6.3% | 0.53 (0.02, 14.55) |
| Toulouse | 2 | 44 | 1 | 86 | 1.9% | 3.9% | 4.05 (0.36, 45.92) |
| Total (95% CI) | 804 | 774 | 100% | 1.08 (0.68, 1.72) | |||
| Total events | 37 | 33 | |||||
| Heterogeneity: Chi2 = 10.43, df = 7 (P = 0.17), I2 = 33%. Test for overall effect: Z = 0.31 (P = 0.75) | |||||||
| Excluding the Malaga cohort | |||||||
| Total (95% CI) | 512 | 506 | 100% | 1.70 (0.93, 3.11) | |||
| Total events | 28 | 16 | |||||
| Heterogeneity: Chi2 = 4.29, df = 6 (P = 0.64), I2 = 0%. Test for overall effect: Z = 1.73 (P = 0.08) | |||||||
Discussion
Inflammasomes are protein complexes that can be activated by pathogen-associated molecular patterns (PAMPs), as well as damage-associated molecular patterns (DAMPs) (Lamkanfi, 2011). They are known to sense a wide range of stimuli including bacteria, fungi, extracellular ATP, amyloid-β and uric acid, as well as various environmental irritants, such as silica, asbestos and alum (Jin and Flavell, 2010). Recent studies have suggested a potential modulation of inflammasome activity by IFN-β in patients with relapsing-remitting multiple sclerosis (Guarda et al., 2011; Beynon et al., 2012). However, these studies did not specifically address the role of inflammasomes in the response to treatment. Bearing this in mind, we measured the expression of the four major inflammasomes (AIM2, NLRC4, NLRP1, NLRP3) and related cytokines (IL1B, IL10, IL18) in PBMC from patients with relapsing-remitting multiple sclerosis classified according to their clinical response to IFN-β. Only the NLRP3 inflammasome and its related cytokine IL1B were found to play a role in the response to IFN-β, based on the finding of a significant increase in the mRNA expression levels of NLRP3 and IL1B in PBMC from non-responders compared to responders at baseline.
The NLRP3 inflammasome, also known as NALP3 or CIAS1, is currently the most fully characterized inflammasome and is comprised of three different proteins: NLRP3, adapter protein apoptosis-associated speck-like protein (ASC), and procaspase-1 (Inoue and Shinohara, 2013). Oligomerization of the NLRP3 inflammasome heterotrimer unit leads to procaspase-1 self-cleavage to generate activated caspase-1, which sequentially processes maturation of IL1B and IL18 and elicits rapid release of these inflammatory cytokines and pyroptosis by inflammasomes (Inoue and Shinohara, 2013).
Clinical criteria of response to IFN-β were applied after 2 years of follow-up based on the presence of relapses and increase in the EDSS, a classification used by the group in previous studies (Comabella et al., 2009; Bustamante et al., 2011). It is important to emphasize that NLRP3 and IL1B findings were still present following the classification of relapsing-remitting multiple sclerosis patients according to different response criteria that incorporated radiological information and shorter follow-up time (Río et al., 2009). In this regard, gene expression levels of NLRP3 and IL1B remained significantly higher in PBMC from non-responders compared to responders classified after 1 year of treatment according to the presence of relapses, progression on the EDSS score, and MRI activity. This ‘survival’ in significant findings regardless of the response criteria used to classify patients possibly reinforces the role of the NLRP3 inflammasome and IL1B in the response to IFN-β.
A critical point in IFN-β response studies is whether proposed biomarkers are in fact true response biomarkers or they simply reflect differences in disease activity between responders and non-responders. The finding of higher NLRP3 and IL1B expression levels in PBMC collected at baseline supports a role of these molecules as disease activity biomarkers. This view may be further sustained by studies in experimental autoimmune encephalomyelitis showing reduced disease severity in Nlrp3-deficient mice (Gris et al., 2010; Inoue et al., 2012). However, studies in PBMC from patients with multiple sclerosis receiving treatment showed a differential effect of IFN-β in the mRNA expression levels of NLRP3 and IL1B in responders and non-responders, findings that support their role also as IFN-β response biomarkers. In a previous study, IL1B production was found to be reduced in blood monocytes from multiple sclerosis patients treated with IFN-β (Guarda et al., 2011). In our study, although baseline expression levels for NLRP3 and IL1B were significantly increased in PBMC from non-responders, the degree of induction in gene expression observed for these genes after IFN-β treatment was much higher in responders than in non-responders, where expression levels were unchanged or even decreased. This behaviour in NLRP3 and IL1B expression between responders and non-responders is somehow similar to the one observed for type I IFN responsive genes following IFN-β treatment (Comabella et al., 2009). How this differential effect of IFN-β in NLRP3 and IL1B expression relates to therapeutic outcome merits further investigation. Considering the key role of IL1B in the generation of Th17 cells (Lasigliè et al., 2011), it would be worthwhile for future research to explore the IL23/IL17 axis in peripheral blood from responders and non-responders. In addition, one potential explanation for this differential effect is the trend for association with the response to IFN-β observed for rs35829419.
