Speech production is a remarkable motor feat, but how the brain orchestrates articulators to produce fluent, well-intonated speech is unclear. Neef et al. verify the proposed uncoupling of motor output cells from motor plan cells in left primary motor cortex in fluent speech, and reveal its disruption in stuttering.
Keywords: motor control; primary motor cortex; transcranial magnetic stimulation; motor evoked potentials, stuttering
Abstract
The precise excitability regulation of neuronal circuits in the primary motor cortex is central to the successful and fluent production of speech. Our question was whether the involuntary execution of undesirable movements, e.g. stuttering, is linked to an insufficient excitability tuning of neural populations in the orofacial region of the primary motor cortex. We determined the speech-related time course of excitability modulation in the left and right primary motor tongue representation. Thirteen fluent speakers (four females, nine males; aged 23–44) and 13 adults who stutter (four females, nine males, aged 21–55) were asked to build verbs with the verbal prefix ‘auf’. Single-pulse transcranial magnetic stimulation was applied over the primary motor cortex during the transition phase between a fixed labiodental articulatory configuration and immediately following articulatory configurations, at different latencies after transition onset. Bilateral electromyography was recorded from self-adhesive electrodes placed on the surface of the tongue. Off-line, we extracted the motor evoked potential amplitudes and normalized these amplitudes to the individual baseline excitability during the fixed configuration. Fluent speakers demonstrated a prominent left hemisphere increase of motor cortex excitability in the transition phase (P = 0.009). In contrast, the excitability of the right primary motor tongue representation was unchanged. Interestingly, adults afflicted with stuttering revealed a lack of left-hemisphere facilitation. Moreover, the magnitude of facilitation was negatively correlated with stuttering frequency. Although orofacial midline muscles are bilaterally innervated from corticobulbar projections of both hemispheres, our results indicate that speech motor plans are controlled primarily in the left primary speech motor cortex. This speech motor planning-related asymmetry towards the left orofacial motor cortex is missing in stuttering. Moreover, a negative correlation between the amount of facilitation and stuttering severity suggests that we discovered a main physiological principle of fluent speech production and its role in stuttering.
Introduction
Effortless and fluent speech production is essential for success in society. Persistent stuttering is a neurodevelopmental speech fluency disorder with a complex genetic basis (Fisher, 2010). It occurs in 5% of all children and persists in about 1% of the adult population (Yairi and Ambrose, 2005), severely compromising quality of life (Yaruss, 2010).
MRI provides evidence that neural structures and neural activity exhibit irregularities in persons with persistent developmental stuttering. The main findings are an imbalanced activation of speech-related auditory and motor cortices (Brown et al., 2005), a reduced activation of subcortical structures, including the basal ganglia and cerebellum (Giraud et al., 2008; Watkins et al., 2008), a reduced white matter integrity of left hemispheric speech motor regions (Sommer et al., 2002; Watkins et al., 2008; Kell et al., 2009; Cai et al., 2014), and a left inferior frontal-premotor functional-connectivity deficit (Chang et al., 2011; Chang and Zhu, 2013). For reports describing the variability across previous imaging investigations see Cai et al. (2014), Ingham et al. (2012) and Nil et al. (2008).
It is plausible to assume that this irregular structural and functional connectivity relates to an irregular up- or downregulation of the local circuitry, or local excitability. Regulation of excitability is the mechanism by which motor programs are organized, selected, and finally executed to perform a coordinated movement (Stinear et al., 2009). Precise regulation of the excitability of neuronal circuits in the primary motor cortex is central to the successful execution of speech movements. These neurons receive input from a large distributed network and, as neurons of the final motor-output stage, integrate this input to command coordinated, voluntary movements. Theories suggest that the executed motor program is the ‘winner’ selected out of a number of competing, simultaneously-activated neural representations of potential actions (Cisek and Kalaska, 2005). The accidental execution of an undesirable movement, e.g. a stutter event, might therefore be triggered by a failure in motor program activation which, on the physiological level, is reflected in a dynamic imbalance between the facilitation and inhibition of neural populations in the primary motor cortex (Michelet et al., 2010). Thus, intracortical inhibitory and excitatory networks of the primary motor cortex, which are driven by large amounts of input information from the cortical and subcortical regions, control the selection and initiation of target movements and suppress undesirable synergism (Stinear et al., 2009). This theory clearly predicts a deviant excitability of the primary motor cortex in stuttering which should be displayed in altered patterns of speech-induced inhibition and facilitation. This prediction is notoriously hard to address because no animal models exist to investigate speech motor control apart from birdsong (Wang et al., 2008), and invasive methods which could directly measure these effects cannot be employed in humans. Neuroimaging studies have shown aberrant activity in the primary motor cortex during stuttering, which has been taken as an indirect sign of deviant excitability, and this has been interpreted in favour of an imbalance of excitability (Ludlow and Loucks, 2003).
Transcranial magnetic stimulation (TMS) is a non-invasive technique that reads out motor cortex excitability by stimulating a subset of neurons in a small part—approximately 1 cm3—of the cerebral cortex (Salvador et al., 2011). The technique uses the principle of electromagnetic induction. A very brief magnetic pulse induces an electrical field, and thereby an electrical current, in conductive brain tissue. Strong, suprathreshold stimuli applied over the motor cortex directly elicit motor evoked potentials (MEPs) in the target muscles of the stimulated area. The MEP peak-to-peak amplitude is a common quantifier of cortical excitability.
Previous TMS examinations of adults who stutter focused on the corticospinal excitability of the primary motor hand representation (Sommer et al., 2009; Alm et al., 2013; Busan et al., 2013). Studies of the motor cortex excitability for orofacial structures with TMS are scarce and challenging (Devlin and Watkins, 2007), only a single study examined the speech motor cortex excitability in adults who stutter (Neef et al., 2011b). In that study, we measured MEP responses at rest and at different intervals after an artificial stimulus, i.e. a conditioning TMS pulse. In adults who stutter, cortical excitability was modulated less compared to fluent speakers. To elucidate whether this reduced dynamic range of excitability modulation also occurs during speech production, ultimately, TMS pulses have to be applied during speaking. To monitor the modulation of excitability in response to a certain change in cortical activity, we replaced the artificial conditioning TMS stimulus by speech-driven, intrinsic modulatory activity. Across trials, the initial excitability state was controlled for cognitive load and myoactivity, as subjects prolonged a fixed articulatory configuration and prepared the pronunciation of a word chosen from a linguistically homogeneous set. Consequently, in the current study, we provide the first online assessment of motor cortex excitability of the tongue representation during speaking. We show that speech production fails to produce an increase in speech-specific left motor cortex excitability in adults who stutter, which contrasts to the situation in fluent speakers.
Materials and methods
Participants
A total of 33 adults were recruited. All were native speakers of German. Data on 13 adults who stutter (four females; mean 34.5 years, SD = 12.0) and 13 fluent speakers (four females, mean 30.1 years, SD=7.8) were included in the analysis. We excluded three subjects because 140% of their motor threshold was >80% of the maximum stimulator output, three other subjects because they perceived the set-up as uncomfortable and refused further participation, and one adult in the group of fluent speakers was excluded because he showed 5% stuttered syllables on speech analysis. Adults who stutter were recruited from the local stuttering support group and from the Institute for Kassel Stuttering Therapy (Euler et al., 2009). Fluent speakers were recruited by advertisement. The groups were matched for age, handedness (Oldfield, 1971), and education (1 = school; 2 = high school; 3 = <2 years college; 4 = 2 years college; 5 = 4 years college; 6 = postgraduate). Eight adults who stutter reported a family history of stuttering. None of the fluent speakers reported having a family history of speech or language disorders. Apart from stuttering in the group of adults who stuttered, participants reported no medical history, neurological impairment, or drug use that would potentially affect their neurological function. Before experimental measures were obtained with TMS, all subjects were screened for exclusion criteria using a standard TMS safety screen (Keel et al., 2001). All subjects provided written informed consent prior to inclusion in the study. All procedures used in this study were approved by the Institutional Review Board of the University Medical Centre Göttingen.
