Figure 2.
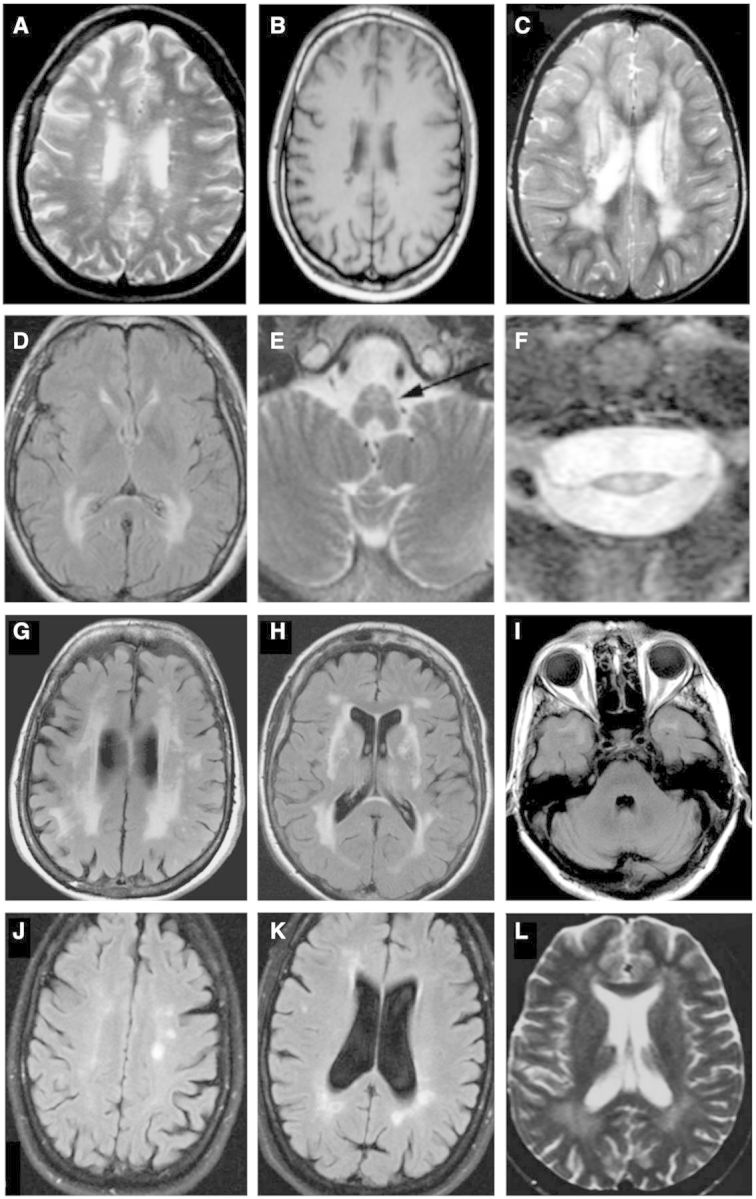
Selected magnetic resonance images from miscellaneous hereditary conditions mimicking multiple sclerosis. (A) Axial FLAIR image of a 36-year-old female with spastic paraparesis and painless visual loss, known to harbour the m.11778G>A mtDNA mutation, diagnostic of LHON-plus, also known as Harding’s disease. Numerous foci of hyperintensity of the periventricular and deep white matter strongly resemble appearances seen in multiple sclerosis (reproduced with permission, Küker et al., 2007). Four years after presentation, this patient developed bilateral optic atrophy, ataxia, and internuclear ophthalmoplegia. (B) Axial T1-weighted image of another patient with LHON showing white matter lesions with hypointense signal, indistinguishable from multiple sclerosis (reproduced with permission, Inglese et al., 2001). (C) Axial T2-weighted image demonstrating more confluent T2 hyperintense white matter disease in a third LHON patient (reproduced with permission, Lerman-Sagie et al., 2005). (D–F) Axial FLAIR and T2-weighted imaging through the brain (D), brainstem (E) and cervical cord (F) in three individual patients with adult-onset Alexander disease. The female patient in (D) presented at 43 years of age with bulbar weakness; patients (E) and (F) presented with lower extremity weakness. The images demonstrate confluent posterior predominant T2 hyperintensity in the periventricular and deep white matter (D) together with atrophy of medullary pyramids (arrow in E) and marked thinning and T2 hyperintensity in the cervical cord (F) (reproduced with permission, Pareyson et al., 2008). (G-I) Axial FLAIR imaging in a patient with CADASIL. Patchy and confluent hyperintensity in the periventricular white matter (G) with involvement of the external capsules (H) and subcortical white matter of the temporal poles (I) are characteristic. (J and K) Axial FLAIR imaging of a 49-year-old female patient with PLP1 disease (hereditary spastic paraplegia, SPG2 type) presenting with a phenotype mimicking primary progressive multiple sclerosis. She had a 10-year history of progressive spastic paraplegia, dysarthria, and ataxia. Her son had died in childhood of a previously undiagnosed severe neurodevelopmental disorder. Multifocal T2 hyperintensities are seen in the periventricular and deep white matter near the atria and occipital horns of the ventricles, together with and other scattered foci of T2 signal in the white matter, mimicking multiple sclerosis (reproduced with permission, Warshawsky et al., 2005). She had abnormal visual-evoked potentials bilaterally and CSF was positive for unmatched oligoclonal bands. (L) Axial T2-weighted image in a 22-year-old female with hereditary spastic paraplegia, SPG11 type, presenting with subacute gait disturbance, urge incontinence, and mild, subjective cognitive disturbance. Confluent T2 hyperintensity is seen in the posterior white matter together with small, scattered foci elsewhere in the brain (reproduced with permission, Romagnolo et al., 2014).
