Abstract
Anti-tumour necrosis factor α (anti-TNFα) therapy is an established treatment in inflammatory bowel disease. However, this treatment is associated with high costs and the possibility of severe adverse events representing a true challenge for patients, clinicians and health care systems. Consequently, a crucial question is raised namely if therapy can be stopped once remission is achieved and if so, how and in whom. Additionally, in a real-life clinical setting, discontinuation may also be considered for other reasons such as the patient’s preference, pregnancy, social reasons as moving to countries or continents with less access, or different local policy or reimbursement. In contrast to initiation of anti-TNFα therapy guidelines regarding stopping of this treatment are missing. As a result, the decision of discontinuation is still a challenging aspect in the use of anti-TNFα therapy. Currently this is typically based on an estimated, case-by-case, benefit-risk ratio. This editorial is intended to provide an overview of recent data on this topic and shed light on the proposed drug withdrawal strategies.
Keywords: Inflammatory bowel disease, Anti-tumour necrosis factor α therapy, Withdrawal, Remission, Infliximab
Core tip: Anti-tumour necrosis factor α (anti-TNFα) therapy is an established treatment in inflammatory bowel disease. Although guidelines exist on initiation of anti-TNFα therapy in inflammatory bowel diseases, information on if, when, how and in whom therapy can be stopped is limited. This is nevertheless an important topic taking under consideration the cost and the possible adverse events associated with biological agents as well as the desire of patients to discontinue medication especially after a long maintained remission. Moreover, although drug discontinuation for reasons other than loss of response is very usual in real-life clinical practice, the optimal withdrawal strategy is still debated.
INTRODUCTION
Anti-tumor necrosis factor α (anti-TNFα) therapy has greatly improved the management of patients with inflammatory bowel diseases (IBD) namely Crohn’s disease (CD) and ulcerative colitis (UC)[1]. However, this treatment is associated with high costs and the possibility of severe adverse events such as opportunistic infections or risk for lymphoma. These aspects represent a true challenge not only for patients and clinicians but also for health care systems[2]. Consequently, a crucial question is raised: can therapy be stopped once remission is achieved and if so, when, how and in whom? Additionally, in a real-life clinical setting, discontinuation may also be considered for other reasons such as the patient’s own preference, pregnancy, moving to places with less access to biological agents, local policy or different reimbursement systems[2].
Nowadays, as supporting data is lacking, there are no stopping rules for anti-TNFα therapy in IBD. There is even less information regarding prognostic factors that could predict relapse or sustained remission after anti-TNFα therapy discontinuation. The only provided evidence regarding CD comes from the landmark STORI trial[3] and a few retrospective observational[4-8] or small prospective studies[9-12], while for UC there are even less data available[7,9,13] (Table 1). As a result, the decision of discontinuation is currently made on the basis of an individual judgement of benefits versus risks and cost-effectiveness[14-18].
Table 1.
Studies on the discontinuation of anti-tumor necrosis factor α therapy in inflammatory bowel disease
| IBD type | Anti-TNFα therapy | n | Median follow up, mo | SCR at the end of follow up, % | Clinical benefit after re-introduction of anti-TNFα therapy for relapse, % | Ref. |
| CD | IFX | 115 | 28 | 55 | 88 | [3] |
| CD | IFX | 48 | 49 | 35 | ND | [4] |
| CD | IFX | 53 | 18 | 12 | 96 | [7] |
| UC | IFX | 28 | 29 | 40 | 71 | [7] |
| CD | IFX or ADM | 121 | 12 | 55 | 55 | [11] |
| UC | IFX | 51 | 12 | 65 | 94 | [13] |
| CD | IFX or ADM | 37 | 1-44 (range) | 26 (1 yr) | ND | [10] |
| CD | IFX or ADM | 17 | 13 | 71 | 100 | [9] |
| UC | IFX | 34 | 13 | 65 | 90 | [9] |
| CD | IFX | 100 | 120 | 52 | ND | [6] |
| CD | IFX or ADM | 86 | 17 | 64 (1 yr) | 93 | [12] |
| CD | IFX | 92 | 47 | 28 | 89 | [5] |
IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis; IFX: Infliximab; ADM: Adalimumab; TNFα: Tumor necrosis factor α; ND: Not defined; SCR: Sustained clinical remission.