Stuttering severity was assessed by collecting samples of reading aloud and spontaneous speech elicited through a standardized interview asking participants to narrate their daily routine, to retell their favourite movie or novella and to give directions when imagining a person asking the way. These samples were video-recorded and analysed offline by a qualified speech–language pathologist. The stuttering severity index (SSI-3) was used to determine the frequency and duration of stuttered syllables as well as physical concomitants of stuttering. Stuttering severity in the adults who stutter was as follows: six were very mild, one was mild, two were moderate, three were severe, and one was very severe. Table 1 summarizes the demographic information of the participants.
Table 1.
Participants demographic information, behavioural results and TMS motor thresholds
| Measures | Stuttering | Controls | Difference |
|---|---|---|---|
| n | 13 | 13 | n/a |
| Females (n) | 4 | 4 | n/a |
| Age in years | 34.5 (7.8) | 30.2 (12.0) | P = 0.3 (n.s.) |
| Age of stuttering onset in years | 4.0 (2.1) | n/a | n/a |
| SSI-3 overall score | 23.5 (8.9) | n/a | n/a |
| % Stuttered disfluencies | 7.6 (6.0) | 0.3 (0.2) | P = 0.001 |
| Handedness | 75.2 (43.6) | 87.6 (17.8) | P = 0.35 (n.s.) |
| Education* | 3 | 4 | P = 0.09 (n.s.) |
| Motor threshold left hemisphere | 41.9 (7.3) | 37.6 (3.2) | P = 0.07 (n.s.) |
| Motor threshold right hemisphere | 42.6 (5.6) | 37.7 (3.4) | P = 0.01 |
*Education is reported as median, group differences were quantified by Mann-Whitney test.
SSI-3 = Stuttering Severity Instrument, third edition; % stuttered disfluencies = stuttered syllables occurring per 100 syllables; n.s. = not significant.
Electromyography
Surface recordings of the lingual muscle were made from the contralateral and ipsilateral tongue-side with two pairs of disposable, pre-gelled, silver/silver chloride ring electrodes (5 mm × 100 mm, Viasys Neurocare). Electrodes were mounted on a customized spoon-shaped silicon mouthpiece. Contact area at the tongue was 5 mm × 10 mm at longitudinal and lateral inter-electrode distances of 25 and 20 mm, respectively. The mouthpiece was placed on the upper surface of the tongue, and the subjects were asked to close their lips and teeth comfortably without additional pressure and to hold the end of the mouthpiece with the hand ipsilateral to the TMS stimulation site, with the elbow comfortably supported. During the recordings, participants were asked to press their tongue slightly against the electrodes and their lower teeth. This procedure was described previously (Neef et al., 2011b). Surface electromyographic signals were amplified (×1000) and Butterworth bandpass-filtered (bandwidth 20 Hz to 2 kHz) with a Digitimer D360 amplifier, acquired at a sampling frequency of 5 kHz, using a 1401 laboratory interface (Cambridge Electronic Design). Recordings were controlled by Signal Software (Cambridge Electronic Design, v 2.13) and stored on a personal computer. An additional channel of the system served to record audio signals of the elicited speech simultaneous with the electromyographic signals. To do so, we attached a wireless microphone (AKG PT 40) to the mouthpiece and fed the acquired audio signal into a third channel of the A/D converter.
Transcranial magnetic stimulation
Single-pulse TMS of the primary motor cortex was delivered using a Magstim 2002 magnetic stimulator with a monophasic current waveform (Magstim Company). The magnetic stimulator was connected to a standard figure-of-eight coil with a mean loop diameter of 7 cm. The intersection of the coil was held tangentially to the skull with the handle pointing backwards and laterally at an angle of 45° to the sagittal plane in order to generate a posterior-anterior current in the brain (Kaneko et al., 1996; Di Lazzaro et al., 2004). The optimal position of the coil was defined as the site where stimulation consistently resulted in the largest MEPs. For localization, the scalp surface was explored systematically, the position for consistently inducing maximal MEPs in the contralateral tongue-site at the lowest stimulus strength was identified as the ‘hot spot’ and marked with a pen to ensure accurate coil placement throughout the experiment (Muellbacher et al., 2001; Neef et al., 2011b). The interstimulus interval between single TMS pulses was 6 s (±10%, ∼0.2 Hz). A maximum of 50 pulses was applied before replacing the self-adhesive electrodes. The frequent change of the electrodes was necessary because of salivation. It took 5 min to clean the mouth piece, precisely mount the new electrodes and prepare the subject for the subsequent measurements. A maximum of 100 pulses was applied to determine the hotspot and the motor threshold. Because of the low rate of pulse repetition we ruled out a modulation of motor cortex excitability by TMS pulses applied during this procedure (Fitzgerald et al., 2006). The hot spot of the motor tongue area was ∼2–3 cm anterior and 1–2 cm lateral to the hand representation, which is consistent with the literature (Svensson et al., 2003). Single TMS pulses were applied to determine the minimal stimulus intensity to the nearest 1% of the maximum stimulator output required to produce MEPs of >100 µV in at least three of six consecutive stimuli. This intensity defines the motor threshold and was set to 120% throughout the experiment.
To check that there is a sufficient dynamic range of excitability modulation and ceiling effects due to saturation are not present, we acquired MEP input-output curves. This procedure was adopted from Rödel et al. (2003). We elicited MEPs by stimulating stepwise during 30 trials, rising from 90% to 100%, 110%, 120%, 130%, and 140% of motor threshold.
Verbal stimuli and speech task
Verbal stimuli consisted of 49 German verbs always starting with the prefix ‘auf’ and always continuing with a consonantal cluster, e.g. ‘aufbleiben’ (to stay up). Words were selected from the database Webcelex (MPI Nijmegen, NL; celex.mpi.nl) and controlled for the linguistic parameters frequency, phonetic complexity, number of letters, number of phonemes, number of syllables, and word accent.
Figure 1A illustrates the time course of a single trial of the speech task. Each trial started with a blank interval of 4000 ms followed by a 1500 ms presentation of a verb without its corresponding prefix. Subjects were instructed to read the verb silently and to remember it. Another blank interval of 3000 ms was followed by a plus sign informing the participant to speak the prefix ‘auf’ and to prolong its labio-dental fricative [f]. After 1500 ms a question mark appeared prompting the articulation of the verb that the participant had previously read. The whole sequence was repeated after 12 s.
Figure 1.
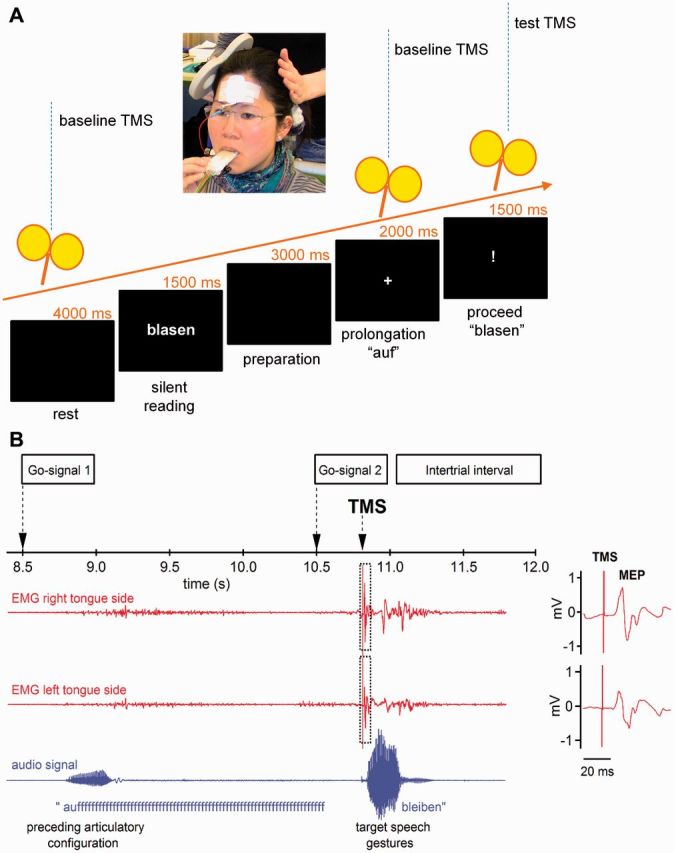
Experimental set-up and procedure. (A) Flowchart of trial. Participants silently read a German verb presented on a screen. A first visual go-signal (+) instructed the participant to start speaking the invariant prefix ‘auf’ and to prolong the ‘fff’ until a second visual go-signal (!) indicated to move onto speaking the previously presented verb. To study a time window of 350 ms preceding the second speech gesture, we used 70 single pulse TMS trials per hemisphere per subject, in which we varied the interval between the single TMS pulse and the speech onset of the verb stem. (B) Traces of the electromyography with MEPs elicited in the contralateral (right) and ipsilateral (left) tongue-side by TMS of the left primary motor cortex. Stimulation intensity was 120% of the individual’s motor threshold. The lower line shows the oscillogram indicating speech onset of the particle ‘auf’ and the subsequent speech signal.
