Foot drop and toe walking are common in cerebral palsy. Willerslev-Olsen et al. report that four weeks of daily training on a treadmill with an incline boosts corticospinal transmission to ankle dorsiflexors and improves gait in affected children. Improvements are greatest in those aged under 10 years.
Keywords: coherence, development, gait
Abstract
Foot drop and toe walking are frequent concerns in children with cerebral palsy. The main underlying cause of these problems is early damage and lack of maturation of the corticospinal tract. In the present study we investigated whether 4 weeks of daily treadmill training with an incline may facilitate corticospinal transmission and improve the control of the ankle joint in children with cerebral palsy. Sixteen children with cerebral palsy (Gross Motor Classification System I:6, II:6, III:4) aged 5–14 years old, were recruited for the study. Evaluation of gait ability and intramuscular coherence was made twice before and twice after training with an interval of 1 month. Gait kinematics were recorded by 3D video analysis during treadmill walking with a velocity chosen by the child at the first evaluation. Foot pressure was measured by force sensitive foot soles during treadmill and over ground walking. EMG-EMG coherence was calculated from two separate electrode recordings placed over the tibialis anterior muscle. Training involved 30 min of walking daily on a treadmill with an incline for 30 days. Gait training was accompanied by significant increases in gait speed, incline on the treadmill, the maximal voluntary dorsiflexion torque, the number and amplitude of toe lifts late in the swing phase during gait and the weight exerted on the heel during the early stance phase of the gait cycle. EMG-EMG coherence in the beta and gamma frequency bands recorded from tibialis anterior muscle increased significantly when compared to coherence before training. The largest changes in coherence with training were observed for children <10 years of age. Importantly, in contrast to training-induced EMG increases, the increase in coherence was maintained at the follow-up measurement 1 month after training. Changes in the strength of coherence in the beta and gamma band were positively correlated with improvements in the subjects’ ability to lift the toes in the swing phase. These data show that daily intensive gait training increases beta and gamma oscillatory drive to ankle dorsiflexor motor neurons and that it improves toe lift and heel strike in children with cerebral palsy. We propose that intensive gait training may produce plastic changes in the corticospinal tract, which are responsible for improvements in gait function.
Introduction
Foot drop and toe walking are among the most frequent clinical problems in children with cerebral palsy (Fowler et al., 2010). The main underlying cause of these problems is reduced force in the ankle dorsiflexor muscles secondary to a lesion of the motor cortex or the corticospinal tract (Bland et al., 2011; Moreau et al., 2012; Petersen et al., 2013; Willerslev-Olsen et al., 2014a). In healthy children, improved control of the ankle dorsiflexors in the swing phase of walking parallels maturation of the corticospinal tract across the age range 4–15 years (Hodapp et al., 2007a; Petersen et al., 2010, 2013). In children with congenital hemiplegic cerebral palsy and foot drop this maturational process is observed to a lesser extent on the side most affected by the CNS lesion (Hodapp et al., 2007b; Petersen et al., 2013).
Coherence analysis between the firing of pairs of single motor units shows peaks in both the beta and gamma frequency ranges. This common drive has been shown to be closely related to motor unit synchronization and both coherence and synchrony have been shown to be dependent on intact central motor pathways including the corticospinal tract (Kirkwood et al., 1984; Farmer et al., 1990, 1993a, b; Datta et al., 1991). Beta and gamma frequency drive to the motor neuron pool may also be accessed through the surface EMG in studies of cortico-muscular coherence (Conway et al., 1995; Salenius et al., 1996; Mima and Hallett, 1999) and through the recording of surface EMG within and between muscles (Halliday et al., 2003; Norton and Gorassini, 2006; Farmer et al., 2007). The oscillatory drive detected through coherence analysis of EMG provides a means of measuring and detecting changes in the output of the motor cortex and its transmission to the spinal cord through the corticospinal tract during functional muscle activation without the need for external neural stimulation. The central drive to the motor neuron pool can be influenced by training and exercise: visuo-motor skill training involving the ankle joint, produces increased EMG-EMG coherence within the tibialis anterior muscle, which relates to the acquisition of the motor task (Perez et al., 2006). EMG-EMG coherence between muscles has also been shown to increase following treadmill training in spinal cord injured subjects (Norton and Gorassini, 2006).
In a previous study we showed that in children with hemiplegic cerebral palsy there is impairment of tibialis anterior EMG-EMG coherence and lack of age-related increase of coherence in the beta and gamma frequency bands and that this diminished coherence relates to impaired control of the ankle dorsiflexor muscles (Petersen et al., 2013). In the present paper we explore the hypothesis that gait training will increase tibialis anterior muscle EMG-EMG coherence and that any change produced by training will be maintained over time and relate to functional measures of lower limb control. The study is a continuation of the study by Willerslev-Olsen et al. (2014b), which investigated the effect of treadmill training on passive ankle muscle stiffness and the kinematics of gait. Similar to that study, we focus on treadmill training with a gradient, which aims to improve the subject’s ability to lift the toes in the end of the swing phase and to place the heel appropriately on the ground in the early stance phase of gait. Walking uphill has been shown to require increased activation of the ankle dorsiflexors when compared to level walking (Leroux et al., 1999; Franz and Kram, 2012; Stern and Gottschall, 2012). Furthermore, the inclination of the treadmill forces subjects to walk with a greater degree of dorsiflexion throughout the stance phase (Leroux et al., 1999; Franz and Kram, 2012). We therefore hypothesized that gait training under these conditions will lead to a progressive and lasting changes in the central motor drive to tibialis anterior motor neurons and will lead to an improvement in the control of ankle joint dorsiflexion during gait.
In this study the children were required to walk on a treadmill with a progressive incline for a period of at least 30 min every day for 1 month. We report that this training effectively increased the ability of the children to lift the toes towards the end of the swing phase and facilitated placement of the heel on the ground. This was accompanied by an initial increase in EMG levels and an initial but also a sustained increase in tibialis anterior EMG-EMG coherence signifying exercise induced changes in the central drive to the tibialis anterior motor neurons.
Materials and methods
Participants
Sixteen children with cerebral palsy were recruited through the Danish Cerebral Palsy Organization (Spastikerforeningen) (average age 9.6 years, range 5–14; 11 males, five females) (Supplementary Table 1). Twelve children had hemiplegic cerebral palsy (eight were primarily affected on the right and four were primarily affected on the left side) and four children had diplegic cerebral palsy (Supplementary Table 1). All children showed impaired gait function with some degree of foot drop and/or toe walking. The study was approved by the local ethics committee (H-B-2009-017) and all procedures were conducted within the standards of the Declaration of Helsinki. Before the study parents and children received written and verbal information, and consent for participation was obtained. Data from these children were also included in the study by Willerslev-Olsen et al. (2014b) in which the effect of gait training on passive muscle stiffness was reported.
The severity of cerebral palsy was classified according to the Gross Motor Classification System (GMFCS; Palisano et al., 1997). The clinical characteristics of the children are summarized in Supplementary Table 1.
Coherence data were also obtained from the tibialis anterior muscle EMGs of 14 healthy age-matched children during walking (average age 10.2 years, range 5–16 years). These children were only tested once and were not asked to perform gait training.
Experimental set-up
Each child with cerebral palsy was involved in the study for a period of 3 months. Gait function, EMG recordings and neurological status were evaluated during four separate test sessions at intervals of approximately 1 month. All test sessions took place at the Helene Elsass Centre (www.elsasscenter.dk; Charlottenlund, Denmark). The first testing session was carried out approximately 1 month before gait training commenced and the second testing session was performed the day before the initiation of gait training. The last two testing sessions took place on the last day of gait training and 1 month after the cessation of gait training.
We used this design so that the children with cerebral palsy could act as their own controls rather than have a separate group of children undergoing no intervention. This approach controls for inter-subject variability, which inevitably complicates the comparison of data from different subjects, especially given the diversity of clinical presentation and functional impairment in children with cerebral palsy. This design also allowed us to determine whether any effect of the intervention could be explained by simple test–retest variability and whether significant changes would be observed in the measured parameters during a control period similar to that of the intervention period.
Measurements were obtained also from a group of healthy children to confirm a lower coherence in children with cerebral palsy as compared to healthy peers (Petersen et al., 2013).
The last testing session 1 month after the end of the intervention was included so that we could ascertain whether there were any sustained training-induced changes in gait function, EMG parameters and/or an increase in overall daily activity.
All four testing sessions included the same measurements made in the same order on every occasion and at the same time of the day. All test sessions began with a neurological examination followed by a video recording of the child’s gait over flat ground. This was followed by evaluation of the ankle joint stiffness and finally by kinematic analysis of gait.