Another important issue when considering cessation of anti-TNFα therapy is whether the drug can safely be restarted when needed and whether efficacy will be similar. Possible lower response rates after re-initiation of biological therapy, limited alternative treatment options and/or immunogenicity concerns are all factors which constitute to the fear of stopping treatment[18,19].
WITHDRAWAL OF ANTI-TNFα THERAPY IN INFLAMMATORY BOWEL DISEASE. IS IT FEASIBLE?
Current guidelines suggest that anti-TNFα therapy should be started early in the course of the IBD to maximize its efficacy before irreversible bowel damage has occurred[20]. On the other hand, there are no rules and/or recommendations with respect to stopping, although it is often empirically proposed not to routinely stop anti-TNFα agents in IBD patients who respond, and especially in patients with disabling features of disease and/or at high-risk for relapse[14-18]. However, recent data indicate that a proportion of patients, in clinical remission can stop anti-TNFα therapy without a major impact on disease control even for a relatively long time period, while on immunomodulators (IMM) (Table 1). The pivotal STORI trial showed that it was possible to identify a subgroup of patients with only a 10% relapse risk 24 mo post-discontinuation[3].
Nevertheless, the results of the various studies may not always be comparable as the type of IBD (CD vs UC), of anti-TNFα therapy (infliximab vs adalimumab), the study design (prospective vs retrospective observational), the studied outcome (clinical vs endoscopic), the duration of remission before stopping anti-TNFα agents and the phenotypic and clinical characteristics of the patients often differ, as were the definition of relapse and the (median) follow up time after anti-TNFα cessation[21]. Moreover, CD patients are very heterogeneous in terms of disease type (luminal or fistulising), location (ileal, ileo-colonic or colonic) and behaviour (inflammatory, stricturing, or penetrating) and the consequences of relapse may widely vary, while establishment of complete mucosal healing and clinical remission is much more straightforward in UC than in CD.
One argument in favour of discontinuation of anti-TNFα therapy is the fact that all studies show that anti-TNFα therapy can be restarted without risking loss of response or adverse events in a large proportion of patients (Table 1). In a recent study by Baert et al[22] re-starting of infliximab therapy re-introduced response in 84.5% of patients at week 14, 70% at 1 year, and in 61% of patients at more than 4 years. Re-introduction of anti-TNFα therapy therefore seems possibly independent of the drug holiday[22], although Laharie et al[23] showed that retreatment with IFX in CD primary responders should be administered within 50 wk after induction, for better efficacy and tolerance.
Discontinuation of anti-TNFα therapy for clinical remission has been shown to be feasible in other autoimmune, chronic diseases such as rheumatoid arthritis[24], Behçet’s uveitis[25], spondyloarthritis[26] and sarcoidosis[27].
WHEN SHOULD WE STOP?
Stopping of anti-TNFα therapy is more likely to succeed in terms of maintaining prolonged remission when complete clinical (CDAI < 150), endoscopic (complete mucosal healing) and serological (normal CRP) remission, were achieved prior to discontinuation[20]. However, the minimum time of remission before stopping anti-TNFα therapy has not been yet well defined. It is proposed that clinical remission for over than one year prior to discontinuation of anti-TNFα therapy is adequate[11,20] while others suggest to stop after a minimum of two years of clinical and endoscopic remission or longer if only clinical remission can be documented[28].
Moreover, preliminary evidence suggests that a long lasting and profound drug-free remission may be achieved in case of a short duration of disease from diagnosis to start of anti-TNFα therapy, probably before irreversible immunological aberrations and more intestinal tissue impairment have occurred[6,7].
HOW SHOULD WE STOP?
The optimal treatment following discontinuation of anti-TNFα therapy in IBD has not yet been clearly defined, as in all previous studies the majority of patients (67%-100%) maintained clinical remission after cessation of treatment, by a continuous administration of an IMM[2,21]. Consequently, it is unclear whether a sustained clinical remission can be achieved during a true drug-free period.
Most will agree that a close follow up is needed when anti-TNF is stopped but how to monitor these patients in the most optimal way is not clear. Monitoring of CRP and fecal calprotectin levels every 8-12 wk may be very useful for predicting early clinical relapse with endoscopic re-evaluation in case of a significant increase of these biomarkers (Figure 1)[29]. If and when endoscopic evaluation should be implemented in the follow up of patients who remain in full remission remains to be elucidated[28].