The insertion of the prefix ‘auf’ was planned to force the cortical dynamics to a predefined state. We wanted to ensure (i) a comparable cognitive load (keep a word in mind); (ii) a comparable myogenic state (prolonging a labiodental fricative); and (iii) a comparable preparation of a motor response (waiting for a go signal).
Experimental design
Participants were tested on both hemispheres, in two different TMS sessions, separated by at least 48 h. The order of the tested hemispheres was balanced between subjects. In each session, we began with baseline assessments of the resting motor threshold and input-output curve. This was followed by a familiarization period where the participants were asked to perform 10 trials of the speech task with the mouthpiece in the correct position but without the delivery of TMS pulses. In the core experiment, 98 trials were split into two blocks of 35 trials and one block of 28 trials. Pauses between blocks lasted ∼5 min. A single TMS pulse was delivered per trial, with an intertrial interval of 12 s. Motor cortex excitability was measured at seven different time points: (i) during the resting state, 2900 ms after the trial started; (ii) while holding the fixed articulatory configuration of the labio-dental fricative [f] 1000 ms after the first go-signal; and (iii) at five different latencies of the transition phase between the fixed articulatory configuration and the target speech gestures, namely 240 ms, 280 ms, 320 ms, 360 ms, and 400 ms after the second go-signal. A total of 14 MEPs were acquired per condition. Importantly, the baseline, resting state MEP measurement occurs before the participants had been asked to read the word and to keep it in their mind directly before probing excitability. Had they already read the word the additional cognitive load and the speech preparatory behaviour might engage different neuronal processes and thus result in incomparable dynamic states of the speech motor cortex. This is suggested by a previous MEG study reporting a reversed cortico-cortical processing chain for delayed single-word reading (Salmelin et al., 2000).
Data analysis
Data analyses were performed using a custom-written EMG-Browser in Igor Pro (Wavemetrics). Acoustic speech waveforms were visually inspected in the data browser and speech onset time was manually determined at the time point where the waveform moved away from the baseline at the first clear pulse of the vocal folds. We visually examined the electromyographic signal of all recordings, manually segmented all valid MEPs, and excluded signals with TMS artefacts lasting beyond the motor-evoked response. All valid recordings were fed into consecutive analyses. To determine pre-TMS tongue activity 200 ms of the EMG signal immediately before the TMS artefact were corrected for offset, rectified and averaged over the two electrode pairs attached to the left side of the tongue and to the right side of the tongue.
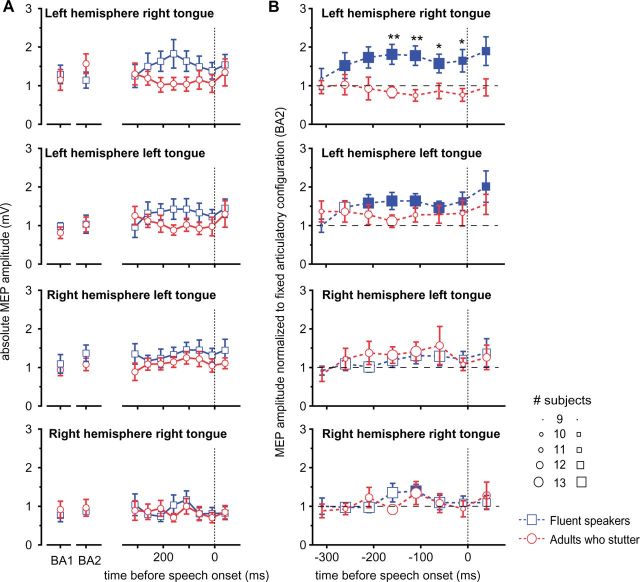
Mean peak-to-peak amplitudes were extracted separately for each condition, for the contralateral and ipsilateral projection in the left and the right hemisphere. To determine the response-locked time course of excitability modulation, we extracted acoustic latencies relative to the individual speech onset of the target. MEP latencies were calculated setting the target speech onset as time zero. The selection of 50 ms bins, starting 40 ms after word onset. No less than nine observations per bin were present for each time bin in each group. For later statistical analyses we performed normalization in two cases. First, MEP amplitudes during the prolongation were related to MEP amplitudes during rest. Grand averages are plotted in Fig. 3. Second, MEP amplitudes of the transition phase were related to the absolute MEP amplitude during prolongation Fig. 4B. All absolute grand average MEP amplitudes are plotted in Fig. 4A.
Figure 3.
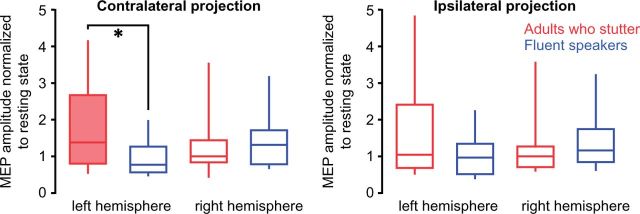
Prolongation induced left hemisphere facilitation in adults who stutter. Box plots illustrate tongue motor cortex excitability during the prolongation of the labio-dental fricative related to resting state. Normalized MEPs are depicted separately for the contralateral and for the ipsilateral tongue projections (± SEM). ANOVA yielded a significant interaction of Group × Hemisphere for the contralateral projections. Post hoc t-tests resulted in a right-lateralized facilitation in fluent speakers and a left-lateralized facilitation in adults who stutter (filled box). A group differences was significant in the left hemisphere contralateral projection. Filled box indicates significant change (P < 0.05). Asterisk indicates level of difference between groups: *P < 0.05.
Figure 4.
Speech preparation facilitated left motor tongue area in fluent speakers only. (A) Group averages of the absolute MEP amplitudes are plotted for resting state excitability (BA1), excitability during prolonged speaking (BA2) and for the trajectory of excitability towards the target gesture. (B) Normalized excitability of the primary motor tongue representation in the transition phase. The x-axis depicts the reaction time of the transition phase between a fixed articulatory configuration and a target articulatory gesture. Towards the target gesture, excitability of the left motor cortex increases significantly in fluent speakers, whereas adults who stutter do not show such a modulation. Filled symbols indicate significant differences from baseline activity. Error bars indicate the standard error of mean. Asterisks indicate differences between groups: *P < 0.05; **P < 0.001. The size of the symbols indicates the number of subjects contributing to the bin.
Statistical analyses
All statistical analyses were performed using SPSS (IBM SPSS, Statistics for Windows, Version 22.0). Data of all metric variables were first fed into Kolmogorov-Smirnov tests for each group separately to evaluate whether the samples come from a population with a specific distribution. Group differences of age, handedness, percentage of stuttered syllables, and motor thresholds were tested by two-tailed unpaired t-tests. Group differences of education, speech onset times, and pre-TMS tongue activity at baseline and during prolongation were tested by Mann-Whitney tests.
To test the variance of pre-TMS tongue activity in the transition phase a mixed-model ANOVA was calculated with Hemisphere (left, right) and Time (−50 ms, −100 ms, −150 ms, −200 ms) as within-subjects factors and Group as between-subjects factor.
To test the variance of motor cortex excitability during prolonging the German prefix ‘auf’ we employed a mixed-model ANOVA with Group as between-subject factor and Hemisphere (left, right), and Tongue projection (ipsilateral, contralateral) as within-subject factor.
