Abstract
Utilization of mesenchymal stromal cells (MSCs) for the treatment of Crohn’s disease and ulcerative colitis is of translational interest. Safety of MSC therapy has been well demonstrated in early phase clinical trials but efficacy in randomized clinical trials needs to be demonstrated. Understanding MSC mechanisms of action to reduce gut injury and inflammation is necessary to improve current ongoing and future clinical trials. However, two major hurdles impede the direct translation of data derived from animal experiments to the clinical situation: (1) limitations of the currently available animal models of colitis that reflect human inflammatory bowel diseases (IBD). The etiology and progression of human IBD are multifactorial and hence a challenge to mimic in animal models; and (2) Species specific differences in the functionality of MSCs derived from mice versus humans. MSCs derived from mice and humans are not identical in their mechanisms of action in suppressing inflammation. Thus, preclinical animal studies with murine derived MSCs cannot be considered as an exact replica of human MSC based clinical trials. In the present review, we discuss the therapeutic properties of MSCs in preclinical and clinical studies of IBD. We also discuss the challenges and approaches of using appropriate animal models of colitis, not only to study putative MSC therapeutic efficacy and their mechanisms of action, but also the suitability of translating findings derived from such studies to the clinic.
Keywords: Mesenchymal stromal cells, Inflammatory bowel disease, Colitis, Animal model, Crohn’s disaese
Core tip: Several clinical trials have investigated the use of mesenchymal stromal cells (MSCs) for the treatment of inflammatory bowel disease. Although MSC therapy has proven to be safe, efficacy remains to be determined. Animal model studies are necessary to evaluate the efficacy and mechanism of action of MSCs, which will improve ongoing clinical trials. However, clinical translation is largely hampered by (1) variability of colitis animal models available; and (2) differences in the biology of murine and human MSC counterparts. Here we discuss the challenges and approaches of translating animal studies to clinical trials.
INTRODUCTION
Mesenchymal stromal cells (MSCs) are under intense clinical investigation worldwide for a number of regenerative and immune disorders. MSCs are attractive for cell therapy due to their immunomodulatory and regenerative properties and robust in vitro proliferative capacity[1,2]. Currently more than 300 clinical trials have been registered to test MSCs as therapeutics for various auto and alloimmune disorders such as inflammatory bowel diseases, graft vs host disease, multiple sclerosis, autoimmune rheumatic diseases, and autoimmune diabetes (clinicaltrials.gov). Isolation and expansion of MSCs for cell therapy can be achieved through harvesting various tissue sources such as bone marrow, adipose, umbilical cord and placenta[3,4]. The International Society for Cellular Therapy (ISCT) defined minimal criteria for the definition of multipotent MSCs such as (1) plastic adherence in standard in vitro culture conditions; (2) expression of CD105, CD73 and CD90, and lack of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR; and (3) differentiation to osteoblasts, adipocytes, and chondroblasts in vitro[5]. As an extension to these standards, ISCT has proposed further criteria for the immunological characterization of MSCs[6]. These include: (1) MSC response to the cytokines IFN-γ and TNF-α; (2) indoleamine 2,3-dioxygenase (IDO) response in cytokine licensing assays; (3) testing the functionality of the expanded cell product; (4) usage of purified immune responders in the functional assays; (5) analysis of mechanistic and efficacy studies of human MSCs in xenotransplantation models; (6) immune reaction to the infused MSCs; and (7) analysis of the lymphocyte populations of patients treated with MSCs[7]. Implementing some of these suggested analyses in preclinical studies involving animal models of tissue injury and inflammatory disorders will provide critical insight into MSC mechanisms of action and improve future clinical trials involving MSCs. Here we review the available data that utilize MSCs for mitigating colitis in animal models and highlight the challenges in translating these studies into effective clinical therapies.