Figure 1.
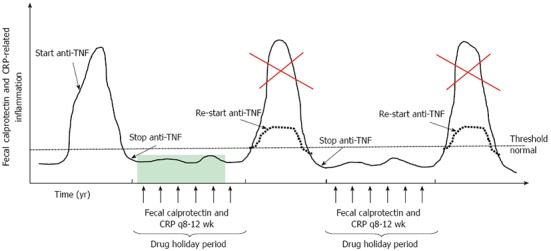
New concept of intermittent anti-tumor necrosis factor α therapy in inflammatory bowel disease. Stopping anti-TNFα agents after achieving a deep remission may result in prolonged clinical remission. Close monitoring of these patients with fecal calprotectin and CRP measurements (arrows) will allow early re-initiation of anti-TNFα therapy, when inflammation is starting to rise, which may result to a sustained clinical benefit (dotted line) preventing a disease flare (red cross). These patients may be considered as treated periodically and not episodically. TNF: Tumor necrosis factor; CRP: C-reactive protein.
IN WHOM?
Another important issue, before applying a stopping strategy for anti-TNFα therapy, is to assess prediction of sustained remission after withdrawal of the drugs, in order to identify the ideal candidate for discontinuation of anti-TNFα treatment. We believe that the decision-making approach to stop anti-TNFα therapy is currently based on limited data (Table 2).
Table 2.
Risk factors for relapse after stopping anti-tumor necrosis factor α therapy for remission (clinical or endoscopic) in inflammatory bowel disease
| Risk factors | Ref. |
| Clinical or phenotypic | |
| Corticosteroid use between 12 and 6 mo before baseline | [3] |
| Male gender | [3] |
| Absence of previous surgical resection | [3] |
| Longer disease duration from diagnosis to first infliximab | [7] |
| Previous biological therapy | [11-13] |
| Dose intensification during the first year of anti-TNFα therapy | [11] |
| Age at CD diagnosis ≥ 25 yr | [6] |
| Ileocolonic disease at diagnosis | [12] |
| Active smoking | [5] |
| Previous antimetabolite failure | [5] |
| Perianal disease | [5] |
| Serological1 | |
| Hemoglobin levels ≤ 14.5 g/dL | [3] |
| White blood count > 6 × 109/L | [3] |
| High sensitive CRP ≥ 5 mg/L | [3] |
| Infliximab trough levels ≥ 2 μg/mL | [3] |
| Serum calprotectin > 5675 ng/mL | [37] |
| Endoscopic1 | |
| CDEIS > 0 | [3] |
| Mucosal1 | |
| Lack of normalization of IL-17A and TNFα expression levels | [10] |
| Microbiological1 (CD-associated dysbiosis) | |
| Low rate of Faecalibacterium prausnitzii in fecal samples | [38] |
| Low rate of Bacteroides in fecal samples | [38] |
| Fecal1 | |
| Fecal calprotectin ≥ 300 μg/g | [3] |
| Genetic | |
| Fc gamma receptor IIIB-NA2/NA2 genotype (fistulising disease) | [39] |
At the time of anti-TNFα therapy discontinuation. CD: Crohn’s disease; CRP: C-reactive protein; CDEIS: Crohn’s Disease Endoscopic index of severity; IL: Interleukin; NA: Neutrophil antigen; TNFα: Tumor necrosis factor α.
Although patients with (perianal) fistulising CD were excluded from many studies including the STORI trial[3] current data suggest that these patients relapse with the same rate as those with luminal disease[5,6,9,11,12]. However, in previous studies infliximab discontinuation led to a higher rate of relapse in patients with perianal fistulising compared to luminal CD[23,30,31]. One possible explanation could be that in most of these studies clinical remission and relapse for the majority of patients with (perianal) fistulising CD were not evaluated by imaging techniques or this information was missing. This is very important taking into account that perianal disease is often active despite external fistulae closure. Consequently, evaluation of CD patients with stricturing and/or penetrating disease, before discontinuation of anti-TNFα therapy, may include also imaging techniques. Patients with internal fistulas, an intestinal stenosis or a complex perianal fistulising disease may have a poor prognosis after discontinuation of the anti-TNFα therapy.
Moreover although complete mucosal healing at the time of IFX discontinuation for clinical remission in CD patients was predictive for sustained clinical remission after cessation of the drug[3,6], this was not confirmed by other studies[11,13,32].