Neurological examination
All cerebral palsy children were examined in the beginning of each test session by a medical doctor with 20 years of experience in neurological examination (J.B.N.). All tests were performed with the children seated comfortably and relaxed on an examination couch with their legs hanging loosely over the side of the couch. Passive range of movement of the ankle was evaluated initially by moving the foot as slowly as possible and as far possible into plantar flexion and then as slowly as possible and as far as possible into dorsiflexion. The position of the joint was noted at both end positions. During this examination any resistance during the slow movement was also noted. The joint was then positioned in a neutral position and quick stretches were applied in the dorsiflexion direction to evaluate the presence of a spastic catch and clonus. Similar quick stretches were applied with the foot in a more plantar-flexed and dorsi-flexed position. Based on this evaluation a score on the modified Ashworth scale was determined. Subsequently, the presence and possible exaggeration of Achilles tendon and patella reflexes was examined using a tendon reflex hammer while gently supporting the foot or limb to enable relaxation. Finally, voluntary muscle force was examined for the following: ankle plantar flexion, ankle dorsi flexion, knee extension, knee flexion and hip flexion. In all of the measurements the examiner resisted the movement with his hand and evaluated the force of contraction on the MRC scale from 1 to 5 (UK Medical Research Council: see Supplementary Table 1).
Gait analysis during treadmill and over ground walking
The gait of the children was analysed by recording leg movements using a 3D motion capture system, by recording the pressure under the foot using pressure sensitive foot soles and by recording EMG activity from the tibialis anterior muscles bilaterally. The children were asked to walk on a treadmill with and without an incline as well as over ground. After 5 min of familiarization, performed at the beginning of the first test session, the children selected their own comfortable walking speed when walking without incline on the treadmill. Walking speed varied between 2 km/h and 4 km/h with a mean of 2.8 ± 0.6 km/h. The same walking speed was used for measurements in all subsequent test sessions for that individual, although the maximal walking speed achieved by the child on the treadmill increased as a result of the training. All children were asked to hold on to the bars of the treadmill even though several of them were able to walk without support. The incline of the treadmill was chosen by the child by slowly increasing the incline while the child was walking. An incline at which the child felt challenged, but still able to walk without major fatigue was chosen for training. The only requirement was that all children should walk with an incline of at least 5%. During training the incline for the different children varied between 5% and 12% with a mean incline of 7.5% ± 2.4. Testing took place without any incline on the treadmill.
The children walked in their own sport shoes without shoe inserts or braces. The same shoes were used in every session. For motion and plantar pressure analysis 2-min data recordings were obtained during level treadmill walking. During this task 2-min recordings were obtained for the purposes of plantar pressure analysis.
3D motion analysis
3D kinematic data were captured using a Qualysis motion capture system (Qualisys) with six, synchronous Oqus 1 cameras operating at a sampling frequency of 200 Hz. Reflective markers (size 12 mm) were placed bilaterally at: (i) the lateral articular line of the knees; (ii) the lateral malleolus; and (iii) the lateral side of the fifth metatarsal.
Plantar pressure measurement
Pressure sensitive soles were placed in the shoes of the child to record the plantar pressure from each foot during walking (F-scan wireless, Tekscan, CA Mätsystem). The pressure sensitive soles were fitted individually for the shoes of each child and reused by that child for each of the four testing sessions. Before each recording the system was calibrated by asking the child to stand relaxed while holding on to the rails of the treadmill with equal weight distribution on the two legs. Data sampling rate was 50 Hz.
EMG recording
EMG activity was recorded by two sets of bipolar electrodes (Ambu Blue sensor N,N-10-A/25. Ambu A/S Ballerup) Recording area 0.5 cm2 interelectrode distance, 2 cm) placed at the most proximal and distal ends of the tibialis anterior muscle, respectively. Recordings were made from both legs, but only data from the most affected leg of the children with cerebral palsy were included in the analysis. The distance between the two sets of EMG electrodes was 6–10 cm. The skin was prepared by first brushing the skin softly with sandpaper (3M red dot; 3M). EMG signals were sampled by a wireless EMG recording system (BTSFreeEMG; BTS Bioengineering Corp.). Data were filtered (band-pass, 10 Hz–400 Hz), sampled at 1000 Hz, and stored on a PC for off-line analysis.
Training protocol
Only children with cerebral palsy underwent gait training. Training sessions were scheduled for 30 min every day for four consecutive weeks at home. A treadmill (ECO ll G6432N) was delivered to the home of all participants several days before initiating gait training. The children were allowed to split up the 30 min of gait training into periods of shorter duration as long as 30 min of total gait training time was achieved by the end of the day.
Speed and incline settings of the treadmill for the initial home training were based on the settings selected at first testing session. As the child’s exercise tolerance improved and the child became more comfortable with the incline and speed of the treadmill the settings could be increased. Parents and children were instructed to increase primarily the incline rather than the speed of the treadmill. The children were also asked to make every effort to place the heel of the foot on the treadmill surface in the early stance phase. The parents were instructed to encourage the child as much as possible both to maintain and achieve progression of the training and to constantly encourage the placement of the heel during the early stance phase. As discussed, the training was focused on treadmill walking with an incline and on facilitating the activation of dorsiflexor muscles (especially tibialis anterior) using a swift but controlled pace without the need to run.
All children and their parents were issued with a diary in order to record the following: (i) the length of time the child had trained each day; (ii) the speed of the treadmill; (iii) the incline of the treadmill; and (iv) what, if any, additional movement activities the child had participated in that day e.g. sport. The parents were asked to report in the diary if illness or if other factors had prevented the child from training on that day. The children were asked to report to their parents how they had felt during the training (i.e. hard work, tired, happy or sweaty) and this was also documented by the parents in the diary.
Off-line data analysis
Signal processing and analysis was performed off line. All 3D motion and plantar pressure data were imported into Matlab (Mathworks) for further analysis. Two measures of foot drop were obtained from the 3D motion analysis: (i) the amplitude of the second toe lift late in the swing phase just prior to heel strike; and (ii) the position of the ankle joint at ground contact. The amplitude of the second toe lift has been shown to provide a functionally important measure of foot drop in patients with spinal cord injury (Barthelemy et al., 2010). This measure was obtained through detection of a peak in the z-axis position of the toe marker of >4 mm within 200 ms before the heel strike. Selected peak displacements were subsequently verified visually. The position of the ankle joint at initial ground contact was determined and compared to the position when the foot was flat on the ground i.e. in early stance within a 150 ms window post ground contact. The difference between the angle at initial contact and the angle in early stance phase with foot flat was taken as an indication of the child’s range of active dorsiflexion.
The plantar pressure data were divided into three recording epochs of equal size: heel, midfoot and forefoot. The peak pressure exerted each of these foot areas was then averaged over all steps for the 2-min data sets. A dynamic index was calculated as: the ratio in per cent of peak pressure in heel area/peak pressure midfoot + peak pressure forefoot. The dynamic index has been shown to provide a valid measure of the quality of heel strike in children with cerebral palsy (Bennett et al., 2007).
Coherence analysis
Frequency domain and statistical analysis
Only data from the most affected leg was used for coherence analysis. Frequency domain analysis of the data was undertaken using the methods set out in detail by Halliday et al. (1995). Briefly, the practice of full wave rectification of normalized surface EMG signals was adopted. This approach has been shown to maximize the information regarding timing of motor unit action potentials (MUAP) while suppressing information regarding MUAP waveform shape (Myers et al., 2003; Halliday and Farmer, 2010; Boonstra and Breakspear 2012; Ward et al., 2013). As a precursor to undertaking population analysis of the data the two rectified tibialis anterior EMG signals were normalized to have unit variance (Halliday and Rosenberg, 2000). Rectified and normalized EMG signals are assumed to be realizations of stationary zero mean time series, denoted by x and y. The results of analysis of individual records generated estimates of the auto-spectra of the two EMGs fxx(λ), fyy(λ), and their cross-spectra fxy (λ). We then estimated three functions that characterize the signals' correlation structure: coherence, |Rxy(λ) |2; phase, φxy(λ); and cumulant density, qxy(u). Coherence estimates are bounded measures of association defined over the range [0, 1]; cumulant density estimates are not bounded, and phase is defined over the range [− π, + π]. For the present data, coherence estimates provide a measure of the fraction of the activity in one surface EMG signal at any given frequency that can be predicted by the activity in the second surface EMG signal. In this way, coherence estimates quantify the strength and range of frequencies of common rhythmic synaptic inputs distributed across the tibialis anterior motor neuron pool. The timing relations between the EMG signals are estimated from the phase. The cumulant density provides an unbounded time-domain representation of the EMG-EMG correlation structure analogous to the motor unit cross-correlogram (Halliday et al., 1995).