CLINICAL TRIALS UTILIZING MSCs FOR INFLAMMATORY BOWEL DISEASES
Crohn’s disease (CD) and ulcerative colitis (UC) are inflammatory diseases of the gastrointestinal system caused by the multiple factors such as genetic susceptibility, breakdown of mucosal immune tolerance, and self-immune activation to gut microbiota[8,9]. Current treatment approaches are predominately aimed at suppressing overt inflammation and include the use of pharmacological agents, biologicals, and surgery to remove sections of inflamed bowel. However, these treatment modalities have limitations due to non-adherence and relapse[10]. In the past decade, alternative cell-based immunosuppressive therapies utilizing MSCs have been tested in clinical trials for both luminal and fistulizing forms of IBD[11,12]. Immunosuppressive and differentiation properties of MSCs are thought to play a major role in ameliorating luminal and fistula conditions, respectively[13]. Almost all the early phase clinical trials are aimed at determining the safety and tolerability of utilizing autologous and allogeneic adipose- or bone marrow-derived sources of MSCs for the treatment of IBD[13]. The results proved MSC therapy is safe but efficacy data remains elusive and conflicting clinical benefit has been reported so far. A large Phase III study for the treatment of complex cryptoglandular perianal fistulas using autologous adipose derived MSCs showed no efficacy[14]. In addition, a recent phase II placebo-controlled, parallel group, multi-center study to investigate the safety and efficacy of allogeneic Multistem®, stem cells derived from adult bone marrow and non-embryonic tissue sources, in subjects with moderate to severe ulcerative colitis showed no clinical benefit (NCT01240915). However, another earlier phase II clinical trial using allogeneic bone marrow-derived MSCs for refractory luminal CD demonstrated that 12 out of 15 patients showed reduction in CD activity index providing evidence of clinical efficacy[15]. Thus clinical trial provide conflicting results on the efficacy of MSCs, which is in contrast to the data derived from preclinical animal model studies. Most of the preclinical in vivo data with animal models of colitis demonstrated the consistent efficacy of MSC’s therapeutic properties. Hence, variations in the clinical outcome could be due to two major factors: (1) discrepancies in MSC source, preparation, and handling methods, all of which may affect the quality of the cellular product. Our group and others demonstrated that thawed MSCs from cryopreservation showed attenuated biodistribution and immunosuppressive properties compared to actively growing MSCs in in vitro cultures. This observation may provide an explanation for variations in industry sponsored clinical trial outcomes[16-18]. Similarly prolonged expansion of MSCs lead to a senescent phenotype, resulting in their failure to mitigate lethal endotoxima in animals[19]. Thus, cellular preparation methods are one of the determining factors of clinical outcome; and (2) variability in immune cellular responses among patients, a complexity not often seen in controlled animal models of inflammation. Etiology and prognosis of human IBD are multifactorial and hence animal models of colitis do not fully represent human IBD. For this reason, translation of cellular or biological therapy from animal model of colitis in to human inflammatory bowel disease remains a challenge. This notion is supported by the failure of biologicals to show clinical benefit despite proven efficacy in animal model studies[10,20]. In addition, MSCs derived from mice and humans do not share identical immunobiology and hence data derived from murine MSCs do not entirely inform MSC based clinical trials in human (Figure 1). Thus, the use of an appropriate and standardized MSC cellular infusion product in animal models of colitis that most closely replicates human bowel inflammation should yield data most suited for clinical translation.
Figure 1.