The Q705K polymorphism (rs35829419) located in exon 3 of the NLRP3 gene is a gain-of-function alteration leading to an overactive NLRP3 inflammasome. IL1B levels were found to be elevated in cells transduced with NLRP3-705K compared to NLRP3-WT (Verma et al., 2012). This observation supported the assertion that rs35829419 was indeed functional in relation to IL1B production. Different alleles of the NLRP3 rs35829419 polymorphism have previously shown protective effects in autoimmune diseases such as Crohn’s disease (Roberts et al., 2010) and coeliac disease (Pontillo et al., 2011), with either the major (C) or the minor (A) allele leading to protection, respectively. In our study, the higher frequency of the rs35829419*A allele observed in responders compared with non-responders may explain the differential increase in NLRP3 and IL1B expression induced by IFN-β in PBMC from patients who will respond to treatment. However, these findings require further confirmation in larger cohorts of patients.
Taken together, the results from the present study point to a role of the NLRP3 inflammasome and its related cytokine IL1B in the response to IFN-β in patients with relapsing-remitting multiple sclerosis. It is important to mention at this point that the regulation of IL1B by the NLRP3 inflammasome occurs at the post-translational level, and hence the contribution of other factors such as NF-κB to the transcriptional differences observed for IL1B cannot be completely ruled out.
Finally, findings in treated patients with multiple sclerosis also suggest a differential effect of IFN-β in NLRP3 and IL1B expression in PBMC from responders and non-responders, which might be related to the subsequent response outcome to IFN-β.
Funding
The authors thank the ‘Red Española de Esclerosis Múltiple (REEM)’ sponsored by the FEDER-FIS and the ‘Ajuts per donar Suport als Grups de Recerca de Catalunya, sponsored by the ‘Agència de Gestió d’Ajuts Universitaris i de Recerca’ (AGAUR), Generalitat de Catalunya, Spain.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- EDSS
Expanded Disability Status Scale
- IFN-β
interferon beta
- PBMC
peripheral blood mononuclear cell
References
- Beynon V, Quintana FJ, Weiner HL. Activated human CD4+CD45RO+ memory T-cells indirectly inhibit NLRP3 inflammasome activation through downregulation of P2X7R signalling. PLoS One. 2012;7:e39576. doi: 10.1371/journal.pone.0039576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante MF, Fissolo N, Río J, Espejo C, Costa C, Mansilla MJ, et al. Implication of the Toll-like receptor 4 pathway in the response to interferon-β in multiple sclerosis. Ann Neurol. 2011;70:634–45. doi: 10.1002/ana.22511. [DOI] [PubMed] [Google Scholar]
- Comabella M, Lünemann JD, Río J, Sánchez A, López C, Julià E, et al. A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain. 2009;132:3353–65. doi: 10.1093/brain/awp228. [DOI] [PubMed] [Google Scholar]
- Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–81. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Förster I, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–23. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Inoue M, Williams KL, Gunn MD, Shinohara ML. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2012;109:10480–5. doi: 10.1073/pnas.1201836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Shinohara ML. The role of interferon-β in the treatment of multiple sclerosis and experimental autoimmune encephalomyelitis - in the perspective of inflammasomes. Immunology. 2013;139:11–18. doi: 10.1111/imm.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha S, Srivastava SY, Brickey WJ, Iocca H, Toews A, Morrison JP, et al. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci. 2010;30:15811–20. doi: 10.1523/JNEUROSCI.4088-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010;30:628–31. doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11:213–20. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- Lasigliè D, Traggiai E, Federici S, Alessio M, Buoncompagni A, Accogli A, et al. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS One. 2011;6:e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14:1054–63. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle C, Goldman M, Goriely S. Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process. J Immunol. 2010;184:1784–92. doi: 10.4049/jimmunol.0902005. [DOI] [PubMed] [Google Scholar]
- Pontillo A, Vendramin A, Catamo E, Fabris A, Crovella S. The missense variation Q705K in CIAS1/NALP3/NLRP3 gene and an NLRP1 haplotype are associated with celiac disease. Am J Gastroenterol. 2011;106:539–44. doi: 10.1038/ajg.2010.474. [DOI] [PubMed] [Google Scholar]
- Río J, Nos C, Tintoré M, Téllez N, Galán I, Pelayo R, et al. Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann Neurol. 2006;59:344–52. doi: 10.1002/ana.20740. [DOI] [PubMed] [Google Scholar]
- Río J, Castilló J, Rovira A, Tintoré M, Sastre-Garriga J, Horga A, et al. Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler. 2009;15:848–53. doi: 10.1177/1352458509104591. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Topless RK, Phipps-Green AJ, Gearry RB, Barclay ML, Merriman TR. Evidence of interaction of CARD8 rs2043211 with NALP3 rs35829419 in Crohn's disease. Genes Immun. 2010;11:351–6. doi: 10.1038/gene.2010.11. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Verma D, Särndahl E, Andersson H, Eriksson P, Fredrikson M, Jönsson JI, et al. The Q705K polymorphism in NLRP3 is a gain-of-function alteration leading to excessive interleukin-1β and IL-18 production. PLoS One. 2012;7:e34977. doi: 10.1371/journal.pone.0034977. [DOI] [PMC free article] [PubMed] [Google Scholar]