Pooled estimates provide a single time or frequency domain measure that describes the correlation structure across a number of normalized data sets (Amjad et al., 1997). Like individual coherence estimates, pooled coherence estimates provide a normative measure of linear association on a scale from 0 to 1 (Halliday and Rosenberg, 2000). Similarly, pooled cumulant density estimates provide a measure of the time-domain correlation across a number of records. Pooled spectra provide a normalized average spectrum for the EMG signals.
The interpretation of pooled estimates of spectrum, phase, coherence and cumulant is similar to those obtained for individual records, except any inferences relate to the population as a whole. Details of pooled coherence analysis and on setting of confidence limits can be found in Amjad et al. (1997). The approach used here to calculate pooled coherence estimates was to pool individual coherency estimates (Halliday and Rosenberg, 2000; Farmer et al., 2007). The individual coherency estimate for record i was denoted as , where this has been calculated from Li segments of data. This coherency function is a complex quantity, the corresponding coherence is its magnitude squared. The pooled coherence across k records, at frequency λ is then:
Estimates of the above pooled coherence provide a single parameter describing the correlation structure, as a function of frequency, within the k records in a single population. This can be considered analogous to single coherence estimate calculated from segments of data.
Inherent within pooled coherence framework is the Chi-Squared extended difference of coherence test with the null hypothesis: no difference in coherence at a given frequency. Like coherence the χ2 test is applied separately at each frequency of interest (Amjad et al., 1997). Changes in the correlation structure between two different subject populations can be ascertained by undertaking a χ2 extended difference of coherence test on the populations to be compared. The resulting χ2 difference test thus provides a metric of the pooled coherence difference at each frequency between the two data populations (Farmer et al., 2007).
Estimates of pooled coherence, pooled spectra, pooled cumulant density functions and pooled phase were used to summarize the EMGs and the EMG-EMG correlation structure in each group of subjects. Estimates of pooled coherence provide a single parameter describing the correlation structure, as a function of frequency, within the records of a single population, where the total number of records to be used equates to the number of subjects for each group.
Statistics
In addition to pooled statistics, the cumulated sum of the logarithmic values of EMG-EMG coherence in the alpha (5–15 Hz) beta (15–30 Hz) and gamma frequency range (30–50 Hz) were collected along with the root mean square EMG magnitude. The root mean square of the EMG signals as well as the amplitude of the peak EMG in the swing phase was averaged over the gait cycles. A window of 400 ms before heel strike was used. Data were tested for normality before analysis using the Shapiro-Wilk test. Data were rank transformed if they were not normally distributed. For the statistical comparison of coherence, root mean square EMG amplitude, maximal voluntary contraction and kinematic measures across the four recording sessions a one-way ANOVA was calculated. Multiple pair wise comparisons were performed using Student-Newman-Keuls test with Bonferroni correction. For correlation analysis we used Pearson product moment correlations. Best subsets regression analysis was used to determine the relationship between coherence, force, EMG and kinematic measurements before and after training. The adjusted R2 correlation value was used in order to take multiple comparisons into account. Subsequent multiple linear regression analysis was used to estimate the relationship between changes in coherence measures in the different frequency bands and changes in the kinematic data with training. All values are given as mean ± 95% confidence intervals (CI). All analyses were performed with Sigmaplot 12.5 (SYSTAT Software) for Windows.
Results
Maximal voluntary contraction
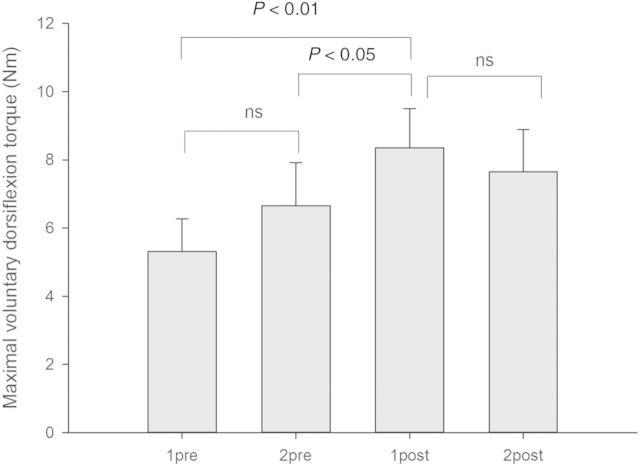
The maximal voluntary dorsiflexion at the ankle increased significantly following the treadmill training when compared to the first pretraining session (one-way repeated measures ANOVA, F = 5.1; Student-Newman-Keuls post hoc test: P < 0.01; Fig. 1) and the second pretraining session (P < 0.05). There were no significant changes between the sessions before training or between the two recording sessions after training (Fig. 1).
Figure 1.
Changes in maximal voluntary dorsiflexion in relation to gait training. The columns show the maximal voluntary dorsi flexion torque at the first (1pre) and second (2pre) sessions prior to training and the first (1post) and second (2post) sessions following training. Statistically significant differences between sessions are indicated by P-values in the figures (ns = no significant difference). Vertical bars are one standard error of the mean. All torque values are in Nm.
Changes in gait ability with training
The children on average walked 25.7 min (range: 14.4–30) daily on the treadmill. By the end of training the children had progressively increased their walking speed from 2.4 km/h [standard deviation (SD): 0.51] to 3.3 km/h (SD: 1.16), corresponding to an increase of 38% and increased the incline on the treadmill from 7.5% (SD: 2.3) to 10% (SD: 2.76), corresponding to an increase of 40%.
The changes in kinematic data following the training in the cerebral palsy group have been reported in Willerslev-Olsen et al. (2014b) and are summarized here. Table 1 shows average measures from the 16 children at the four testing sessions. In the present study the values obtained from each individual child were used for correlation with their tibialis anterior muscle EMG coherence (see below). It can be seen that gait training produced changes in all kinematic parameters: increases in the amplitude of toe lifts, joint range and dynamic index.
Table 1.
Average information about gait ability for the children with cerebral palsy
| Test | Mean amplitude of toe lift late swing (average mm) | Angle difference from gound at heel strike (average degrees) | Dynamic index (heel impulse % of total) |
|---|---|---|---|
| First pretest | 33.83 (SD: 15.27) | 5.47 (SD:3.27) | 28.7 (SD:18.68) |
| Second pretest | 39.85 (SD: 16.19) | 7.23 (SD: 4.10) | 36.38 (SD: 17.38) |
| First post-test | 43.70 (SD: 21.61) | 7.86 (SD: 4.34) | 45.55 (SD: 16.45) |
| Second post-test | 45.58 (SD: 22.38 | 7.49 (SD: 5.53) | 36.78 (SD: 14.55) |
Treadmill training changes in EMG coherence
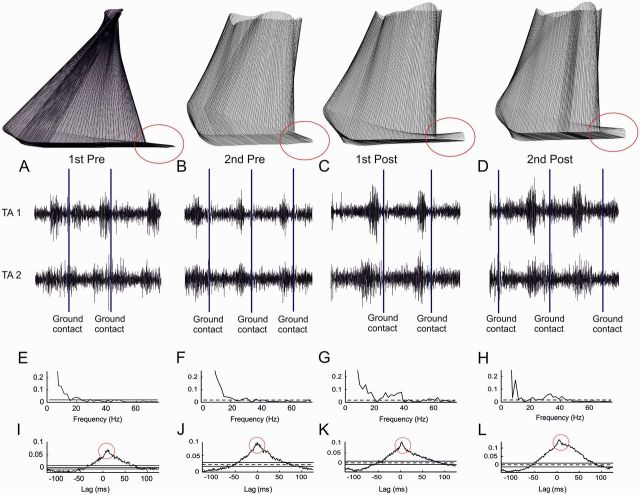
Figure 2 shows an example of kinematics and EMG-EMG coherence and cumulant data from a single child with cerebral palsy (age 10 years). Before treadmill training the child was unable to lift the toes at end of the swing phase and the foot engaged with the floor primarily through exerting pressure through the mid- and forefoot (indicated by red circles in Fig. 2A and B). Following treadmill training a clear toe lift and heel strike can be observed (red circles in Fig. 2C and D).
Figure 2.