Finally, regarding the role of therapeutic drug monitoring on the decision making of stopping anti-TNF therapy for clinical remission preliminary evidence points out that low IFX trough levels at the time of discontinuation are predictive of sustained remission after drug cessation in CD, while the role of antibodies to IFX has not been yet clearly defined[3,6]. It seems that these patients do not need the drug anymore to maintain remission which is in agreement with the results of the TAXIT trial where 9% of the patients being in remission had undetectable trough levels and were stopped successfully without relapse[33].
NEW CONCEPT OF INTERMITTENT ANTI-TNFα THERAPY IN INFLAMMATORY BOWEL DISEASE
Tailoring of anti-TNFα maintenance therapy for patients achieving remission is becoming nowadays a necessity as safety and costs issues may hinder the long-term, sustained clinical benefit deriving from this therapy. This could be achieved either by lowering the dose of these drugs[33], based on therapeutic drug monitoring[34] or by stopping them following the intermittent non-continuous pharmacological treatment approach[2]. Patients following the latter strategy may be considered as treated periodically rather than episodically (Figure 1)[35]. A paradigm of this therapeutic pharmacological approach from real-life clinical practice is described in Figure 2. We believe that prediction of sustained clinical remission after discontinuation of anti-TNFα therapy along with the close monitoring of these patients so as to avoid an upcoming disease flare by early re-introduction of these drugs may be a first step for optimizing maintenance anti-TNFα treatment in patients achieving remission.
Figure 2.
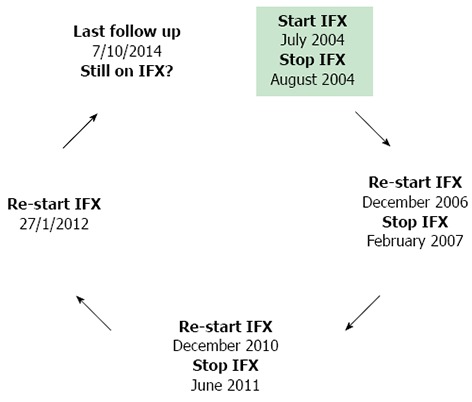
Successful intermittent infliximab therapy in a patient with ulcerative colitis: A paradigm from real-life clinical practice. This is an example of a UC patient (male, age at diagnosis 33 years) with pancolitis, treated in our center, who received successfully intermittent infliximab (IFX) therapy (black arrows represent time periods of clinical remission). He has been treated with IFX for relapse, after discontinuation of the drug on his own preference while in clinical remission continuing on azathioprine. At the last time of follow up he was still in clinical and biochemical (normal CRP) remission under IFX maintenance monotherapy. This patient has never developed antibodies to IFX despite receiving interrupted therapy, while the last available (25/2/2014) trough concentrations of IFX were 8.09 μg/mL (q5).
CONCLUSION
Intentional cessation of anti-TNFα treatment will become a more prevalent practice in the future not only for safety and cost reasons but probably also due to newly available non TNFα neutralizing pharmaceutical therapeutics options, although with the introduction of biosimilars, costs will probably become less important. Anti-TNFα withdrawal strategy to achieve disease control based on an on-demand use of anti-TNFα therapy (when relapse is suspected after discontinuation of the drugs) and a continuous treatment with IMM, could be an option to reduce chronic exposure to biologics, at least to a highly selected IBD population.
Nevertheless, in order to elucidate whether discontinuation of anti-TNFα therapy for remission will become a routine strategy in the future for the long-term management of IBD patients and to define the optimal withdrawal strategy more studies are needed. One of them would definitely be the SPARE study from the Groupe d’Etude Therapeutique des Affections Inflammatoires Digestives group, a Phase IV, prospective, open-label, randomized controlled trial comparing infliximab antimetabolites combination therapy to anti-metabolites monotherapy and infliximab monotherapy in CD patients in sustained steroid-free remission on combination therapy (ClinicalTrials.gov Identifier: NCT02177071)[36]. The main goal of this study is to demonstrate that infliximab scheduled maintenance with or without antimetabolites is superior to antimetabolites alone to maintain sustained steroid-free remission over 2 years, while the latter is non inferior with regards to the mean time spent in remission over the same duration[36].