To test for group differences in speech-specific modulation of motor cortex excitability, we used two-tailed unpaired t-tests and thus compared the normalized MEP amplitudes (normalized to the augmented baseline excitability, while holding the fixed articulatory configuration of the labio-dental fricative). Paired two-tailed t-tests were used to evaluate differences between speech-related excitability and baseline excitability. Not all bins contained data from all subjects due to the somewhat random delay between go signal and speech onset (Fig. 4B). The t-tests were performed for eight time bins. Because data in the bins are not independent, because of the inherently limited speed with which excitability is modulated, it is not clear to which degree a correction for multiple comparisons is needed; uncorrected values are reported.
For the group of adults who stutter, we related the mean normalized MEP amplitude across all time bins with the percentage of syllables stuttered using an exponential function. This was done separately for the two hemispheres and their merged projections.
A mixed-model ANOVA was used to test the variance of motor thresholds with Group (adults who stutter, fluent speakers) as between-subject factor and Hemisphere (left, right) as within-subject factor. Variance in the MEP input-output curves was tested with a mixed-model ANOVA with Group as between-subject factor and Hemisphere, Tongue projection (ipsilateral, contralateral), and Intensity (90, 100, 110, 120, 130, 140% motor threshold) as within-subject factors.
To control whether speaking with the mouthpiece in place resulted in a change of the motor cortex excitability we compared MEP amplitudes recorded before and after familiarization. The before familiarization condition was characterized by the input-output curve MEPs at 120% motor threshold. Responses recorded during resting state within a trial served as after-familiarization MEPs. These MEPs were elicited with the same stimulation intensity, 2900 ms after trial onset, but before word presentation. We conducted a mixed-model ANOVA with Hemisphere (left, right) and Task (within trial, input-output curve MEP at 120% motor threshold) as within-subjects factors and Group as between-subjects factor. We compared motor cortex excitability at the beginning of the test phase (first 20 MEPs) with motor cortex excitability at the end of the test phase (last 20 MEP) to exhaustedly test whether speaking with the mouthpiece affects excitability of the tongue motor cortex over the whole time course of the experiment. Only MEPs of the transition phase were considered, i.e. the data that entered Fig. 4A and B, but here, MEPs were pooled within subjects regardless of the exact time point when stimulation occurred in the transition phase. We conducted another mixed-model ANOVA with Hemisphere (left, right) and Time (first 20 MEPs, last 20 MEPs) as within-subjects factors and Group as between-subjects factor.
Significant effects in ANOVAs were further evaluated with ANOVAS or t-test.
Results
Adults who stutter were fluent and as fast as fluent speakers when responding to the second go-signal
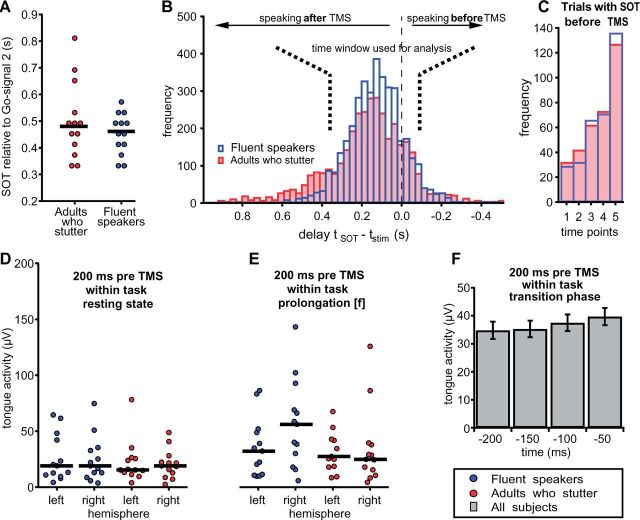
Intelligibility of the utterances was limited due to the mouthpiece. As a consequence, an analysis of the frequency of stuttering events in the phrases uttered during the experiment would not be reliable. Nevertheless, we can use the speech onset time distribution to draw conclusions on the performance of the speakers because most disfluencies occur at word initial position (Bloodstein and Ratner, 2008) and hence would cause longer speech onset times. Figure 2A and B illustrate that adults who stutter were as fast (speech onset time = 480 ms) as fluent speakers (speech onset time = 461 ms, U = 92, P = 0.724). This implies a minimal amount of stuttering events throughout the experiment. Figure 2B demonstrates speech onset times of all trials normalized to the timing of the TMS pulse. Adults who stutter show a longer tail at the left illustrating that slightly more trials were characterized by longer speech onset times and maybe stuttering events but those trials were not included in the statistical analysis. Respondents reported that they had not stuttered during the experiment when asked.
Figure 2.
No group differences for speech onset time and tongue’s pre-TMS innervation. Speech onset times of participants. (A) The medians of the speech onset times are plotted for every subject. A Mann-Whitney test detected no differences between groups (P = 0.724). The solid lines represent the group medians. (B) Histograms illustrate the distribution of speech onset time normalized to the time points of the TMS pulse. Bins that appear at the left side of line zero contain the number of trials where the TMS pulse was applied before speech onset. (C) Amount of trials in which TMS occurred after speech onset as shown on the right side of line zero in the histogram. Time points 1 to 5 represent the times of the TMS pulse with regard to the second go-signal (240 ms, 280 ms, 320 ms, 360 ms and 400 ms). (D and E) Tongue activity quantified as the average over the offset corrected, rectified EMG signal during a 200 ms interval prior to transcranial magnetic stimulation. EMG signals were averaged over the two electrode pairs attached to the left side of the tongue and to the right side of the tongue. (C) For the transition phase after the second go-signal the time course of tongue activity is separated into 50 ms bins over a period of 200 ms. Tongue pressure was comparable between groups across conditions and rose slightly towards the transition phase. SOT = speech onset time.
The number of trials in which the subject started voicing before the TMS pulse was applied is 334 for control subjects and 336 for adults who stutter (Fig. 2C).
Adults who stutter show the same level of the pre-TMS tongue innervation as fluent speakers
Figure 2D–F depicts tongue activity before TMS. During the within-task resting state condition (2700–2895 ms) tongue pressure was similar between groups (left hemisphere grand median = 16 µV, P > 0.05; right hemisphere grand median = 19 µV, P > 0.05). The same holds true for the prolongation of the voiceless, labio-dental fricative (9300–9495 ms, left hemisphere grand median = 30 µV, P > 0.05). Right hemisphere projections show a higher median in fluent speakers (56 µV) compared to adults who stutter (25 µV) with a grand median = 34 µV, but no significant group difference (P > 0.05). Kolmogorov-Smirnov tests reject the hypothesis of normal distribution of pre-TMS tongue activity during resting state and during the prolongation of the fricative. Therefore, all comparisons were calculated with independent samples median tests. For the transition phase after the second go-signal the time course of tongue activity divided into 50 ms bins over a period of 200 ms. In this case Kolmogorov-Smirnov tests indicate normality of pre-TMS tongue activity for all conditions. A mixed model ANOVA yielded no effect of Group, no effect of Hemisphere, and no interactions, but an effect of Time [F(3,22) = 7.85, P = 0.006, Greenhouse-Geisser corrected]. To summarize, tongue pressure was comparable between groups across conditions and rose slightly towards the transition phase.
Adults who stutter exhibit a left-lateralized increase of excitability
To test the level of excitation during speaking, we normalized MEP amplitudes to resting state activity. While fluent speakers showed a right-lateralized increase of excitability, adults who stutter showed a left-lateralized increase of excitability (Fig. 3). This is evident in the interaction of Group × Hemisphere [F(24,1) = 5.0, P = 0.036, Greenhouse-Geisser corrected]. Post hoc two-tailed paired t-tests revealed a marginally increased excitation towards the right hemisphere in fluent speakers (t = 2.06, P =0.062) and a significantly increased excitation towards the left hemisphere in adults who stutter (t = 2.43, P = 0.032).