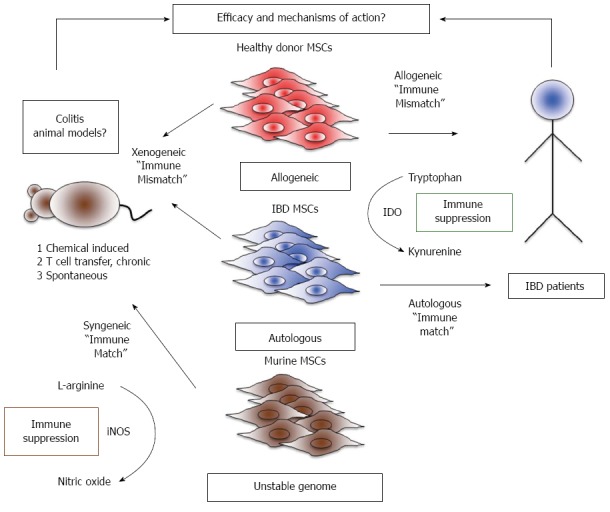
Challenges in modelling efficacy and mechanisms of action of mesenchymal stromal cells between preclinical and clinical studies. Right: Current clinical trials fall into two major groups: (1) allogeneic studies where inflammatory bowel diseases patients receive mesenchymal stromal cells (MSCs) from a random MHC unmatched randomdonor; and (2) autologous studies where patients are given their own MSCs. In human MSCs, immunosuppression is dependent on Indoleamine 2, 3 dioxygenase (IDO) activity, an enzyme that converts the essential amino acid tryptophan into the immunosuppressive catabolite, kynurenine. Left: Murine MSCs differ from human MSCs in their primary mechanism of immune suppression, utilizing inducible nitric oxide synthase (iNOS) to create nitric oxide as a product of L-arginine catabolism. The therapeutic effects of murine MSCs can be tested by engraftment into a colitic mouse, and is syngeneic if the MSCs are derived from a mouse with the same strain/background as the colitic mouse. However, murine MSCs may potentially transform after prolonged in vitro culture due to genomic instability. IBD: Inflammatory bowel diseases.
ANIMAL MODELS OF INFLAMMATORY BOWEL DISEASES
Animal models for colitis can be divided into: (1) chemical induced acute colitis; (2) adoptive T cell transfer mediated chronic colitis; and (3) spontaneous colitis (Figure 1 Left). Chemical induced colitis models commonly utilize 2,4,6, trinitrobenzene sulfonic acid (TNBS), oxazolone, and dextran sodium sulfate (DSS)[21]. In the TNBS model, colitis is induced in the rodents by intrarectal administration of alcohol containing TNBS. Alcohol serves not only as a carrier but also inflicts damage to the mucosal barrier. This aids TNBS in acting as a hapten for mucosal colonizing microbiota and subsequent Th1-dependent immune activation and infiltration of CD4+ T cells[22,23]. Studies in IFNγ-/- BALB/c mice demonstrated that TNBS induced colitis also involves the elaboration of Th2 cytokines, which is often seen in ulcerative colitis[24-26]. Although the TNBS model reflects human intestinal bowel inflammation to some degree, it is limited by the fact that it is a mouse strain dependent model and it does not depict the spontaneous relapse often seen in human ulcerative colitis[21,27]. Thus, while the TNBS induced colitis model can be used to study the potential efficacy of MSCs, the mechanism behind these effects may be difficult to elucidate due to the lack of involvement of entire lympho myeloid cellular compartments and disease reoccurrence seen in human disease. Similar to the TNBS colitis model, intrarectal administration of oxazolone as a hapten induces colitis in the animals but the inflammation is largely mediated by Th2 cytokines[28]. Hence this model is used to study Th2 associated inflammation in colitis, an effect more pronounced in UC compared to CD[29]. Human bone marrow derived MSCs induce Th2-dependent immune responses and have been shown to have therapeutic benefits in the animal model of multiple sclerosis. Currently, it is unknown whether human MSCs exert such an effect in colitic animals[30]. Due to the confounding nature of Th2 polarizing responses with both human MSCs and oxozalone-induced colitis, this model is not well suited for studying the mechanistic underpinnings of MSC therapy.