ACKNOWLEDGMENTS
Papamichael K received a fellowship grant from the Hellenic Gastroenterology Society and the European Crohn’s and Colitis Organization; Vermeire S is a Senior Clinical Investigator of the Research Foundation-Flanders, Belgium.
Footnotes
Conflict-of-interest: Papamichael K has received a consultancy fee from MSD Hellas; and Vermeire S has received research funding from UCB Pharma, Abbvie and UCB Pharma, lecture fees from Abbott, Abbvie, MSD, Ferring Pharmaceuticals and UCB Pharma and consultancy fees from Pfizer, Ferring Pharmaceuticals, Shire Pharmaceuticals Group, MSD, and AstraZeneca Pharmaceuticals.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 19, 2014
First decision: December 11, 2014
Article in press: February 11, 2015
P- Reviewer: Ahluwalia NK, Mendall MA, Negreanu L, Trifan A S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Billiet T, Rutgeerts P, Ferrante M, Van Assche G, Vermeire S. Targeting TNF-α for the treatment of inflammatory bowel disease. Expert Opin Biol Ther. 2014;14:75–101. doi: 10.1517/14712598.2014.858695. [DOI] [PubMed] [Google Scholar]
- 2.Sorrentino D, Nash P, Viladomiu M, Hontecillas R, Bassaganya-Riera J. Stopping anti-TNF agents in patients with Crohn’s disease in remission: is it a feasible long-term strategy? Inflamm Bowel Dis. 2014;20:757–766. doi: 10.1097/01.MIB.0000442680.47427.bf. [DOI] [PubMed] [Google Scholar]
- 3.Louis E, Mary JY, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70.e5; quiz e31. doi: 10.1053/j.gastro.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Waugh AW, Garg S, Matic K, Gramlich L, Wong C, Sadowski DC, Millan M, Bailey R, Todoruk D, Cherry R, et al. Maintenance of clinical benefit in Crohn’s disease patients after discontinuation of infliximab: long-term follow-up of a single centre cohort. Aliment Pharmacol Ther. 2010;32:1129–1134. doi: 10.1111/j.1365-2036.2010.04446.x. [DOI] [PubMed] [Google Scholar]
- 5.Chauvin A, Le Thuaut A, Belhassan M, Le Baleur Y, Mesli F, Bastuji-Garin S, Delchier JC, Amiot A. Infliximab as a bridge to remission maintained by antimetabolite therapy in Crohn’s disease: A retrospective study. Dig Liver Dis. 2014;46:695–700. doi: 10.1016/j.dld.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Papamichael K, Vande Casteele N, Gils A, Tops S, Hauenstein S, Singh S, Princen F, Van Assche G, Rutgeerts P, Vermeire S, et al. Long-Term Outcome of Patients With Crohn’s Disease Who Discontinued Infliximab Therapy Upon Clinical Remission. Clin Gastroenterol Hepatol. 2014:Epub ahead of print. doi: 10.1016/j.cgh.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Steenholdt C, Molazahi A, Ainsworth MA, Brynskov J, Østergaard Thomsen O, Seidelin JB. Outcome after discontinuation of infliximab in patients with inflammatory bowel disease in clinical remission: an observational Danish single center study. Scand J Gastroenterol. 2012;47:518–527. doi: 10.3109/00365521.2012.660541. [DOI] [PubMed] [Google Scholar]
- 8.Lu C, Waugh A, Bailey RJ, Cherry R, Dieleman LA, Gramlich L, Matic K, Millan M, Kroeker KI, Sadowski D, et al. Crohn’s disease genotypes of patients in remission vs relapses after infliximab discontinuation. World J Gastroenterol. 2012;18:5058–5064. doi: 10.3748/wjg.v18.i36.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molander P, Färkkilä M, Salminen K, Kemppainen H, Blomster T, Koskela R, Jussila A, Rautiainen H, Nissinen M, Haapamäki J, et al. Outcome after discontinuation of TNFα-blocking therapy in patients with inflammatory bowel disease in deep remission. Inflamm Bowel Dis. 2014;20:1021–1028. doi: 10.1097/MIB.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 10.Rismo R, Olsen T, Cui G, Paulssen EJ, Christiansen I, Johnsen K, Florholmen J, Goll R. Normalization of mucosal cytokine gene expression levels predicts long-term remission after discontinuation of anti-TNF therapy in Crohn’s disease. Scand J Gastroenterol. 2013;48:311–319. doi: 10.3109/00365521.2012.758773. [DOI] [PubMed] [Google Scholar]