Fluent speakers have a left-lateralized facilitation of the primary motor tongue representation before the speech segment is executed
To assess the modulation of excitability in the transition phase from a fixed articulatory configuration towards target speech movements, we normalized MEP amplitudes at stimulus-locked time points in the transition phase in relation to the excitation level during the prolongation of the fricative. Only fluent speakers showed an additional facilitation of neurons in the left hemisphere primary motor tongue representation (Fig. 4B). Response-locked normalized MEP amplitudes were significantly increased up to 260 ms before speech onset of the target speech movements (0.009 < P < 0.049, uncorrected; for all P-values see Supplementary Table 1). In contrast, right hemisphere projections did not show this facilitation in the transition phase to the subsequent speech gestures, except for the ipsilateral projection 110 ms to 60 ms before speech onset (P = 0.039, uncorrected). Adults who stutter lacked a facilitation of neurons in the left hemisphere primary motor tongue representation (for the P-values see Supplementary Table 1). To check for differences between adults who stutter and fluent speakers, we ran two-tailed unpaired t-tests. Fluent speakers showed a significantly increased excitation in the contralateral left hemisphere projection up to −160 ms before speech onset (0.004 < P < 0.041 uncorrected; for all P-values see Supplementary Table 2).
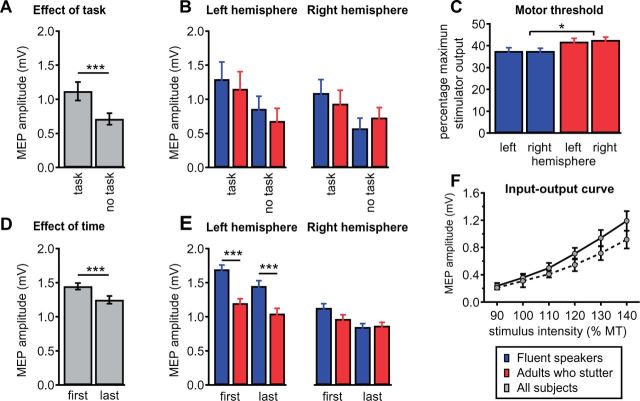
Speaking with the mouthpiece affected excitability of the tongue motor cortex over the whole time course of the experiment. This is the result of the comparison of non-normalized, absolute MEP amplitudes taken from the transition phase at the beginning (first 20 MEPs) and at the end of the test phase (last 20 MEP). ANOVA showed an effect of Time [F(1,24) = 10.12, P = 0.004, Greenhouse-Geisser corrected], an effect of Hemisphere [F(1,24) = 113.95, P <0.001], an effect of Group [F(1,24) = 26.0, P < 0.001] and a Group × Hemisphere interaction [F(1,24) = 26.18, P < 0.001]. To further disentangle the Group ×Hemisphere interaction we calculated two mixed-model ANOVAs separately for each hemisphere. Left hemisphere analysis yielded an effect of Time [F(1,24) = 6.31 P = 0.019, Greenhouse-Geisser corrected] and an effect of Group [F(1,24) = 47.93, P < 0.001] but no Group × Time interaction. Right hemisphere analysis yielded an effect of Time [F(1,24) = 12.97 P = 0.001, Greenhouse-Geisser corrected] but no effect of Group and no interaction. Regardless of hemisphere or group, MEPs were smaller at the end of the experiment than at the beginning, as shown in Fig. 5D. In summary, the observed decrease in MEP amplitude from the first third of test pulses in the transition phase to the last third of pulses could indicate habituation. However, this occurs to the same degree in fluent speakers and in adults who stutter. The finding that differentiates the two groups is the larger MEP response to left hemispheric TMS in the control group (Fig. 5E). This is the effect that we also found in the normalized data and report in Fig. 4B.
Figure 5.
Motor cortex excitability. (A and B) The tongue motor cortex is more excitable during resting state within the experimental speech trial (task) compared to resting state during the recording of the input-output curve (no task). Stimulation intensity was 120% of the individual motor threshold in both conditions. Depicted are MEP amplitudes averaged across all subjects. Grand average MEP amplitudes for fluent speakers and adults who stutter are comparable in magnitude within conditions and within hemispheres. (C) Motor thresholds are plotted as percentage of the maximum stimulator output. Adults who stutter exhibit higher motor thresholds than fluent speakers. (D and E) To exhaustively test whether speaking with the mouthpiece affects excitability of the tongue motor cortex, we compared grand average amplitudes of the first 20 MEPs with the last 20 MEPs acquired in the transition phase after the second go-signal. The first MEP amplitudes were larger in magnitude compared to the last MEP amplitudes. When separated for hemisphere and group, only left hemisphere stimulation evoked larger MEP amplitudes in fluent speakers than in adults who stutter. (F) Shown are group mean MEP amplitudes of the input-output curve averaged over both hemispheres. MEP amplitudes increase with rising stimulus intensity. The contralateral projection of the tongue (solid line) shows a slightly stronger attenuation compared to the ipsilateral projection (dashed line). Error bars indicate the standard error of mean. Asterisks indicate significance levels: *P < 0.05, ***P < 0.001.
The magnitude of left-lateralized facilitation was correlated with stuttering severity
If the observation of a modulated excitability of left hemisphere primary motor tongue representation was indeed related to speech fluency, one would expect a correlation between the excitability modulation and stuttered syllables. To assess this relationship, we plotted these parameters against one other and observed a tight covariance, which could be tentatively described by an exponential fit (Fig. 6). In this phenomenological description, the correlation between the M1 excitability modulation and the percentage of stuttered syllables was significant for the left projection (P = 0.004) but only marginally significant for right projections (P = 0.055).
Figure 6.
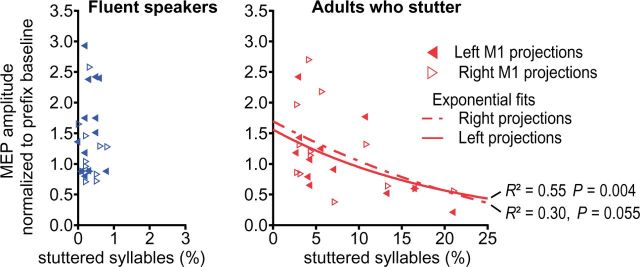
Stuttering severity negatively correlated with speech induced facilitation. In adults who stutter, regression analysis yielded a significant negative correlation between percentage of stuttered syllables in a speech sample of 1000 syllables and lack of facilitation of the MEP in the transition phase between speech gestures.
Adults who stutter have an attenuated motor threshold of the primary motor tongue representation
The motor threshold was assessed to adjust stimulation intensity individually and is given as per cent of the maximum stimulator output. This is a well-established procedure to avoid a group effect induced by different motor thresholds of the recruited subjects. The motor thresholds differed between groups [ANOVA, effect of group, F(1, 24) = 6.09, P < 0.021], with no effect of hemisphere, and no interaction (Fig. 5C). Post hoc t-tests demonstrated higher thresholds in adults who stutter (left 41.9, SD 7.3; right 42.6, SD 5.6) compared to fluent speakers [left 37.6, SD 3.2; right 37.7, SD 3.4; t(50) = −3.33, P = 0.0016 with Bonferroni correction]. This difference should be interpreted carefully. Macroanatomical features dominate the motor threshold (Kozel et al., 2000; Salvador et al., 2011) and the intersubject variability is high, especially for stimulation of neurons of the primary motor tongue area, which extend into the depth of the central sulcus’ ventrorostral part (Grabski et al., 2012). Possibly due to the high intersubject variability, previous reports on motor thresholds in stuttering are ambiguous (Neef et al., 2011 a; Alm et al., 2013). Throughout the experiment, the applied stimuli were normalized to the subjects motor threshold. This levels the intersubject variability and as a result, input-output curves show no effect of group, as reported in the next section.
In resting state, adults who stutter exhibit a normal dynamic range of motor cortex excitability
The MEP input-output curve was assessed to ensure a broad dynamic range of excitability modulation in both groups and thus to avoid ceiling effects due to saturation of the stimulated neural populations at a stimulation intensity of 120% of motor threshold. The analysis of the MEP input-output curve (Fig. 5F) yielded a main effect of Tongue projection [ANOVA, F(1,23) = 10.15, P = 0.004], a main effect of Intensity [F(5,19) = 33.1, P < 0.0001], and an interaction of Tongue projection × Intensity [F(5,19) = 4.5, P = 0.028], but no effect of Group [F(1,23) = 0.01, P = 0.91] as well as no other effects. As shown in Fig. 5F, MEP peak-to-peak amplitudes increase with increasing stimulation intensity. In both groups, the facilitation was particularly marked in the contralateral tongue projection after stimulation of the left hemisphere as shown in Fig. 5F.