In the widely used DSS colitis model, mice consume drinking water containing DSS to induce colitis. DSS causes severe injury in the intestinal epithelium leading to the infiltration of polymorphonuclear leukocytes, intestinal wall thickening, diarrhea, and decreases in body weight[31]. DSS mediated acute colitis models do not faithfully recapitulate T cell mediated gut injury seen in human IBD. Moreover, the secondary inflammation seen in DSS induced colitis is mediated by innate immune cells (as opposed to T-cells) and hence it is not equivalent to IBD of humans[32]. Due to these limitations, caution needs to be drawn on utilizing this model to study both efficacy and mechanisms of action of MSCs. Alternatively, in the T cell transfer colitis model, adoptive transfer of flow cytometry sorted naïve CD4+CD45RBhi+ cells induce chronic colitis in severe combined immunodeficient (scid) mice, lacking endogenous T and B cells[33]. This model has the advantage to study early and late immunological changes associated with IBD. Adoptive transfer of CD4+CD25+ regulatory T cells ameliorates colitis induced by CD4+CD45RBhi+ cells, suggesting that this model may be well suited to study cell therapy[34]. However, unlike regulatory T cells, MSCs possess a larger array of immunoregulatory properties and lack of the involvement complex systemic immune response in scid mice makes this model difficult to study MSC’s mechanism of action.
An alternative approach to study chronic IBD progression is by examining the spontaneous colitis in genetic knockout animals lacking key immunoregulatory molecules. IL-10-/- mice are a perfect example for such studies, which develop spontaneous chronic colitis starting from week 4-6 to week 16-20[35,36]. Spontaneous colitis in IL-10-/- mice is analogous to chronic IBD seen in humans[37], which involves Th1 CD4+ T cells and the lack of mucosal immune tolerance due to the absence of immunosuppressive IL-10 cytokine[38]. Histopathological changes, total body weight loss, and IFN-γ and TNF-α upregulation have been reported in these animals during chronic colitis progression. The therapeutic effect of cytokines, antibodies, and chemical compounds have been tested in this model as a proof-of-concept in treating chronic IBD involving T cell mediated gut pathology[39-41]. Altogether, each colitis model has advantages and disadvantages for the study of MSC efficacy and mechanisms of action. Investigators may require the use of at least two of the models, for example an acute and a chronic colitis model, described above to validate their findings relevant to the clinical trials.
DIFFERENCES IN MSC BIOLOGY DERIVED FROM MICE AND HUMANS
MSC transplantation studies in the animal model of colitis can be divided into two categories (1) syngeneic/allogeneic; and (2) xenogeneic (Figure 1 Left). In the case of syngeneic/allogeneic studies, MSCs are derived from mouse species with/without difference in genetic background to the recipient animals. This is in contrast to xenogeneic studies, where human MSCs are used as a therapeutic agent in immune competent colitic animals. In syngeneic transplantation studies, MSCs are major histocompatibility complex (MHC) matched. These studies provide critical proof-of-concept data for clinical trials involving autologous MSC therapy. However, the major limitation for translating data from such studies to clinical trials is the differential characteristics of murine derived MSCs compared to human MSC counterparts. In vitro expansion and biological properties of MSCs are examples of such important differences. Human MSCs can readily be expanded ex vivo from the bone marrow or other tissues and can be cultured in basal growth medium. Indeed, robust in vitro proliferation properties of human MSCs make them an especially attractive cellular pharmaceutical for use in clinical trials. Despite their ability to proliferate in vitro, prolonged culture leads human MSCs to undergo cellular senescence[42]. This is in stark contrast to murine MSCs, which do not exhibit this property, as they do not proliferate in vitro like human MSCs. Moreover, murine MSCs are sensitive to oxygen and prolonged culture selects for hypoxia-resistant immortalized clones[43]. Immortalized MSCs with chromosomal aberrations show differential properties compared to primary MSCs[44], including reduced expressions of phenotype marker CD105, and go on to form osteosarcoma-like tumors when implanted into mice[45]. In addition, immortalized murine MSCs lose their multilineage differentiation potential in vitro and differentiate only into the osteoblastic lineage[44]. Hence, primary but not immortalized murine MSCs need to be used in the cell therapy experiments, posing a hurdle for the use of murine MSCs in animal model studies.