Kinematics of gait and coherence estimates from a single child. The stick diagrams in the top row indicate the movement of the affected lower limb during one gait cycle in the typical child with cerebral palsy. Note that the child failed to lift the toes in the end of the swing phase prior to training (1st Pre and 2nd Pre) and made contact with the ground primarily with the mid- and forefoot. Following training, clear lift of the toes was observed (1st Post and 2nd Post). (A–D) Sampled EMG from the two sets of electrodes placed over the tibialis anterior muscle (tibialis anterior 1 and tibialis anterior 2). Ground contact is indicated by vertical solid lines. (E–H) Coherence calculated between the two EMG recordings obtained for the 400 ms time period prior to ground contact. (I–L) Cumulant density functions associated with the coherence obtained between the two EMG recordings. Red circles are used to highlight the absence (I and J) and presence (K and L) of a short-lasting central peak in the cumulant density function indicating short-term EMG-EMG synchronization sitting on top of the broader peak produced EMG fluctuation over this section of the gait cycle. A, E and I are from recordings in the first pretraining session; B, F and J from the second pretraining session; C, G and K from the first post-training session; and D, H and L are from the second post-training session.
In the same subject in both of the two pretraining data collection sessions coherence between the EMGs recorded from the two sets of electrodes placed over the tibialis anterior muscle for the 400 ms period before ground contact revealed significant coupling primarily for frequencies <10 Hz with weak coherence at 20 Hz (Fig. 2E and F). Low frequency (<10 Hz) coherence primarily reflects the envelope of the EMG activity during the gait cycle (Halliday et al., 2003). Recordings obtained immediately after training and 1 month after training showed significant additional coherence in a broad frequency band from 20 to 40 Hz (Fig. 2G and H). In addition, it can be seen from Fig. 2I–L that the cumulant density function was characterized by a broad peak from −50 ms to +50 ms before the training, but following the training a small short-lasting peak either side of time zero (duration <10 ms) is observed on top of the broader peak, indicative of short term synchronization in addition to the broad synchronization resulting from EMG amplitude modulation (Kirkwood et al., 1982).
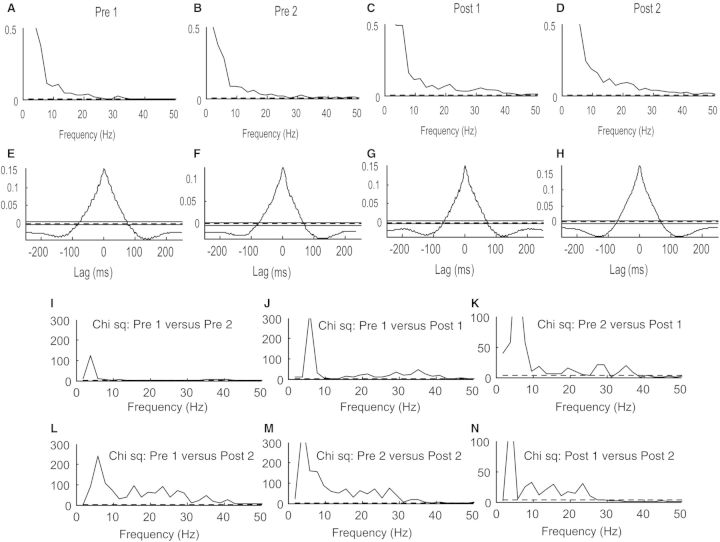
In Fig. 3 EMG-EMG coherence and cumulant data from all 16 children were pooled and additional analysis was performed using the χ2 difference of coherence test. The pooled results were consistent with those illustrated in Fig. 2 for a single subject. Figure 3 shows that there are increases in coherence following treadmill training compared to pretraining coherence in the frequency range 10 to 50 Hz (incorporating the 15–30 Hz beta and 30–50 Hz gamma frequency ranges). This coherence increase was detected immediately after treadmill training (Fig. 3C) and persisted for 1 month after the training (Fig. 3D). See for comparison the two results of recordings before the training (Fig. 3A and B) and the two post-training results (Fig. 3C and D).
Figure 3.
Pooled data from all 16 children with cerebral palsy. (A–D) Pooled coherence measures from pairwise tibialis anterior EMG recordings for the 400 ms time period prior to ground contact at the first (A) and second (B) test session before training and the first (C) and second (D) test sessions after training. The abscissa is the frequency in Hz. (E–H) Equivalent pooled measures of the cumulant density function. The abscissa is the lag in ms with respect to events in the first EMG recording. (I–N) show pairwise χ2 comparisons of coherence between the first and second test sessions before training (I), the first test session prior to training and the first test session following training (J), the second test session before training and the first test session after training (K), the first test session before training and the second test session after training (L), the second test session before training and the second test session after training (M) and finally the first test session after training and the second test session after training (N). The abscissa is the frequency in Hz.
Due to averaging effects no clear differences between the pooled cumulant density functions could be discerned when comparing the results before and after the training (Fig. 3E–H). In all cases a broad peak was observed, which reflects EMG amplitude modulation resulting from the gait cycle itself (see Kirkwood and Sears, 1978 for similar results in which a broad central peak in the cross-correlation histogram reflects periodicity in the respiratory drive to motor neurons). However, a narrow central peak on top of this broad peak could be discerned in some individual cases (as in Fig. 2K and L). Inspection of the cumulant density functions for data from each individual child revealed a short-duration central peak in 5 of 16 children before training. The prevalence of a central peak increased significantly to 11 of 16 children after training (Wilcoxon signed rank test; P = 0.03; Table 2). The average duration of the peak was slightly longer before training than after the training (Table 2; 13 ms versus 11 ms). Furthermore, the amplitude of the narrow central peak was twice as large at both post-training sessions than at the two pretraining session, but this did not reach a statistically significant level (Table 2; one-way repeated measures ANOVA; F = 2.18; P = 0.1). The differences in EMG-EMG coherence resulting from treadmill training were evaluated using the extended χ2 difference of coherence test (Farmer et al., 2007) and the results of this analysis are shown for each frequency comparison in Fig. 3I–N. The χ2 test revealed no differences between recordings obtained for the two sessions prior to training at frequencies in excess of 10 Hz (Fig. 3I). In contrast, the χ2 test showed significant coherence differences in the frequency band 15–40 Hz between all the pretraining data and the immediate post-training data indicative of a training related increase in tibialis anterior EMG-EMG coherence over a broad frequency band between 15 and 40 Hz (Fig. 3J and K). An increase in 15–30 Hz tibialis anterior EMG-EMG coherence was also observed when the χ2 difference was calculated between the pretraining data and the data obtained 1 month after training had ceased, indicating a long-term effect on tibialis anterior muscle EMG-EMG coherence as a result of treadmill training (Fig. 3L and M). Interestingly, the χ2 test indicated coherence in the 15–30 Hz band to be significantly greater 1 month after the training when compared to that obtained immediately after treadmill training (Fig. 3N).
Table 2.
Central peaks in the cumulant density function
| First pretest | Second pretest | First post-test | Second post-test | |
|---|---|---|---|---|
| No. of peaks | 5 | 5 | 11 | 11 |
| Duration (ms) | 13.60 | 13.64 | 11.42 | 11.11 |
| Amplitude | 0.0143 (±0.0214) | 0.0119 (±0.0190) | 0.0252 (±0.0222) | 0.0246 (±0.0294) |
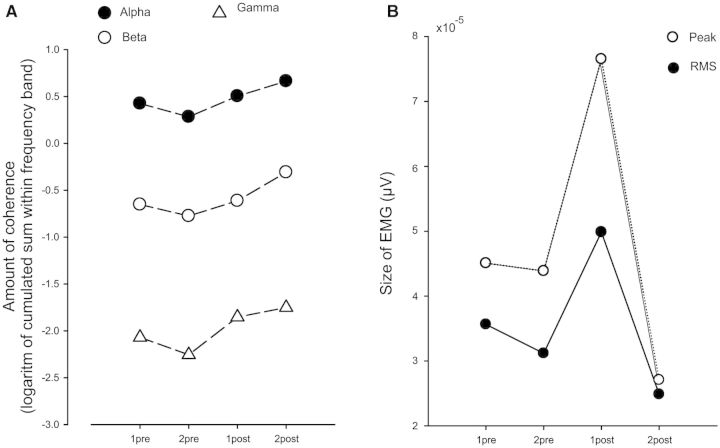
To further quantify changes in EMG-EMG coherence, the logarithmic value of the sum of coherence in the alpha (5–15 Hz), beta (15–35 Hz) and gamma bands (35–65 Hz) was calculated and compared between the four data collection sessions (Fig. 4A). One-way repeated measures ANOVA showed a significant interaction between test sessions for the gamma band (F = 6.98; P < 0.001) and post hoc analysis (Student-Newman-Keuls) revealed significant increase of coherence after the training when compared to the sessions before treadmill training (P < 0.01). There were no significant changes in the alpha or beta bands using a similar analysis.