The MEP input-output curve mirrors cortical excitability under resting state conditions. To investigate a potential effect due to speaking with the mouthpiece we compared MEP amplitudes before and after familiarization. Before familiarization, as part of the input-output curve, MEPs were elicited at 120% motor threshold. The same intensity was applied throughout the experimental phase and the within-trial resting state condition, 2900 ms post-trial onset. The mixed-model ANOVA resulted in an effect of Task [F(1,24) = 18.0, P < 0.001] with an increased excitability observed in the within-trial condition (Fig. 5A and B). No other effects or interactions occurred. This might be caused by a familiarization to the new speech environment (Xivry et al., 2013; Kadota et al., 2014), or by training (Svensson et al., 2006). An alternative cause could be a general arousal effect due to the visual attention required in the task (Ruge et al., 2014). However, this occurred to the same degree in fluent speakers and in adults who stutter and was therefore not discussed.
Discussion
Fluent speech requires the highly integrated control of the orofacial and respiratory muscles. During speech the orofacial primary motor cortex integrates excitatory and inhibitory signals from various cortical and subcortical sites in order to generate excitatory patterns orchestrating speech movements. Speech flow is often interrupted in stuttering, as sound prolongations, sound and syllable repetitions, and speech blocks indicate an inability to move onto subsequent speech segments. Using TMS, we tracked the modulation of motor cortex excitability during processes of speech motor planning and the timed initiation of sensorimotor speech programs. Specifically, we acquired motor cortex excitability (i) during voluntary prolongation of a speech sound; and (ii) during the transition phase between an invariant articulatory configuration and various subsequent speech movements. The major finding of our current study was the unilateral facilitation of the orofacial primary motor cortex in fluent speakers but the absence of such facilitation in adults who stutter. The critical pathophysiological role of the primary motor cortices is stressed by the inverse correlation of stuttering frequency and inter-gestural facilitation (Fig. 6).
How can we explain the left lateralized increase of motor cortex excitability in fluent speakers and the lack thereof in adults who stutter?
The complex interactions of brain areas and the results of related experiments are best discussed in the context of concrete models of cognitive function. The GODIVA model (Gradient Order Directions into Velocities of Articulators; Bohland et al., 2010) postulates two independent mechanisms in the primary motor cortex finalizing the cortical and subcortical control of fluent speech output: (i) motor output cells; and (ii) motor plan cells. Motor output cells coordinate the timing and initiation of sensorimotor speech programs. Here the pre-supplementary motor area triggers the release of planned speech sounds in interaction with the putamen, globus pallidus, and thalamus. This cortico-striato-thalamo-cortical network interacts in both the left and the right primary motor cortex to bilaterally innervate the orofacial structures during speaking. In contrast to the motor output cells, the motor plan cells are hypothesized to occur only in the left orofacial motor cortex, which is supported by the observation of a left hemisphere specialization for phonation and vocalization (Terumitsu et al., 2006). The motor plan cells integrate selected sensorimotor programs driven by the left posterior inferior frontal gyrus, the adjoining left ventral premotor cortex [Brodmann area (BA) 44/BA6], and the left supplementary motor area (Peeva et al., 2010). An uncoupling of motor output cells from motor plan cells would allow the system to prepare subsequent sensorimotor programs while a former program is executed (Bohland et al., 2010).
Our data show that the excitability of the left orofacial motor cortex is transiently increased just before a novel speech gesture is executed. It thereby provides evidence for such an uncoupling of speech motor planning in the left primary motor cortex from speech output initiation in both motor cortices. It is tempting to speculate that the increased excitation of the left orofacial motor cortex in the transition phase mirrors activated motor programs in the primary motor cortex of fluent speakers waiting for the initiation to release. This interpretation is supported by the fact that phonological manipulation specifically involves the left primary motor cortex but not the right primary motor cortex in fluent speakers (Peeva et al., 2010).
The fact that adults who stutter did not generate a facilitation of left motor cortex is likely related to weaker structural connectivity and altered interplay between left hemisphere speech-related brain regions. A huge body of literature supports the theory that stuttering is a disconnection syndrome because white matter integrity is reduced in the fibre bundles of the left hemisphere speech-related network (Sommer et al., 2002; Chang et al., 2008; Watkins et al., 2008; Cykowski et al., 2010; Cai et al., 2014). In a fibre-tracking study (Chang et al., 2011), adults who stutter showed a significantly smaller number of white matter voxels passing through the motor cortex in the left superior longitudinal fasciculus compared to fluent speakers. This structural deficit might impede the transfer of selected sensorimotor programs from left BA44/BA6 to the orofacial motor cortex and, as a consequence, motor planning could exert a smaller influence on motor cortex excitability via cortico-cortical connections (Greenlee et al., 2004). An alternative view is that the reduced white matter integrity hampers cortico-striatal connectivity (Civier et al., 2013) interrupting the smooth sequencing of subsequent speech segments via the cortico-thalamo-cortical loop (Alm, 2004).
The lack of excitability facilitation found here in adults who stutter could also be caused by an imbalanced neurotransmitter system because dopamine affects cortical excitability and activity in a non-linear, dose-dependent manner (Nitsche et al., 2010; Lissek et al., 2014). Indeed, in adults who stutter, increased dopaminergic activity has been reported (Wu et al., 1997) and pharmacological interventions with dopamine antagonists have been shown to improve symptoms of stuttering (Maguire et al., 2004). When given to control subjects, the dopamine agonists bromocriptin and carbergoline reduce intracortical facilitation at resting state (Ziemann et al., 1997; Korchounov et al., 2007). In an earlier study we found that in adults who stutter the intracortical facilitation is likewise reduced (Neef et al., 2011b). This complementary neurobiological theory of stuttering supports the view that a chronic hyperdopaminergic activity could hinder response competition and thus the timed selection of motor output cells (Civier et al., 2013) and also connects to the findings of impaired motor learning in adults who stutter (Smits-Bandstra et al., 2006; Smits-Bandstra and De Nil, 2009).
In either case, disconnections, elevated dopamine or a combination of both might result in a weakened transfer of sensorimotor programs to the motor plan cells in the left M1 or effect a delayed selection of motor output cells. The reduced dynamic range for intracortical facilitation indicated a weakening of the capability to encode specialized sensorimotor programs at the level of the primary motor cortex. Both processes seem to be plausible mechanisms that could result in intermittent interruptions of fluent speech.
Speech sound prolongation elevates left orofacial primary motor cortex in stuttering
The voluntary prolongation of a speech sound is a rather artificial condition for fluent speakers, whereas it happens involuntarily in stuttering. In the current experiment, we asked adults who stutter as well as fluent speakers to voluntarily prolong the fricative of the German prefix ‘auf’ in order to acquire the state-dependent motor cortex excitability for speaking. Accordingly, we controlled for cognitive load and pre-TMS innervation of orofacial muscles. The articulation of the fricative requires a constriction between the lower lip and the upper incisors, but the tongue rests with its tip gently touching the lower incisors. Although the tongue does not play an active part in this, motor cortex excitability was increased compared to the resting state (Fig. 3). Considering the bilateral innervation of articulatory structures, one would expect a comparable increase of excitability in both hemispheres. But our data show a left-lateralized increase in adults who stutter, contrary to a trend towards a right lateralized increase in fluent speakers. Besides the prolongation of the prefix ‘auf’, the cognitive state during this time in the task also reflects preparation for the production of the next word following the second go signal. Therefore the observed elevated excitation may reflect a different preparatory behaviour with an excessive activation of the primary motor cortex instead of the inferior frontal areas in adults who stutter (Salmelin et al., 2000).