In addition to the phenotypical differences, murine and human MSCs differ in their mechanisms of immunosuppression. Human MSCs suppresses T cell proliferation through indoleamine 2,3-dioxygenase (IDO)[46,47]. IFN-γ secreted by activated T cells upregulates IDO expression in MSCs, an enzyme that converts tryptophan to the immunosuppressive metabolite, kynurenine[46]. Blocking of IDO activity abolishes MSC’s suppressive activity, which points to the immunological significance of this pathway in human MSCs[46-48]. Murine MSCs, on the other hand, do not suppress via IDO, alternatively utilizing nitric oxide (NO) to dampen inflammation[49,50]. Blocking of IFN-γ inducible nitric oxide synthase (iNOS) activity negates the suppressive capacity of murine MSCs[49,50]. Although, IDO knockout murine MSCs induce partial immune tolerance in mice with kidney allografts, IFN-γ does not induce IDO substantially in murine MSCs, suggesting a less important role for IDO in the immunobiology of murine derived MSCs[51]. While both of these enzymes exert their immunosuppressive effects differentially, studying the IDO pathway of human MSCs in a mouse model of colitis will likely yield the most relevant data for translation to clinical trials (Figure 1 Right).
Xenotransplantation methods afford an opportunity to study the effect of human MSCs in mice with colitis[52-58]. A recently published study from our group demonstrated that intravenously infused human MSCs in immune competent mice could be detected only up to 24 h in the lungs and not in the colon or any other organs in any time points tested[17]. Hence human MSCs get immune rejected rapidly in immune competent mice and studying their therapeutic effect despite of immune rejection is challenging. Another variable to be considered is the health status of the MSC donors. MSCs derived from patients with IBD or healthy individuals represent the cellular products used in autologous and allogeneic clinical trials, respectively. Currently, it is unclear if MSCs derived from IBD patients are functionally comparable to healthy donor MSCs. MSCs derived from patients with rheumatoid arthritis, immune thrombocytopenia, Gaucher’s Disease, Sjögren’s syndrome, Myelodysplastic syndromes, and systemic lupus erythematous show defective phenotype and function[59-63]. Although MSCs derived from Crohn’s patients show intact phenotype and function in one study, the mechanism of suppression was not established[64]. Defects in the autophagy pathway have been shown to predispose Crohn’s patients to disease progression, but whether such defects alter human MSC suppressive functions remain to be investigated[65]. Blocking of autophagy in murine MSCs actually enhances their immunosuppressive properties in T cell-mediated experimental autoimmune encephalomyelitis (EAE), a mouse model of the human demyelinating disease, multiple sclerosis[66]. It is unknown whether this observation can be translated to human MSCs and in patients with IBD. Further studies are required to understand the efficacy and mechanism of action of MSCs derived from patients with IBD, which inform insights for autologous MSC therapy for IBD (Figure 1 Right). In summary, both syngeneic and xenogeneic transplantation of MSCs from patients with IBD or random healthy donors in animal model of colitis have limitations, impeding the direct translation of the findings to the clinic.
EFFICACY AND MECHANISM OF ACTION OF MSCs IN MOUSE MODELS OF COLITIS
Efficacy of human MSCs derived from adipose tissue, umbilical cord and bone marrow has been shown for TNBS and DSS induced colitis in mice and rats[52-58]. Multiple mechanisms have been demonstrated such as induction of IL-10 secreting regulatory T cells[58], down regulation of IL-23/IL-17 regulated inflammatory reactions[57], and the modulation of Treg/Th17 cells[54], but the key factor in human MSCs responsible for therapeutic effect in colitic mice remains unknown. Hence, the efficacy of human MSCs in immune competent colitic mice is suggestive of the role of soluble factors produced by MSCs within a short period of time before clearance by the recipient immune system.
