Abstract
This scientific commentary refers to ‘In vivo detection of nerve injury in familial amyloid polyneuropathy by magnetic resonance neurography’ by Kolmer et al. (10.1093/brain/awu344).
This scientific commentary refers to ‘In vivo detection of nerve injury in familial amyloid polyneuropathy by magnetic resonance neurography’ by Kollmer et al. (10.1093/brain/awu344).
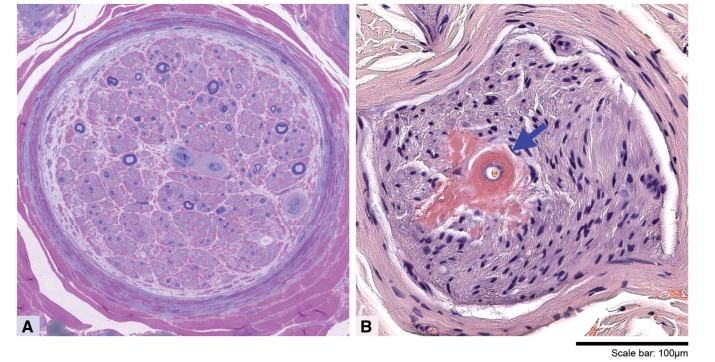
Transthyretin-related familial amyloid polyneuropathy (TTR-FAP) is a severe adult-onset autosomal dominant peripheral neuropathy with a median survival of less than 10 years from symptom onset (Planté-Bordeneuve et al., 1998). Mutations in the TTR gene facilitate the dissociation of the stable tetrameric TTR protein into pro-amyloidogenic monomers. These misfold, aggregate and are deposited as TTR amyloid fibrils within the endoneurium—the layer of connective tissue surrounding the myelin sheath (Fig. 1)—and other tissues. Common clinical features of TTR-FAP include a peripheral neuropathy (most commonly presenting as a small fibre length-dependent neuropathy), an autonomic neuropathy and a cardiomyopathy. The estimated overall disease prevalence is 1:1 000 000. However, prevalence varies widely and may be as high as 1:1000 in parts of Portugal, Spain and Japan. Diagnosis can be difficult and is often delayed, particularly in non-endemic areas, as a result of the wide spectrum of clinical presentations and incomplete penetrance (Adams et al., 2014). One of the major ongoing challenges in the disease is to identify early and sensitive biomarkers to allow earlier initiation of therapies and to enable clinical trials in early stage patients, before irreversible axonal neural degeneration has occurred. In this issue of Brain, Kollmer and colleagues address this challenge and show that nerve injury in the lower limbs can be detected, localized and quantified using quantitative MRI in both symptomatic patients and presymptomatic gene carriers (Kollmer et al., 2014).
Figure 1.
Morphological appearances of sural nerve biopsy in a patient with amyloid neuropathy. Semi-thin resin preparation (A) stained with methylene blue azure–basic fuchsin shows a transverse section of a nerve fascicle in which there is a substantial loss of large and small myelinated fibres and no evidence of regeneration. Some of the endoneurial blood vessels with concentrically markedly thickened hyalinized walls are positively stained with Congo red (B, arrow). Congo red-positive amyloid deposits are also seen freely in the endoneurium in the vicinity of the affected blood vessel. Scale bar: A and B = 100 μm.