This observed group difference during the state we normalized to may raise the question whether normalizing the data of the transition phase to the phase of prolongation could have driven the apparent differences in MEPs during speech production. To demonstrate that between-group differences were not driven by the normalization, we plotted the grand averages of the non-normalized absolute MEP amplitudes for all conditions in Fig. 4A. Kolmogorov-Smirnov tests indicated non-normality of MEP amplitudes in the bins characterizing the response-locked trajectory of speech motor preparation. To overcome this problem of variability, we pooled absolute MEP amplitudes for all time points before speaking but separately for each hemisphere. The same data points that entered the analysis on normalized data were analysed here. An additional mixed-model ANOVA with Hemisphere as within-subjects factor and Group as between-subjects factor resulted in an effect of Group [F(24,1) = 18.86, P < 0.001], an effect of Hemisphere [F(24,1) = 140.24, P < 0.001, Greenhouse-Geisser corrected], and a Group ×Hemisphere interaction [F(24,1) = 70.22, P < 0.001]. Left hemisphere MEP amplitudes were significantly larger in fluent speakers (1.67 mV, SD 0.25 mV) compared to adults who stutter [1.12 mV, SD 0.19 mV; t(24) = 6.629, P < 0.001]. Right hemisphere MEP amplitudes were not different between groups (fluent speakers 1.06 mV, SD 0.17 mV; adults who stutter 1.0 mV, SD 0.18 mV). The dramatic effect is in line with our interpretation and as it occurs in the raw MEP amplitudes, it excludes the possibility that the normalization created the effect of group.
However, for the sake of our study question we decided to normalize MEP amplitudes of the transition phase to the state of cortical excitability during prolongation. MEPs are variable in size and shape even when stimulation intensity and the coil positioning are kept constant (Rösler et al., 2008). This variability is particularly pronounced when recording from orofacial muscles (Devlin and Watkins, 2007). The neurogenic origin of this variability lies partly in the fluctuating number of excited motor neurons as a result of variation of facilitation by voluntary contraction or cognitive events. Speaking integrates both voluntary contraction of orofacial muscles and cognitive control. During prolongation of the introductory syllable ‘auf’ the orofacial cortical neurons descend into a reference state of excitability, an appropriate reference to normalize MEP amplitudes during the transition phase.
Conclusion and outlook
Our data integrate structural and neurophysiological findings into a plausible model of speech pathophysiology in persistent developmental stuttering. Moreover, our results support a theoretical framework of speech motor control, namely the GODIVA model (Bohland et al., 2010). With regard to this model, our data suggest that stuttering is associated with the inadequate activation of sensorimotor plans in the left orofacial motor cortex. Because it is postulated that cortico-cortical connections from BA44/BA6 and the supplementary motor area provide the segmental and suprasegmental plan, one could speculate that a facilitation of those regions, for instance with non-invasive neurostimulation, would facilitate the stable activation of the sensorimotor plans in the left primary motor cortex. This should be evident in a normalization of the motor cortex excitability in the transition phase between articulatory gestures.
Stuttering was not overtly present in the vast majority of our trials. Our finding therefore indicates an abnormal motor preparation in adults who stutter, even in mostly fluent parts of speech. This points to a chronic aberration of the physiology not permanently accompanied by clinical symptoms (Fox et al., 1996). Our method allows probing the functional integrity of speech production networks in a performance independent manner.
Our data do not allow conclusions with regard to subcortical pathological mechanism of stuttering. This is problematic because a large body of literature emphasizes subcortical irregularities (Wu et al., 1997; Alm, 2004; Brown et al., 2005; Giraud et al., 2008; Chang et al., 2011; Ingham et al., 2012). A combination of a virtual lesion approach with our new method would allow one to indirectly prove the impact of the timed initiation of speech gestures and thus the functionality of the cortico-striato-thalamo-cortical loop. Inhibition of the pre-supplementary motor area as part of this loop would hinder the balance of input from the thalamus to the bilaterally organized motor output cells. If this balance played a role in the planning phase, one would observe a decrease of facilitation in the left primary motor cortex of fluent speakers. Likewise, a facilitation of this loop would normalize the left motor cortex excitability in adults who stutter. It will be important to test these hypotheses in future experiments.
Acknowledgements
We are grateful for the assistance of Alexander Wolff von Gudenberg for helping us to recruit adults who stutter. We thank Géza Ambrus for technical assistance, and Michael Nitsche and Melanie Wilke for discussions. We would also like to thank the referees and editors for engaged reading and comments and Steven Brown and Elizabeth Kelly for improvement in grammar and spelling. Part of this work was previously presented at the 6th International Conference on Speech Motor Control, June 8–11, 2011, Groningen, Nijmegen, Netherlands; at the International Seminar on Speech Production, June 20–23, 2011, Montreal, Canada; and at a Nanosymposium at the Annual Meeting of the Society for Neuroscience, October 13–17, 2012, New Orleans, USA.
Glossary
Abbreviations
- BA
Brodmann area
- MEP
motor evoked potential
- TMS
transcranial magnetic stimulation
Funding
This work was funded by the Dorothea Schlözer Fellowship Programme of the University of Goettingen (to N.E.N.), by the Deutsche Forschungsgemeinschaft (NE 1841/1-1 to N.E.N; and SO 429/2-2; SO 429/4-1 to M.S.), by the Rose Foundation (W.P.), and by Bernstein Focus Neurotechnology (01GQ0810 to A.N.).
Supplementary material
Supplementary material is available at Brain online.
References
- Alm PA. Stuttering and the basal ganglia circuits: a critical review of possible relations. J Commun Disord. 2004;37:325–69. doi: 10.1016/j.jcomdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Alm PA, Karlsson R, Sundberg M, Axelson HW. Hemispheric lateralization of motor thresholds in relation to stuttering. PLoS One. 2013;8:e76824. doi: 10.1371/journal.pone.0076824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodstein O, Ratner NB. A handbook on stuttering. 6th edn. Clifton Park, NY: Delmar Learning; 2008. [Google Scholar]
- Bohland JW, Bullock D, Guenther FH. Neural representations and mechanisms for the performance of simple speech sequences. J Cogn Neurosci. 2010;22:1504–29. doi: 10.1162/jocn.2009.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: an ALE meta-analysis of functional neuroimaging studies. Hum Brain Mapp. 2005;25:105–17. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busan P, D’Ausilio A, Borelli M, Monti F, Pelamatti G, Pizzolato G, et al. Motor excitability evaluation in developmental stuttering: a transcranial magnetic stimulation study. Cortex. 2013;49:781–92. doi: 10.1016/j.cortex.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Cai S, Tourville JA, Beal DS, Perkell JS, Guenther FH, Ghosh SS. Diffusion imaging of cerebral white matter in persons who stutter: evidence for network-level anomalies. Front Hum Neurosci. 2014;8:54. doi: 10.3389/fnhum.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage. 2008;39:1333. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Horwitz B, Ostuni J, Reynolds R, Ludlow CL. Evidence of Left Inferior frontal–premotor structural and functional connectivity deficits in adults who stutter. Cereb Cortex. 2011;21:2507–18. doi: 10.1093/cercor/bhr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Zhu DC. Neural network connectivity differences in children who stutter. Brain. 2013;136:3709–26. doi: 10.1093/brain/awt275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–14. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Civier O, Bullock D, Max L, Guenther FH. Computational modeling of stuttering caused by impairments in a basal ganglia thalamo-cortical circuit involved in syllable selection and initiation. Brain Lang. 2013;126:263–78. doi: 10.1016/j.bandl.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski MD, Fox PT, Ingham RJ, Ingham JC, Robin DA. A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: a potential role for impaired myelination. Neuroimage. 2010;52:1495–504. doi: 10.1016/j.neuroimage.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Watkins KE. Stimulating language: insights from TMS. Brain. 2007;130:610–22. doi: 10.1093/brain/awl331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler HA, Gudenberg AW von, Jung K, Neumann K. Computergestützte therapie bei redeflussstörungen: die langfristige wirksamkeit der kasseler stottertherapie. Sprache Stimme Gehör. 2009;33:193–7. [Google Scholar]