In an alternative approach in three independent studies, human MSCs preactivated in vitro with IL-1β, IFN-γ, and nucleotide-binding oligomerization domain 2 (NOD2)-activating ligands before engraftment showed enhanced therapeutic benefits[55,56,67]. Activation of NOD2 in human MSCs has been shown to suppress mononuclear cell proliferation through prostaglandin E2 production[55]. IL-1β primed MSCs express higher levels of CXCR4 on the surface and hence migrate to the inflammatory sites more efficiently[67]. Human MSCs pretreated with IFN-γ ameliorate colitis through the attenuation of Th1 inflammatory responses[56]. However, these studies still do not fully explain the mechanism of xenogeneic or allogeneic MSC mediated suppression of inflammation in colitic mice. Interestingly, IFN-γ pretreated syngeneic but not allogeneic MSCs ameliorate EAE[68]. In addition, allogeneic MSCs are immune rejected by MHC class I- and class II-mismatched recipient mice and hence they are not intrinsically immunoprivileged and cannot serve as a “universal donor” in immunocompetent MHC-mismatched recipients[69]. The therapeutic effect seen with xeno- and allogeneic transplantation studies in colitic mice can be described as a “hit and vanish” phenomenon of MSC action.
Biodistribution properties of MSCs are important for their therapeutic efficacy. MSC engraftment to the colon may play a part in their therapeutic effects as topical administration of MSCs engraft to the inflamed colon and attenuates inflammation[70]. Castelo-Branco et al[71] demonstrated that intraperitoneal but not intravenous injected cryopreserved allogeneic MSCs migrate to the colon and ameliorate TNBS colitis This study suggested the defective distribution of intravenously infused cryopreserved, allogeneic MSCs, ultimately resulting in their failure to engraft into the colon. In this study, distribution was affected by two factors: (1) cryopreservation; and (2) allogeneic source of the MSCs. Industry sponsored clinical trials largely utilize thawed MSCs from cryopreservation while most preclinical studies utilize live MSCs from culture[72]. Our group has demonstrated that cryopreserved MSCs exhibit a heat shock response and defective actin polymerization, which affects their immunosuppressive and engraftment properties[16,17]. Altogether, cell preparation, handling methods, and the MHC matching of the cellular product likely affects the ensuing clinical outcome. How these factors mechanistically alter the disease course in colitic animals require further investigation.
APPROACHES TO STUDY THE MECHANISM OF ACTION OF MSCs IN MOUSE MODELS OF COLITIS
Two approaches can be employed to study the mechanism of action of MSCs in animal models of colitis, which can be translated to human MSC based clinical trials. (1) overexpression of key effector pathways specific to human MSCs in their murine counterparts; and (2) investigation of pathways shared by human and murine MSCs. Ling et al[73] pioneered the first approach by overexpressing human IDO protein in murine MSCs. The human IDO gene was cloned downstream of the murine iNOS promoter and transfected into MSCs derived from iNOS knockout animals. In this way, inflammatory stimuli induced transcription of human IDO transcription in place of iNOS. These IDO humanized murine MSCs were able to inhibit T cell proliferation and blockade of IDO activity with 1-methyl tryptophan (1-MT) abolished their suppressive effects. In addition, IDO overexpressing murine MSCs promoted tumor growth in melanoma and lymphoma models, an effect that was also reversed by 1-MT administration. While this is a novel approach to study human specific effector pathways in murine MSCs, caution needs to be taken when such MSCs are generated since the genome of murine MSCs is unstable and may result in the transformation of these primary MSCs into immortalized cells. In addition, transfection of primary cells and subsequent culture under selection pressure to generate clones may lead to the loss of key immunosuppressive and regenerative properties. Despite these shortcomings, such an approach is important for identifying the in vivo characteristics of a critical immunoregulatory pathway specific to human MSCs.