- 11.Molnár T, Lakatos PL, Farkas K, Nagy F, Szepes Z, Miheller P, Horváth G, Papp M, Palatka K, Nyári T, et al. Predictors of relapse in patients with Crohn's disease in remission after 1 year of biological therapy. Aliment Pharmacol Ther. 2013;37:225–233. doi: 10.1111/apt.12160. [DOI] [PubMed] [Google Scholar]
- 12.Brooks AJ, Sebastian S, Cross SS, Robinson K, Warren L, Wright A, Marsh AM, Tsai H, Majeed F, McAlindon ME, et al. Outcome of elective withdrawal of anti-tumour necrosis factor-α therapy in patients with Crohn’s disease in established remission. J Crohns Colitis. 2014:Epub ahead of print. doi: 10.1016/j.crohns.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Farkas K, Lakatos PL, Nagy F, Szepes Z, Miheller P, Papp M, Palatka K, Bálint A, Bor R, Wittmann T, et al. Predictors of relapse in patients with ulcerative colitis in remission after one-year of infliximab therapy. Scand J Gastroenterol. 2013;48:1394–1398. doi: 10.3109/00365521.2013.845906. [DOI] [PubMed] [Google Scholar]
- 14.Louis E, Belaiche J, Reenaers C. Are we giving biologics too much time? When should we stop treatment? World J Gastroenterol. 2008;14:5528–5531. doi: 10.3748/wjg.14.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peyrin-Biroulet L, Danese S. Stopping infliximab in Crohn’s disease: still an ongoing STORI. Inflamm Bowel Dis. 2012;18:2201–2202. doi: 10.1002/ibd.23016. [DOI] [PubMed] [Google Scholar]
- 16.Kamm MA, Ng SC, De Cruz P, Allen P, Hanauer SB. Practical application of anti-TNF therapy for luminal Crohn’s disease. Inflamm Bowel Dis. 2011;17:2366–2391. doi: 10.1002/ibd.21655. [DOI] [PubMed] [Google Scholar]
- 17.Hashash JG, Regueiro MD. The great debate: stopping immunomodulators and biologics in Crohn’s disease patients in remission. Expert Rev Gastroenterol Hepatol. 2013;7:501–503. doi: 10.1586/17474124.2013.814933. [DOI] [PubMed] [Google Scholar]
- 18.Clarke K, Regueiro M. Stopping immunomodulators and biologics in inflammatory bowel disease patients in remission. Inflamm Bowel Dis. 2012;18:174–179. doi: 10.1002/ibd.21792. [DOI] [PubMed] [Google Scholar]
- 19.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013;108:40–47; quiz 48. doi: 10.1038/ajg.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Haens GR, Panaccione R, Higgins PD, Vermeire S, Gassull M, Chowers Y, Hanauer SB, Herfarth H, Hommes DW, Kamm M, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–212; quiz 213. doi: 10.1038/ajg.2010.392. [DOI] [PubMed] [Google Scholar]
- 21.Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;40:338–353. doi: 10.1111/apt.12838. [DOI] [PubMed] [Google Scholar]
- 22.Baert F, Drobne D, Gils A, Vande Casteele N, Hauenstein S, Singh S, Lockton S, Rutgeerts P, Vermeire S. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol. 2014;12:1474–81.e2; quiz e91. doi: 10.1016/j.cgh.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 23.Laharie D, Chanteloup E, Chabrun E, Subtil C, Kowo M, El Hanafi K, DE Lédinghen V. The tolerance and efficacy of a postponed retreatment with infliximab in Crohn’s disease primary responders. Aliment Pharmacol Ther. 2009;29:1240–1248. doi: 10.1111/j.1365-2036.2009.03997.x. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y, Hirata S. Is it possible to withdraw biologics from therapy in rheumatoid arthritis? Clin Ther. 2013;35:2028–2035. doi: 10.1016/j.clinthera.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguchi T, Kawazoe Y, Kamoi K, Miyanaga M, Takase H, Sugita S, Mochizuki M. Clinical course of patients with Behçet’s uveitis following discontinuation of infliximab therapy. Jpn J Ophthalmol. 2014;58:75–80. doi: 10.1007/s10384-013-0283-3. [DOI] [PubMed] [Google Scholar]