The longest established treatment in TTR-FAP is liver transplantation, with a 5-year survival of 82% in patients with the most common Val30Met mutation; however, 5-year survival in patients with other mutations is only 59% (Wilczek et al., 2011). Transplantation is also limited by organ availability and patient suitability. Safer, more efficacious therapies are clearly needed. Tafamidis and diflunisal, small molecules that stabilize TTR tetramers and reduce amyloid formation, are recently introduced therapies that have been assessed in randomized double-blind placebo-controlled clinical trials (Coelho et al., 2012; Berk et al., 2013). The tafamidis trial enrolled 125 patients and was of 18 months’ duration. Although the comparison of tafamidis and placebo did not reach significance on the primary endpoints in an intention-to-treat analysis, benefit was seen in the 87 efficacy-evaluable patients and for most secondary endpoints (Coelho et al., 2012). The diflunisal trial of 130 patients treated for 2 years showed treatment benefit for the primary endpoint: change in a modified Neuropathy Impairment Score. Emerging therapies for TTR-FAP include TTR gene silencing strategies with either antisense oligonucleotides or small interfering RNAs (Adams et al., 2014). Experience from liver transplantation and observations from the trials of the newer therapies provide important insights into optimal treatment timing. Patients do best from liver transplantation when it is performed early in the disease course. Tafamidis and diflunisal appear to slow progression rather than reverse existing disability (Coelho et al., 2012; Berk et al., 2013). Both of these observations emphasize that treatment ideally should be instigated in early-stage disease, or even at a pre-clinical stage. Identifying sensitive biomarkers of early disease will become increasingly important as newer therapies safer than liver transplantation are introduced. It is likely that earlier treatment will still give the best results as treating an established neuropathy will always be challenging, especially when other organs such as the heart are involved. Ideally, biomarkers will be identified that are not only useful for early disease detection, but are responsive to change in natural history studies. Such biomarkers will thus be suitable for use both in clinical trials and for establishing the optimal timing of a therapy in an individual patient.
Kollmer and colleagues investigated the potential use of magnetic resonance neurography biomarkers in TTR-FAP (Kollmer et al., 2014). MRI has an established role in the diagnosis of focal nerve lesions and inflammatory neuropathies such as chronic inflammatory demyelinating polyradiculoneuropathy, where nerve root enlargement and/or gadolinium enhancement are listed as supportive criteria for diagnosis (Hughes et al., 2006). Diagnostic judgements are generally qualitative, while quantitative assessments using standardized magnetic resonance protocols are necessary to provide potential disease biomarkers. MRI allows the quantification of both anatomical parameters such as nerve size, and tissue magnetic resonance contrast parameters such as the T2 intensity of the nerve tissue. The latter are inferred to reflect changes in the microstructural tissue integrity. In this study by Kollmer and colleagues, lower limb MRI was performed at 3 T in 13 symptomatic TTR-FAP patients, seven presymptomatic gene carriers and 40 healthy controls. Anatomically, nerve cross-sectional area was increased in both symptomatic and presymptomatic patients compared with controls at thigh level, but not at calf level. The difference between symptomatic and presymptomatic patients at thigh level was significant for the tibial fascicles, but did not quite achieve statistical significance for the peroneal fascicles of the sciatic nerve. This demonstrates that increased nerve size is predominantly proximal and also suggests that it occurs early in the disease process. Nerve size may therefore possibly be useful as an early marker of disease penetrance and may be less responsive as a biomarker of disease progression. In terms of nerve tissue magnetic resonance contrast parameters, the main analysis was based on defining ‘lesion voxels’ as all those with T2 signal intensity >20% greater than the average (mode) signal intensity of healthy controls. By this method the total lesion-voxel number was highest in symptomatic patients, intermediate in asymptomatic gene carriers and lowest in controls. Similar to the anatomical data, there was greater lesion burden in the thigh than the calf, even when corrected for differences in nerve size.
Kollmer and co-workers probed the nature of these tissue magnetic resonance contrast abnormalities further within the distal thigh block by estimating the underlying parameters which determine T2 signal intensity: relative proton spin density and T2 relaxation time. Relative proton spin density relates to the density of water hydrogen atoms within the tissue, and absolute proton-density weighted signal intensity was increased compared with controls in both presymptomatic gene carriers and symptomatic patients. Kollmer et al. suggest that this might be due to the endoneurial deposition of TTR amyloid occurring early in pathogenesis. On the other hand they observed an increase in T2 relaxation time in symptomatic patients only, with no difference between presymptomatic gene carriers and controls. They hypothesized that this reflects endoneurial oedema, occurring at a later stage in disease pathogenesis.