- Fisher SE. Genetic susceptibility to stuttering. N Engl J Med. 2010;362:750–2. doi: 10.1056/NEJMe0912594. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–96. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C, et al. A PET study of the neural systems of stuttering. Nature. 1996;382:158–62. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- Giraud A-L, Neumann K, Bachoud-Levi A-C, von Gudenberg AW, Euler HA, Lanfermann H, et al. Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain Lang. 2008;104:190–9. doi: 10.1016/j.bandl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Grabski K, Lamalle L, Vilain C, Schwartz J-L, Vallée N, Tropres I, et al. Functional MRI assessment of orofacial articulators: Neural correlates of lip, jaw, larynx, and tongue movements. Hum Brain Mapp. 2012;33:2306–21. doi: 10.1002/hbm.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee JDW, Oya H, Kawasaki H, Volkov IO, Kaufman OP, Kovach C, et al. A functional connection between inferior frontal gyrus and orofacial motor cortex in human. J Neurophysiol. 2004;92:1153–64. doi: 10.1152/jn.00609.2003. [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Grafton ST, Bothe AK, Ingham JC. Brain activity in adults who stutter: similarities across speaking tasks and correlations with stuttering frequency and speaking rate. Brain Lang. 2012;122:11–24. doi: 10.1016/j.bandl.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota H, Hirashima M, Nozaki D. Functional modulation of corticospinal excitability with adaptation of wrist movements to novel dynamical environments. J Neurosci. 2014;34:12415–24. doi: 10.1523/JNEUROSCI.2565-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalogr Clin Neurophysiol. 1996;101:478–82. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Kell CA, Neumann K, Kriegstein K von, Posenenske C, Gudenberg AW von, Euler H, et al. How the brain repairs stuttering. Brain. 2009;132:2747–60. doi: 10.1093/brain/awp185. [DOI] [PubMed] [Google Scholar]
- Korchounov A, Ilić TV, Ziemann U. TMS-assisted neurophysiological profiling of the dopamine receptor agonist cabergoline in human motor cortex. J Neural Transm Vienna Austria 1996. 2007;114:223–9. doi: 10.1007/s00702-006-0523-5. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum JP, Bohning D, et al. How coil–cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci. 2000;12:376–84. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–66. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Lissek S, Vallana GS, Schlaffke L, Lenz M, Dinse HR, Tegenthoff M. Opposing effects of dopamine antagonism in a motor sequence task—tiapride increases cortical excitability and impairs motor learning. Front Behav Neurosci. 2014;8:201. doi: 10.3389/fnbeh.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL, Loucks T. Stuttering: a dynamic motor control disorder. J Fluen Disord. 2003;28:273–95. doi: 10.1016/j.jfludis.2003.07.001. quiz 295. [DOI] [PubMed] [Google Scholar]
- Maguire GA, Yu BP, Franklin DL, Riley GD. Alleviating stuttering with pharmacological interventions. Expert Opin Pharmacother. 2004;5:1565–71. doi: 10.1517/14656566.5.7.1565. [DOI] [PubMed] [Google Scholar]
- Michelet T, Duncan GH, Cisek P. Response competition in the primary motor cortex: corticospinal excitability reflects response replacement during simple decisions. J Neurophysiol. 2010;104:119–27. doi: 10.1152/jn.00819.2009. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Boroojerdi B, Ziemann U, Hallett M. Analogous corticocortical inhibition and facilitation in ipsilateral and contralateral human motor cortex representations of the tongue. J Clin Neurophysiol. 2001;18:550–8. doi: 10.1097/00004691-200111000-00005. [DOI] [PubMed] [Google Scholar]
- Neef NE, Jung K, Rothkegel H, Pollok B, von Gudenberg AW, Paulus W, et al. Right-shift for non-speech motor processing in adults who stutter. Cortex. 2011a;47:945–54. doi: 10.1016/j.cortex.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Neef NE, Paulus W, Neef A, von Gudenberg AW, Sommer M. Reduced intracortical inhibition and facilitation in the primary motor tongue representation of adults who stutter. Clin Neurophysiol. 2011b;122:1802–11. doi: 10.1016/j.clinph.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Nil LFD, Beal DS, Lafaille SJ, Kroll RM, Crawley AP, Gracco VL. The effects of simulated stuttering and prolonged speech on the neural activation patterns of stuttering and nonstuttering adults. Brain Lang. 2008;107:114–23. doi: 10.1016/j.bandl.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Monte-Silva K, Kuo M-F, Paulus W. Dopaminergic impact on cortical excitability in humans. Rev Neurosci. 2010;21:289–98. doi: 10.1515/revneuro.2010.21.4.289. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peeva MG, Guenther FH, Tourville JA, Nieto-Castanon A, Anton J-L, Nazarian B, et al. Distinct representations of phonemes, syllables, and supra-syllabic sequences in the speech production network. Neuroimage. 2010;50:626–38. doi: 10.1016/j.neuroimage.2009.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel RMW, Laskawi R, Markus H. Tongue representation in the lateral cortical motor region of the human brain as assessed by transcranial magnetic stimulation. Ann Otol Rhinol Laryngol. 2003;112:71–6. doi: 10.1177/000348940311200114. [DOI] [PubMed] [Google Scholar]
- Rösler KM, Roth DM, Magistris MR. Trial-to-trial size variability of motor-evoked potentials. A study using the triple stimulation technique. Exp Brain Res. 2008;187:51–9. doi: 10.1007/s00221-008-1278-z. [DOI] [PubMed] [Google Scholar]
- Ruge D, Muggleton N, Hoad D, Caronni A, Rothwell JC. An unavoidable modulation? Sensory attention and human primary motor cortex excitability. Eur J Neurosci. 2014;40:2850–8. doi: 10.1111/ejn.12651. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Freund H-J. Single word reading in developmental stutterers and fluent speakers. Brain. 2000;123:1184–202. doi: 10.1093/brain/123.6.1184. [DOI] [PubMed] [Google Scholar]
- Salvador R, Silva S, Basser PJ, Miranda PC. Determining which mechanisms lead to activation in the motor cortex: a modeling study of transcranial magnetic stimulation using realistic stimulus waveforms and sulcal geometry. Clin Neurophysiol. 2011;122:748–58. doi: 10.1016/j.clinph.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits-Bandstra S, De Nil L. Speech skill learning of persons who stutter and fluent speakers under single and dual task conditions. Clin Linguist Phon. 2009;23:38–57. doi: 10.1080/02699200802394914. [DOI] [PubMed] [Google Scholar]
- Smits-Bandstra S, De Nil LF, Saint-Cyr JA. Speech and nonspeech sequence skill learning in adults who stutter. J Fluen Disord. 2006;31:116–36. doi: 10.1016/j.jfludis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Sommer M, Knappmeyer K, Hunter EJ, Gudenberg AW, Neef N, Paulus W. Normal interhemispheric inhibition in persistent developmental stuttering. Mov Disord. 2009;24:769–73. doi: 10.1002/mds.22383. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Büchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360:380–3. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Coxon JP, Byblow WD. Primary motor cortex and movement prevention: where Stop meets Go. Neurosci Biobehav Rev. 2009;33:662–73. doi: 10.1016/j.neubiorev.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Svensson P, Romaniello A, Wang K, Arendt-Nielsen L, Sessle BJ. One hour of tongue-task training is associated with plasticity in corticomotor control of the human tongue musculature. Exp Brain Res. 2006;173:165–73. doi: 10.1007/s00221-006-0380-3. [DOI] [PubMed] [Google Scholar]
- Terumitsu M, Fujii Y, Suzuki K, Kwee IL, Nakada T. Human primary motor cortex shows hemispheric specialization for speech. Neuroreport. 2006;17:1091–5. doi: 10.1097/01.wnr.0000224778.97399.c4. [DOI] [PubMed] [Google Scholar]
- Wang CZH, Herbst JA, Keller GB, Hahnloser RHR. Rapid interhemispheric switching during vocal production in a songbird. PLoS Biol. 2008;6:e250. doi: 10.1371/journal.pbio.0060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131:50–9. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Maguire G, Riley G, Lee A, Keator D, Tang C, et al. Increased dopamine activity associated with stuttering. Neuroreport. 1997;8:767–70. doi: 10.1097/00001756-199702100-00037. [DOI] [PubMed] [Google Scholar]
- Xivry J-JO de, Ahmadi-Pajouh MA, Harran MD, Salimpour Y, Shadmehr R. Changes in corticospinal excitability during reach adaptation in force fields. J Neurophysiol. 2013;109:124–36. doi: 10.1152/jn.00785.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yairi E, Ambrose NG. Early childhood stuttering. Austin, TX: Pro-Ed.; 2005. [Google Scholar]
- Yaruss JS. Assessing quality of life in stuttering treatment outcomes research. J Fluen Disord. 2010;35:190–202. doi: 10.1016/j.jfludis.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroencephalogr Clin Neurophysiol. 1997;105:430–7. doi: 10.1016/s0924-980x(97)00050-7. [DOI] [PubMed] [Google Scholar]