Figure 4.
Pooled estimates of coherence and EMG in all 16 children with cerebral palsy. The cumulated sum of the logarithmic values of coherence was estimated for all children in the alpha (5–15 Hz), the beta (15–35 Hz) and gamma bands (35–65 Hz). The respective values are indicated for the four test sessions in A. The size of the largest peak of tibialis anterior EMG was calculated in all children for the most proximal electrode recording and displayed as open circles in B. Filled circles indicate the root mean square (RMS) value calculated for the same recordings. Note that lines depicting standard deviations have been omitted due to their large size.
The EMG peak and EMG root mean square amplitude recorded from the tibialis anterior muscle in the swing phase in each of the children during the four test sessions was quantified and this is plotted as the EMG peak and EMG root mean square values in Fig. 4B. The largest tibialis anterior EMGs were observed in the session immediately after training when compared to any of the other sessions. However, the change did not reach statistical significance because of the large inter- and intrasubject variability in the EMG amplitude measurements (one-way repeated measures ANOVA on ranks; χ2 = 3.8; P = 0.28).
Kinematic parameters and EMG coherence
Baseline measurements
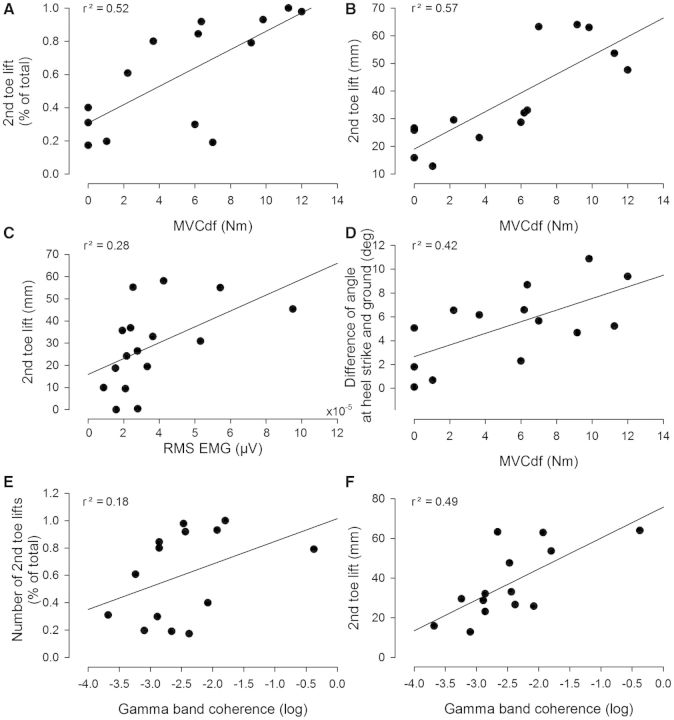
For the data obtained at the first pretraining session best subsets regression analysis was used to determine whether the magnitude of coherence in the different frequency bands, the maximal voluntary dorsiflexion force and the magnitude of EMG in the swing phase (root mean square amplitude) alone or in combination, predicted changes in the kinematic gait parameters. The strongest contributor on its own was the maximal voluntary dorsiflexion, which was positively correlated to the following: (i) the number of second toe lifts (Fig. 5A; P < 0.01); (ii) the amplitude of second toe lifts (Fig. 5B; P < 0.01); and (iii) the difference between the angle of the ankle joint at heel strike and the ground (Fig. 5D; P < 0.01). Separate Pearson product analysis revealed that significant correlations were also found between the magnitude of EMG (root mean square) in the swing phase and the amplitude of second toe lifts (Fig. 5C, P < 0.01) and the dynamic index (not illustrated; P < 0.01). Coherence in the gamma band showed a positive correlation with the number and amplitude of second toe lifts (Fig. 5E and F; P < 0.05). No correlation was found between coherence in any of the frequency bands and the magnitude of the EMG (P > 0.3).
Figure 5.
Correlations between maximal voluntary contraction, gait kinematics and coherence in the population of children with cerebral palsy. All correlations are based on measurements from the first test session before training in the individual children. (A and B) Correlation between maximal voluntary dorsiflexion force (in Nm) and the number of 2nd toe lifts (A) and the amplitude of the peak of the second toe lift (in mm; B). (C) Correlation between the root mean square EMG calculated for the most proximal tibialis anterior EMG recording and the amplitude of the peak of the second toe lift. (D) Correlation between the maximal dorsiflexion force (MVCdf) in Nm and the difference in the angle at ground contact and the ground. (E and F) Correlation between the sum of the logarithmic values of the coherence in the gamma band and the number of second toe lifts and the amplitude of the peak of the second toe lift (F). In all cases the correlation value is given as the r2 in the top of each graph. The lines depict the linear regression lines for the data.
Training induced changes
Best subsets regression analysis and subsequent multiple linear regression analysis was also used to determine the extent to which changes in coherence, maximal voluntary dorsiflexion and the magnitude of EMG in the swing phase (root mean square amplitude) with training could predict the improvements in gait function. Combined changes in beta and gamma band coherence predicted changes in the amplitude of the peak amplitude of second toe lifts (r2 = 0.36) with gamma coherence as the main contributor (P < 0.05). Combined changes in gamma and beta coherence also predicted changes in dynamic index (r2 = 0.68, with gamma coherence as the main contributor; P < 0.001). Changes in alpha band coherence, maximal voluntary dorsiflexion or EMG root mean square amplitude were unable in any combination to predict any of the changes in gait kinematics or foot pressure (P > 0.2).
Age-related changes in coherence
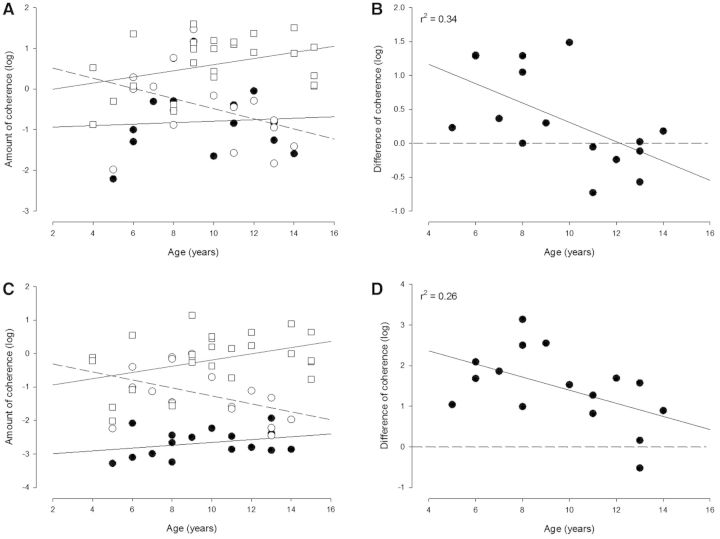
In Fig. 6 the magnitude of coherence in the beta (Fig. 6A) and gamma bands (Fig. 6C) is shown before and after the training as a function of age in the children with cerebral palsy. For comparison results are shown for typically developing healthy children. Healthy children showed an increase of beta and gamma coherence with increasing age (r2 = 0.4; P < 0.05) as has been reported previously (Petersen et al., 2010). Coherence in the children with cerebral palsy before training was overall reduced compared to healthy children and showed no clear increase with the subjects’ age. Interestingly, after training, a much larger increase in coherence was observed in both beta and gamma frequency bands for younger children with cerebral palsy compared to older children with cerebral palsy. All children below the age of 10 years showed an increase in beta and gamma band EMG-EMG coherence with training. This is visualized most clearly in Fig. 6B and D where the magnitude of coherence after training has been subtracted from the magnitude of coherence before training. Pearson product analysis showed a significant decrease in the training-induced change of coherence with age for both beta and gamma frequency bands (r2 = −0.34 and −0.26, respectively; P < 0.05).
Figure 6.
Relation between changes in coherence and age in the population of children with cerebral palsy and comparison to healthy children. The magnitude of coherence in the beta (A and B) and gamma (C and D) band is shown for each child with cerebral palsy before (filled circles in A and C) and after training (open circles in A and C) as a function of age. The amount of coherence before training is the average of the amount of coherence measured at the two sessions before training. For comparison, the magnitude of coherence in the two frequency bands is also shown for healthy children (open squares). In B and D the difference in coherence in the beta (B) and gamma (D) frequency bands before and after training is shown for the children with cerebral palsy and plotted against age (coherence values in the first test session post-training were subtracted from the values of coherence obtained in the first session pretraining). The full lines in A and C depict the linear regression lines for the data from children with cerebral palsy before training, the dotted lines regression lines from healthy children and the dashed lines regression lines for the difference in coherence before and after training for the children with cerebral palsy. In B and D the horizontal dashed line depicts zero, whereas the full line depicts the regression line for the data.