Taken together with available histopathological data in this and previous studies, and notwithstanding the limitations of cross-sectional data, the authors suggest a potential schema for anatomical and pathological disease progression. In the presymptomatic stage, endoneurial amyloid deposition occurs in proximal nerves resulting in increased nerve size and increased proton spin density. In the early symptomatic stage, endoneurial oedema occurs with prolongation of T2 relaxation time detected. This proximal pathology results in secondary axonal degeneration in distal nerves (Fig. 1A). In late stage disease, the pathology spreads to involve more distal nerves, at which point sural nerve biopsy may demonstrate amyloid deposition (Fig. 1B).
This paper represents an exciting development in TTR-FAP as it identifies the earliest biomarker of neuropathy to date and also provides interesting insights into disease pathogenesis. To translate this work into clinical practice, further studies are needed. First, the methods used here are time- and resource-intensive. Over 30 000 regions of interest were drawn by hand prior to analysis, and a large number of healthy controls were studied to allow normalization of signal intensities. Second, the data are presented as group analyses rather than to classify individual patients as ‘normal’ or ‘abnormal’. This would be necessary if these MRI techniques are to be used to distinguish mutation carriers who are presymptomatic and therefore would benefit from intervention, from those who are non-penetrant and would presumably receive no such benefit. Finally, as the authors acknowledge, longitudinal natural history studies are now needed to determine the usefulness of these biomarkers in identifying the optimal timing of a therapy in an individual patient, and the responsiveness of the biomarkers as outcome measures, a key determinant of study power. Note should also be made of the increasing volume of research using MRI to quantify muscle changes secondary to neuropathic processes (Sinclair et al., 2012), which is also a potential source of responsive biomarkers in this disease group.
TTR-related FAP is a devastating disease for which there are now a number of new and emerging therapies. As these treatments slow progression rather than reverse established damage, biomarkers of early stage and subclinical disease, before nerve conduction studies are abnormal, are urgently needed. Quantitative magnetic resonance neurography as demonstrated in the paper by Kollmer and colleagues not only provides intriguing insights into the anatomical and pathological progression of this devastating disease, but also shows promise as an early marker of the neuropathy that is the first manifestation of the disease in most patients.
Acknowledgement
The authors wish to thank Dr Zane Jaunmuktane for preparing the figure.
References
- Adams D, Théaudin M, Cauquil C, Algalarrondo V, Slama M. FAP neuropathy and emerging treatments. Curr Neurol Neurosci Rep. 2014;14:435. doi: 10.1007/s11910-013-0435-3. [DOI] [PubMed] [Google Scholar]
- Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–67. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho T, Maia LF, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79:785–92. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RAC, Bouche P, Cornblath DR, et al. European federation of neurological societies/peripheral nerve society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol Off J Eur Fed Neurol Soc. 2006;13:326. doi: 10.1111/j.1468-1331.2006.01278.x. [DOI] [PubMed] [Google Scholar]
- Kollmer J, Pham M. In-vivo detection of nerve injury in familial amyloid polyneuropathy by magnetic resonance-neurography. Brain. 2015;138:549–62. doi: 10.1093/brain/awu344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planté-Bordeneuve V, Lalu T, Misrahi M, et al. Genotypic-phenotypic variations in a series of 65 patients with familial amyloid polyneuropathy. Neurology. 1998;51:708–14. doi: 10.1212/wnl.51.3.708. [DOI] [PubMed] [Google Scholar]
- Sinclair CDJ, Morrow JM, Miranda MA, et al. Skeletal muscle MRI magnetisation transfer ratio reflects clinical severity in peripheral neuropathies. J Neurol Neurosurg Psychiatry. 2012;83:29–32. doi: 10.1136/jnnp.2011.246116. [DOI] [PubMed] [Google Scholar]
- Wilczek HE, Larsson M, Ericzon B-G FAPWTR. Long-term data from the Familial Amyloidotic Polyneuropathy World Transplant Registry (FAPWTR) Amyloid Int J Exp Clin Investig Off J Int Soc Amyloidosis. 2011;18(Suppl 1):193–5. doi: 10.3109/13506129.2011.574354072. [DOI] [PubMed] [Google Scholar]