- 26.Olivieri I, D’Angelo S, Padula A, Leccese P, Nigro A, Palazzi C. Can we reduce the dosage of biologics in spondyloarthritis? Autoimmun Rev. 2013;12:691–693. doi: 10.1016/j.autrev.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Vorselaars AD, Verwoerd A, van Moorsel CH, Keijsers RG, Rijkers GT, Grutters JC. Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. Eur Respir J. 2014;43:602–609. doi: 10.1183/09031936.00055213. [DOI] [PubMed] [Google Scholar]
- 28.Pittet V, Froehlich F, Maillard MH, Mottet C, Gonvers JJ, Felley C, Vader JP, Burnand B, Michetti P, Schoepfer A. When do we dare to stop biological or immunomodulatory therapy for Crohn’s disease? Results of a multidisciplinary European expert panel. J Crohns Colitis. 2013;7:820–826. doi: 10.1016/j.crohns.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 29.De Suray N, Salleron J, Vernier-Massouille G. Close monitoring of CRP and fecal calprotectin is able to predict clinical relapse in patients with Crohn’s disease in remission after infliximab withdrawal. A subanalysis of the STORI study. Gastroenterology. 2012;142:S–149. [Google Scholar]
- 30.Molnár T, Farkas K, Miheller P, Nyári T, Szepes Z, Herszényi L, Müzes G, Nagy F, Tulassay Z, Wittmann T. Is the efficacy of successful infliximab induction therapy maintained for one year lasting without retreatment in different behavior types of Crohn’s disease? J Crohns Colitis. 2008;2:322–326. doi: 10.1016/j.crohns.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Domènech E, Hinojosa J, Nos P, Garcia-Planella E, Cabré E, Bernal I, Gassull MA. Clinical evolution of luminal and perianal Crohn’s disease after inducing remission with infliximab: how long should patients be treated? Aliment Pharmacol Ther. 2005;22:1107–1113. doi: 10.1111/j.1365-2036.2005.02670.x. [DOI] [PubMed] [Google Scholar]
- 32.Dai C, Liu WX, Jiang M, Sun MJ. Mucosal healing did not predict sustained clinical remission in patients with IBD after discontinuation of one-year infliximab therapy. PLoS One. 2014;9:e110797. doi: 10.1371/journal.pone.0110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorrentino D, Paviotti A, Terrosu G, Avellini C, Geraci M, Zarifi D. Low-dose maintenance therapy with infliximab prevents postsurgical recurrence of Crohn’s disease. Clin Gastroenterol Hepatol. 2010;8:591–599.e1; quiz e78-79. doi: 10.1016/j.cgh.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Vande Casteele N, Gils A, Ballet V, Compernolle G, Peeters M, Van Steen K, Simoens S, Ferrante M, Van Assche G, Vermeire S, et al. Randomised controlled trial of drug level versus clinically based dosing of infliximab maintenance therapy in IBD: Final results of the TAXIT study. UEG J. 2013;1:A1. [Google Scholar]
- 35.Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014;13:24–30. doi: 10.1016/j.autrev.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Groupe d’Etude Therapeutique des Affections Inflammatoires Digestives. A proSpective Randomized Controlled Trial comParing infliximAb-antimetabolites Combination Therapy to Anti-metabolites monotheRapy and Infliximab monothErapy in Crohn’s Disease Patients in Sustained Steroid-free Remission on Combination Therapy (SPARE). Last Updated Date June 26, 2014. Available from: https://clinicaltrials.gov/ct2/show/record/NCT02177071?term=spare&rank=3.
- 37.Meuwis MA, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Piver E, Seidel L, Colombel JF, Louis E. Serum calprotectin as a biomarker for Crohn’s disease. J Crohns Colitis. 2013;7:e678–e683. doi: 10.1016/j.crohns.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Rajca S, Grondin V, Louis E, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, et al. Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in Crohn’s disease. Inflamm Bowel Dis. 2014;20:978–986. doi: 10.1097/MIB.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 39.Papamichail K, Arias M, Ferrante M, Ballet V, Claes K, Wollants WJ, Van Assche G, Rutgeerts PJ, Vermeire S. Fc gamma receptor mutations for prediction of sustained clinical remission after infliximab discontinuation in Crohn’s disease patients. UEG J. 2014;2:A374. [Google Scholar]