Figure 7 illustrates the difference in coherence recorded 1 month and immediately after training. It can be seen that coherence in both frequency bands was generally increased at 1 month following training compared to the values obtained immediately after training and that there is no significant relationship between this difference and the subjects’ age. The level of coherence obtained through training was thus maintained 1 month post-training in all cerebral palsy children regardless of age.
Figure 7.
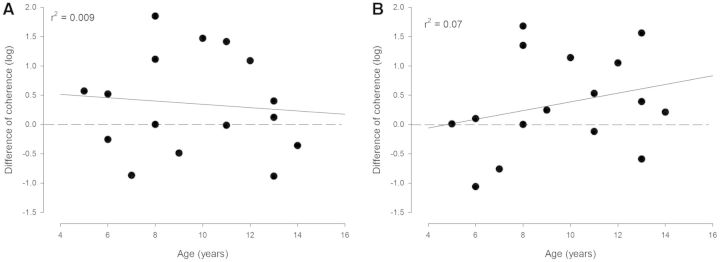
Comparison of coherence immediately after and 1 month after training in the population of children with cerebral palsy. The difference in the magnitude of coherence 1 month and immediately after the training in the beta (A) and gamma (B) frequency bands is shown for each child with cerebral palsy as a function of age. The horizontal dashed line depicts zero, whereas the continuous line depicts the regression line for the data.
Discussion
This study has demonstrated that daily home-based gait training on a treadmill with an incline in children with cerebral palsy changes intramuscular beta and gamma frequency band EMG-EMG coherence calculated for an ankle dorsiflexor muscle (tibialis anterior) activated during the gait cycle. Furthermore, we show that gait training in children with cerebral palsy is associated with an increase in toe lift in the end of swing phase and that it facilitates heel strike.
Gait training has been shown in a number of studies to produce clinically relevant improvements in gait function in children with cerebral palsy (Damiano and DeJong, 2009; Mutlu et al., 2009; Willerslev-Olsen et al., 2014b). In our previous study (Willerslev-Olsen et al., 2014b) we demonstrated that daily treadmill walking with an incline for 1 month reduces ankle joint stiffness, increases toe lift and improves heel strike in children with cerebral palsy, which translates into clinically relevant improvements not only in the quality of walking (i.e. reduced foot drop and toe walking), but also in walking speed and endurance (Willerslev-Olsen et al., 2014b). Gait at an incline is associated with a larger toe lift and thus increased activation of the tibialis anterior muscle (Leroux et al., 1999; Franz and Kram 2012; Stern and Gottschall, 2012). We therefore hypothesized that gait training at an incline would facilitate the central drive to the ankle dorsiflexors in children with cerebral palsy and that this would provide an explanation of the improved toe lift and heel strike in the children. To document central drive and its change with training we used coherence analysis of the discharge of populations of tibialis anterior motor units during gait activity in the children.
In healthy adults, coherence in the 10–50 Hz frequency band between populations of tibialis anterior motor units is observed in the early and late swing phase of the gait cycle (Halliday et al., 2003). This coherence is greatly reduced following stroke (Nielsen et al., 2008) and following spinal cord injury (Hansen et al., 2005; Barthelemy et al., 2010). It has been shown that for individual muscles and for co-contracting synergistic muscles both at the level of single motor units and at the level of the surface EMG that beta and gamma band coherence reflects descending corticospinal (central) drive to the lower motor neuron pool (Farmer et al., 1993a; Farmer, 1998; Barthelemy et al., 2011). The detection of beta and gamma band cortico-muscular coherence in upper limb muscles and the presence of 15-45 Hz coherence between the leg area of the motor cortex and tibialis anterior EMG activity during gait are supportive of the role of the motor cortex oscillations in the production of EMG oscillatory drive (Conway et al., 1995; Salenius et al., 1996; Halliday et al., 1998; Brown et al., 1999; Mima and Hallett, 1999; Petersen et al., 2012).
Healthy children below the age of 4–6 years have been shown to have very little coherence between tibialis anterior motor units during gait, whereas healthy children older than 12–14 years have coherence values similar to that of adults (Petersen et al., 2010). A similar increase in coherence with age in the beta and gamma bands was observed for healthy children in the present study (Fig. 6). This developmental increase in coherence is likely to reflect maturation of the corticospinal tract and the resulting increase in central drive to the motor neuron pool in healthy children [see Farmer et al. (2007) and James et al. (2008) for similar results on upper limb maturation of EMG-EMG coherence and CMC]. In children with cerebral palsy a similar pattern of EMG-EMG coherence maturation is not observed (Petersen et al., 2013) and this finding was confirmed in the present study which contains a different group of cerebral palsy subjects. The cerebral palsy subjects in the present study showed a lack of age-related increases in EMG-EMG coherence and an overall reduced magnitude of coherence when compared to healthy children. We suggest that this loss of central drive indicates impaired development of functional corticospinal connectivity in the children with cerebral palsy.
Corticospinal development has been shown in animals to be an activity-dependent mechanism (Friel et al., 2007; Martin et al., 2007, 2011; Chakrabarty et al., 2009a, b). We suggest that reduced physical activity and gait practice also contribute to impaired corticospinal development in children with cerebral palsy. Indeed, children with cerebral palsy are generally much less active than their healthy peers and perform <50% of the 10 000–15 000 recommended number of daily steps (Carlon et al., 2013). Importantly during the period of gait training the number of daily steps achieved by the children with cerebral palsy in the present study increased by ∼50% from a baseline of 4329 steps per day to a mean of 6000 steps per day (Willerslev-Olsen et al., 2014b). We suggest that the training with an incline and the resulting increase in activity underlies the observed changes in central common drive to the tibialis anterior motor neuron pool.
It should be noted from Fig. 6 that children with cerebral palsy still showed much lower coherence values than healthy children despite the significant increase in coherence following training. The coherence values were thus far from normalized leaving plenty of room for additional improvements in both coherence and function with further training. Our findings suggest that implementing additional gait training as a long-term rehabilitation strategy in children with cerebral palsy could facilitate development of the corticospinal tract especially if it is initiated during the ages in which corticospinal drive to the spinal motor neurons has not reached adult levels (i.e. pre 10–12 years of age) (Farmer et al., 2007; James et al., 2008; Petersen et al., 2010). Petersen et al. (2010) reported in typically developing children that the most significant changes in both gait characteristics and tibialis anterior muscle EMG-EMG coherence occur before the age range 10–12 years, suggesting an age window within which the gait training effects on the central drive to the motor neuron pool might be maximal.
The strongest effects of gait training in the present study were detected for children below ∼10 years in whom there were the most striking increases in beta and gamma range intramuscular coherence. In older children (>10 years) the training effects on gamma band coherence were smaller and in certain subjects and especially in the beta frequency range reversed (Fig. 6). We suggest that this finding reflects training induced alterations in the central drive to the spinal motor neurons in the younger children during a time period when in healthy subjects there is particular scope for optimization of gait performance and an increase in EMG-EMG coherence. Interestingly, in comparison to the beta frequency band, older children did show increases in gamma band coherence with training, suggesting that the frequency at which common drive can be affected by training also changes with age.
It is important to note that training-induced changes in coherence are not simply explained by increased amount of EMG activity or changes in motor unit recruitment. Although increased tibialis anterior EMG activity was also observed in the first session following the training period this was variable between subjects and no correlation between increased coherence and increased EMG activity was observed across the population of children. In addition, increased coherence was maintained at the second test session 1 month after the end of training, whereas the tibialis anterior EMG activity had fallen back to baseline pretraining levels at this time point. The increases in coherence are also unlikely to be caused by muscle hypertrophy even though the children did show increased maximal voluntary dorsiflexion force (cf. Fig. 1). This is for the following reasons. First, muscle hypertrophy is unlikely with a low-intensity training intervention as in the present study (Schoenfeld, 2010), and more so as the majority of children had not reached puberty. Even intense weight training fails to produce any significant muscle hypertrophy in children before puberty (Behm et al., 2008; Granacher et al., 2011). The increased voluntary dorsiflexion force following the training is therefore more likely caused by an increase in central neural drive to the tibialis anterior muscle, which is consistent with the improved ability to activate the muscle in the swing phase during gait. Secondly, coherence measures reflect synchronization of the rhythmic drive to the muscles, which is unrelated to muscle size. In fact coherence is much stronger for smaller distal than for larger proximal muscles i.e. it favours those muscles with strongest direct corticospinal input (Farmer et al., 1993a). Finally, previous studies have shown inconsistent effects of strength training on motor unit synchronization and EMG coherence within and across subjects (Kidgell et al., 2006; Ushiyama et al., 2010; Carroll et al., 2011).