In support of the second approach, we studied the in vivo significance of an effector pathway shared between mouse and human MSCs. Both human and murine MSCs secrete high levels of IL-6. When BALB/c mice were sublethally irradiated and subsequently given single intraperitoneal injection of MHC mismatched C57Bl/6 MSCs, mortality was reduced in a dose dependent manner[74]. This effect was abrogated when similarly conditioned mice were given MSCs from IL-6-/- animals. MSCs accelerated the recovery of damaged intestinal epithelium by stimulating the proliferation of intestinal crypt cell pool. Thus, IL-6 produced by murine MSCs ameliorate gut injury in the absence of immune rejection in irradiated mice[74]. However MSCs contain a large array of immunoregulatory and regenerative molecules and some of these are specific to murine immunobiology (e.g., iNOS), which could dominate the effect of other pathways that are shared by human and mice. The field of comparative MSC biology to identify shared and differentially operating pathways between mice and human is still in its infancy and requires further investigation. Regardless of the approach used in these mechanistic assays, in vivo colitic animal model studies are required for their validation as a companion to translation into clinical trials.
CONCLUSION
MSCs are an attractive agent for cell-based therapies not only for IBD, but also other auto- and allo-immune ailments. Lack of a comprehensive, comparative characterization of murine and human MSCs impede the direct translation of the preclinical animal model findings to clinical trials. Conflicting results seen in MSC efficacy studies are likely to reflect differences in MSC source, cell preparation and handling methods. Utilization of appropriate animal models and MSCs derived from animal and human origin to address these issues will help to understand their mechanism of action in inflammatory and regenerative settings.
Footnotes
Conflict-of-interest: Raghavan Chinnadurai, Spencer Ng, Vijayakumar Velu, and Jacques Galipeau have no conflict of interest to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 9, 2014
First decision: January 22, 2015
Article in press: March 27, 2015
P- Reviewer: Miheller P, Naito Y, Sorrentino D, Stocco G, Yamakawa M S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013;13:856–867. doi: 10.2174/1566524011313050016. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 3.Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19:1449–1470. doi: 10.1089/scd.2010.0140. [DOI] [PubMed] [Google Scholar]
- 4.Hoogduijn MJ, Betjes MG, Baan CC. Mesenchymal stromal cells for organ transplantation: different sources and unique characteristics? Curr Opin Organ Transplant. 2014;19:41–46. doi: 10.1097/MOT.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 5.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 6.Phinney DG, Galipeau J, Krampera M, Martin I, Shi Y, Sensebe L. MSCs: science and trials. Nat Med. 2013;19:812. doi: 10.1038/nm.3220. [DOI] [PubMed] [Google Scholar]
- 7.Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054–1061. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 10.Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut. 2012;61:918–932. doi: 10.1136/gutjnl-2011-300904. [DOI] [PubMed] [Google Scholar]
- 11.Ricart E, Jauregui-Amezaga A, Ordás I, Pinó S, Ramírez AM, Panés J. Cell therapies for IBD: what works? Curr Drug Targets. 2013;14:1453–1459. doi: 10.2174/13894501113146660234. [DOI] [PubMed] [Google Scholar]
- 12.Voswinkel J, Francois S, Simon JM, Benderitter M, Gorin NC, Mohty M, Fouillard L, Chapel A. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:180–192. doi: 10.1007/s12016-012-8347-6. [DOI] [PubMed] [Google Scholar]
- 13.van Deen WK, Oikonomopoulos A, Hommes DW. Stem cell therapy in inflammatory bowel disease: which, when and how? Curr Opin Gastroenterol. 2013;29:384–390. doi: 10.1097/MOG.0b013e328361f763. [DOI] [PubMed] [Google Scholar]
- 14.Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Dis Colon Rectum. 2012;55:762–772. doi: 10.1097/DCR.0b013e318255364a. [DOI] [PubMed] [Google Scholar]