A distinction should be made between the changes in coherence observed in relation to long-term training in children during development observed in the present study and the transient changes in coherence observed in relation to acquisition of new motor skills in adults (Perez et al., 2006; Geertsen et al., 2013). In adults, short-lasting sessions (30 min) of visuo-motor skill learning involving the ankle joint have been shown to increase corticomuscular and intramuscular coherence in the tibialis anterior muscle for ∼20 min. These coherence changes then return to the levels observed prior to training despite maintained improvement of performance (Perez et al., 2006). Such changes in coherence appear to reflect transient plastic changes in the oscillatory discharge of corticospinal neurons in relation to the acquisition of new tasks; possibly in much the same—and possibly closely related to—the transient changes in corticospinal excitability that have been documented in relation to acquisition of new motor skills (Perez et al., 2004, 2006; Pascual-Leone et al., 2005). The observation that the increase in coherence observed in the present study was maintained for 1 month after the end of training and that this was related to the functional outcome following the training suggests that other mechanisms are involved. We suggest that the increase—and in some cases occurrence—of beta and gamma frequency coherence in the children reflects a more long-lasting strengthening of corticospinal connections to spinal motor neurons.
The observation that following training coherence increased less and sometimes decreased in children older than ∼10 years of age suggests that plastic changes may preferentially be induced in children before the end of the period of time where development of corticospinal function still contributes to improvements of gait function (Petersen et al., 2010). However, it is important to note that this does not exclude that such plastic changes may be evoked also in older children and adults if a longer and more challenging training regime were to be adopted and we cannot exclude the possibility that older children would show training induced increases in coherence if a different training regime were adopted. Differences in type and intensity of training may indeed explain the changes in coherence observed in adults with spinal cord injury following gait training (Norton and Gorassini, 2006). Further studies are necessary to confirm if there are age-related limitations of training induced alterations in coherence and the functional consequences of this in relation to rehabilitation (Bravo-Esteban et al., 2014).
It is difficult to determine whether the common drive reflected as coherence and the changes in common drive induced by training play a functional role in muscle activation or whether the training-induced changes in oscillatory drive to the motor neuron pool are a surrogate marker of other corticospinal (central drive) changes that may be more functionally relevant. In this regard we note that prior to training the amount of coherence in the beta band was correlated with maximal voluntary dorsiflexion and the number and amplitude of toe lifts in the end of swing. Training-induced changes in coherence in both the beta and gamma bands were also correlated with changes in toe lifts as well as the efficiency of heel strike (dynamic index) following the gait training. This raises the possibility that the observed changes in coherence provide relevant information about the motor neuronal drive to the ankle muscles and thus are genuine markers of functional plastic changes in corticospinal (central) drive. This is consistent with the view that oscillatory corticospinal drive provides a more efficient mechanism with which to recruit spinal motor neurons (Baker et al., 1999), a mechanism that can be therapeutically exploited though adopting a regime of gait training in children with cerebral palsy.
Conclusion
These data show that daily intensive gait training increases beta and gamma oscillatory central drive to ankle dorsiflexor motor neurons and that it improves toe lift and heel strike in children with cerebral palsy. We propose these findings indicate that intensive gait training produces plastic changes in the corticospinal tract and that the resulting increase in central drive is responsible for improvements in gait function.
Acknowledgements
We are grateful to Michael Hedegård for taking care of the logistics of the project. We are grateful to Dr Lucinda Carr for her careful reading of the revised manuscript.
Funding
The study was supported by a grant from Ludvig and Sara Elsass Foundation. Simon Farmer acknowledges support from the National Institute for Health Service Research and the University College London Hospitals Biomedical Research Centre (UCLH BRC).
Supplementary material
Supplementary material is available at Brain online.
References
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods. 1997;73:69–79. doi: 10.1016/s0165-0270(96)02214-5. [DOI] [PubMed] [Google Scholar]
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Experimental Brain Res. 1999;128:109–17. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- Barthelemy D, Grey MJ, Nielsen JB, Bouyer L. Involvement of the corticospinal tract in the control of human gait. Progress Brain Res. 2011;192:181–97. doi: 10.1016/B978-0-444-53355-5.00012-9. [DOI] [PubMed] [Google Scholar]
- Barthelemy D, Willerslev-Olsen M, Lundell H, Conway BA, Knudsen H, Biering-Sørensen F, et al. Impaired transmission in the corticospinal tract and gait disability in spinal cord injured persons. J Neurophysiol. 2010;104:1167–76. doi: 10.1152/jn.00382.2010. [DOI] [PubMed] [Google Scholar]
- Behm DG, Faigenbaum AD, Falk B, Klentrou P. Canadian Society for Exercise Physiology position paper: resistance training in children and adolescents. Appl Physiol Nutr Metab. 2008;33:547–61. doi: 10.1139/H08-020. [DOI] [PubMed] [Google Scholar]
- Bennett D, Walsh M, O'Sullivan R, Gallagher J, O'Brien T, Newman CJ. Use of a dynamic foot pressure index to monitor the effects of treatment for equinus gait in children with cerebral palsy. J Pediatr Orthopedics. 2007;27:288–94. doi: 10.1097/BPO.0b013e3180342899. [DOI] [PubMed] [Google Scholar]
- Bland DC, Prosser LA, Bellini LA, Alter KE, Damiano DL. Tibialis anterior architecture, strength, and gait in individuals with cerebral palsy. Muscle Nerve. 2011;44:509–17. doi: 10.1002/mus.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra TW, Breakspear M. Neural mechanisms of intermuscular coherence: implications for the rectification of surface electromyography. J Neurophysiol. 2012;107:796–807. doi: 10.1152/jn.00066.2011. [DOI] [PubMed] [Google Scholar]
- Bravo-Esteban E, Taylor J, Aleixandre M, Simon-Martinez C, Torricelli D, Pons JL, et al. Tibialis Anterior muscle coherence during controlled voluntary activation in patients with spinal cord injury: diagnostic potential for muscle strength, gait and spasticity. J Neuroeng Rehabil. 2014;11:23. doi: 10.1186/1743-0003-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Farmer SF, Halliday DM, Marsden J, Rosenberg JR. Coherent cortical and muscle discharge in cortical myoclonus. Brain. 1999;122(Pt 3):461–72. doi: 10.1093/brain/122.3.461. [DOI] [PubMed] [Google Scholar]
- Carlon SL, Taylor NF, Dodd KJ, Shields N. Differences in habitual physical activity levels of young people with cerebral palsy and their typically developing peers: a systematic review. Disabil Rehabil. 2013;35:647–55. doi: 10.3109/09638288.2012.715721. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Selvanayagam VS, Riek S, Semmler JG. Neural adaptations to strength training: moving beyond transcranial magnetic stimulation and reflex studies. Acta Physiologica. 2011;202:119–40. doi: 10.1111/j.1748-1716.2011.02271.x. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S, Friel KM, Martin JH. Activity-dependent plasticity improves M1 motor representation and corticospinal tract connectivity. J Neurophysiol. 2009a;101:1283–93. doi: 10.1152/jn.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S, Shulman B, Martin JH. Activity-dependent codevelopment of the corticospinal system and target interneurons in the cervical spinal cord. J Neurosci. 2009b;29:8816–27. doi: 10.1523/JNEUROSCI.0735-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, et al. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol. 1995;489(Pt 3):917–24. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano DL, DeJong SL. A systematic review of the effectiveness of treadmill training and body weight support in pediatric rehabilitation. J Neurol Phys Ther. 2009;33:27–44. doi: 10.1097/NPT.0b013e31819800e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AK, Farmer SF, Stephens JA. Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. J Physiol. 1991;432:401–25. doi: 10.1113/jphysiol.1991.sp018391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF. Rhythmicity, synchronization and binding in human and primate motor systems. J Physiol. 1998;509(Pt 1):3–14. doi: 10.1111/j.1469-7793.1998.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J Physiol. 1993a;470:127–55. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Gibbs J, Halliday DM, Harrison LM, James LM, Mayston MJ, et al. Changes in EMG coherence between long and short thumb abductor muscles during human development. J Physiol. 2007;579:389–402. doi: 10.1113/jphysiol.2006.123174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Ingram DA, Stephens JA. Mirror movements studied in a patient with Klippel-Feil syndrome. J Physiol. 1990;428:467–84. doi: 10.1113/jphysiol.1990.sp018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. J Physiol. 1993b;463:83–105. doi: 10.1113/jphysiol.1993.sp019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler EG, Staudt LA, Greenberg MB. Lower-extremity selective voluntary motor control in patients with spastic cerebral palsy: increased distal motor impairment. Dev Med Child Neurol. 2010;52:264–9. doi: 10.1111/j.1469-8749.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- Franz JR, Kram R. The effects of grade and speed on leg muscle activations during walking. Gait Posture. 2012;35:143–7. doi: 10.1016/j.gaitpost.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel KM, Drew T, Martin JH. Differential activity-dependent development of corticospinal control of movement and final limb position during visually guided locomotion. JNeurophysiol. 2007;97:3396–406. doi: 10.1152/jn.00750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geertsen SS, Kjaer M, Pedersen KK, Petersen TH, Perez MA, Nielsen JB. Central common drive to antagonistic ankle muscles in relation to short-term cocontraction training in nondancers and professional ballet dancers. J Appl Physiol. 2013;115:1075–81. doi: 10.1152/japplphysiol.00707.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granacher U, Goesele A, Roggo K, Wischer T, Fischer S, Zuerny C, et al. Effects and mechanisms of strength training in children. Int J Sport Med. 2011;32:357–64. doi: 10.1055/s-0031-1271677. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Christensen LO, Hansen NL, Petersen NP, Nielsen JB. Functional coupling of motor units is modulated during walking in human subjects. J Neurophysiol. 2003;89:960–8. doi: 10.1152/jn.00844.2002. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241:5–8. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Farmer SF. On the need for rectification of surface EMG. J Neurophysiol. 2010;103:3547; author reply 48–9. doi: 10.1152/jn.00222.2010. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR. On the application, estimation and interpretation of coherence and pooled coherence. J Neurosci Methods. 2000;100:173–4. doi: 10.1016/s0165-0270(00)00267-3. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data—theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–78. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hansen NL, Conway BA, Halliday DM, Hansen S, Pyndt HS, Biering-Sørensen F, et al. Reduction of common synaptic drive to ankle dorsiflexor motoneurons during walking in patients with spinal cord lesion. J Neurophysiol. 2005;94:934–42. doi: 10.1152/jn.00082.2005. [DOI] [PubMed] [Google Scholar]
- Hodapp M, Klisch C, Berger W, Mall V, Faist M. Modulation of soleus H-reflexes during gait in healthy children. Exp Brain Res. 2007a;178:252–60. doi: 10.1007/s00221-006-0730-1. [DOI] [PubMed] [Google Scholar]
- Hodapp M, Klisch C, Mall V, Vry J, Berger W, Faist M. Modulation of soleus H-reflexes during gait in children with cerebral palsy. J Neurophysiol. 2007b;98:3263–8. doi: 10.1152/jn.00471.2007. [DOI] [PubMed] [Google Scholar]
- James LM, Halliday DM, Stephens JA, Farmer SF. On the development of human corticospinal oscillations: age-related changes in EEG-EMG coherence and cumulant. Eur J Neurosci. 2008;27:3369–79. doi: 10.1111/j.1460-9568.2008.06277.x. [DOI] [PubMed] [Google Scholar]
- Kidgell DJ, Sale MV, Semmler JG. Motor unit synchronization measured by cross-correlation is not influenced by short-term strength training of a hand muscle. Exp Brain Res. 2006;175:745–53. doi: 10.1007/s00221-006-0724-z. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978;275:103–34. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA, Tuck DL, Westgaard RH. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982;327:105–35. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA, Westgaard RH. Restoration of function in external intercostal motoneurones of the cat following partial central deafferentation. J Physiol. 1984;350:225–51. doi: 10.1113/jphysiol.1984.sp015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux A, Fung J, Barbeau H. Adaptation of the walking pattern to uphill walking in normal and spinal-cord injured subjects. Exp Brain Res. 1999;126:359–68. doi: 10.1007/s002210050743. [DOI] [PubMed] [Google Scholar]
- Martin JH, Chakrabarty S, Friel KM. Harnessing activity-dependent plasticity to repair the damaged corticospinal tract in an animal model of cerebral palsy. Dev Med Child Neurol. 2011;53(Suppl. 4):9–13. doi: 10.1111/j.1469-8749.2011.04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH, Friel KM, Salimi I, Chakrabarty S. Activity- and use-dependent plasticity of the developing corticospinal system. Neurosc Biobehav Rev. 2007;31:1125–35. doi: 10.1016/j.neubiorev.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima T, Hallett M. Corticomuscular coherence: a review. J Clin Neurophysiol. 1999;16:501–11. doi: 10.1097/00004691-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Moreau NG, Falvo MJ, Damiano DL. Rapid force generation is impaired in cerebral palsy and is related to decreased muscle size and functional mobility. Gait Posture. 2012;35:154–8. doi: 10.1016/j.gaitpost.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu A, Krosschell K, Spira DG. Treadmill training with partial body-weight support in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2009;51:268–75. doi: 10.1111/j.1469-8749.2008.03221.x. [DOI] [PubMed] [Google Scholar]
- Myers LJ, Lowery M, O'Malley M, Vaughan CL, Heneghan C, St Clair Gibson A, et al. Rectification and non-linear pre-processing of EMG signals for cortico-muscular analysis. J Neurosci Methods. 2003;124:157–65. doi: 10.1016/s0165-0270(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Brittain JS, Halliday DM, Marchand-Pauvert V, Mazevet D, Conway BA. Reduction of common motoneuronal drive on the affected side during walking in hemiplegic stroke patients. Clin Neurophysiol. 2008;119:2813–8. doi: 10.1016/j.clinph.2008.07.283. [DOI] [PubMed] [Google Scholar]
- Norton JA, Gorassini MA. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J Neurophysiol. 2006;95:2580–9. doi: 10.1152/jn.01289.2005. [DOI] [PubMed] [Google Scholar]
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Ann Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lundbye-Jensen J, Nielsen JB. Changes in corticospinal drive to spinal motoneurones following visuo-motor skill learning in humans. J Physiol. 2006;573:843–55. doi: 10.1113/jphysiol.2006.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Petersen TH, Farmer SF, Kliim-Due M, Nielsen JB. Failure of normal development of central drive to ankle dorsiflexors relates to gait deficits in children with cerebral palsy. J Neurophysiol. 2013;109:625–39. doi: 10.1152/jn.00218.2012. [DOI] [PubMed] [Google Scholar]
- Petersen TH, Kliim-Due M, Farmer SF, Nielsen JB. Childhood development of common drive to a human leg muscle during ankle dorsiflexion and gait. J Physiol. 2010;588:4387–400. doi: 10.1113/jphysiol.2010.195735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TH, Willerslev-Olsen M, Conway BA, Nielsen JB. The motor cortex drives the muscles during walking in human subjects. J Physiol. 2012;590:2443–52. doi: 10.1113/jphysiol.2012.227397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salenius S, Salmelin R, Neuper C, Pfurtscheller G, Hari R. Human cortical 40 Hz rhythm is closely related to EMG rhythmicity. Neurosci Lett. 1996;213:75–8. doi: 10.1016/0304-3940(96)12796-8. [DOI] [PubMed] [Google Scholar]
- Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. 2010;24:2857–72. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- Stern KA, Gottschall JS. Child temporal-spatial gait characteristics and variability during uphill and downhill walking. Pediatr Phys Therap. 2012;24:285–90. doi: 10.1097/PEP.0b013e31824d732d. [DOI] [PubMed] [Google Scholar]
- Ushiyama J, Takahashi Y, Ushiba J. Muscle dependency of corticomuscular coherence in upper and lower limb muscles and training-related alterations in ballet dancers and weightlifters. J Appl Physiol. 2010;109:1086–95. doi: 10.1152/japplphysiol.00869.2009. [DOI] [PubMed] [Google Scholar]
- Ward NJ, Farmer SF, Berthouze L, Halliday DM. Rectification of EMG in low force contractions improves detection of motor unit coherence in the beta-frequency band. J Neurophysiol. 2013;110:1744–50. doi: 10.1152/jn.00296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Andersen JB, Sinkjaer T, Nielsen JB. Sensory feedback to ankle plantar flexors is not exaggerated during gait in spastic children with cerebral palsy. J Neurophysiol. 2014a;111:746–54. doi: 10.1152/jn.00372.2013. [DOI] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Lorentzen J, Nielsen JB. Gait training reduces ankle joint stiffness and facilitates heel strike in children with Cerebral Palsy. NeuroRehabilitation. 2014b;35:643–55. doi: 10.3233/NRE-141180. [DOI] [PubMed] [Google Scholar]