- 15.Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 16.François M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy. 2012;14:147–152. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinnadurai R, Garcia MA, Sakurai Y, Lam WA, Kirk AD, Galipeau J, Copland IB. Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Reports. 2014;3:60–72. doi: 10.1016/j.stemcr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, Hamad OA, Hinsch R, Ignatowicz L, Locke M, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32:2430–2442. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobrowolska G, Boldyreff B, Issinger OG. Cloning and sequencing of the casein kinase 2 alpha subunit from Zea mays. Biochim Biophys Acta. 1991;1129:139–140. doi: 10.1016/0167-4781(91)90230-j. [DOI] [PubMed] [Google Scholar]
- 20.Auer K, Trachter R, Van den Bogaerde J, Bassaganya-Riera J, Sorrentino D. Translational research and efficacy of biologics in Crohn’s disease: a cautionary tale. Expert Rev Clin Immunol. 2014;10:219–229. doi: 10.1586/1744666X.2014.877839. [DOI] [PubMed] [Google Scholar]
- 21.Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18:279–288. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 23.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 24.Dohi T, Fujihashi K, Kiyono H, Elson CO, McGhee JR. Mice deficient in Th1- and Th2-type cytokines develop distinct forms of hapten-induced colitis. Gastroenterology. 2000;119:724–733. doi: 10.1053/gast.2000.16500. [DOI] [PubMed] [Google Scholar]
- 25.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 26.Inoue S, Matsumoto T, Iida M, Mizuno M, Kuroki F, Hoshika K, Shimizu M. Characterization of cytokine expression in the rectal mucosa of ulcerative colitis: correlation with disease activity. Am J Gastroenterol. 1999;94:2441–2446. doi: 10.1111/j.1572-0241.1999.01372.x. [DOI] [PubMed] [Google Scholar]
- 27.Motavallian-Naeini A, Andalib S, Rabbani M, Mahzouni P, Afsharipour M, Minaiyan M. Validation and optimization of experimental colitis induction in rats using 2, 4, 6-trinitrobenzene sulfonic acid. Res Pharm Sci. 2012;7:159–169. [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima R, Kuroda S, Ohkishi T, Nakamaru K, Hatakeyama S. Oxazolone-induced colitis in BALB/C mice: a new method to evaluate the efficacy of therapeutic agents for ulcerative colitis. J Pharmacol Sci. 2004;96:307–313. doi: 10.1254/jphs.fp0040214. [DOI] [PubMed] [Google Scholar]
- 29.Fuss IJ. Is the Th1/Th2 paradigm of immune regulation applicable to IBD? Inflamm Bowel Dis. 2008;14 Suppl 2:S110–S112. doi: 10.1002/ibd.20683. [DOI] [PubMed] [Google Scholar]
- 30.Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, Miller RH. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whittem CG, Williams AD, Williams CS. Murine Colitis modeling using Dextran Sulfate Sodium (DSS) J Vis Exp. 2010;(35):1652. doi: 10.3791/1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 34.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 35.Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy RJ, Hoper M, Deodhar K, Erwin PJ, Kirk SJ, Gardiner KR. Interleukin 10-deficient colitis: new similarities to human inflammatory bowel disease. Br J Surg. 2000;87:1346–1351. doi: 10.1046/j.1365-2168.2000.01615.x. [DOI] [PubMed] [Google Scholar]
- 38.Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kühn R, Müller W, Berg DJ, Rennick DM. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubé PE, Yan F, Punit S, Girish N, McElroy SJ, Washington MK, Polk DB. Epidermal growth factor receptor inhibits colitis-associated cancer in mice. J Clin Invest. 2012;122:2780–2792. doi: 10.1172/JCI62888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolachala V, Ruble B, Vijay-Kumar M, Wang L, Mwangi S, Figler H, Figler R, Srinivasan S, Gewirtz A, Linden J, et al. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol. 2008;155:127–137. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med. 2005;202:1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 43.Krishnappa V, Boregowda SV, Phinney DG. The peculiar biology of mouse mesenchymal stromal cells--oxygen is the key. Cytotherapy. 2013;15:536–541. doi: 10.1016/j.jcyt.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Romieu-Mourez R, Coutu DL, Galipeau J. The immune plasticity of mesenchymal stromal cells from mice and men: concordances and discrepancies. Front Biosci (Elite Ed) 2012;4:824–837. doi: 10.2741/E422. [DOI] [PubMed] [Google Scholar]
- 45.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 46.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 47.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 48.Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J Immunol. 2014;192:1491–1501. doi: 10.4049/jimmunol.1301828. [DOI] [PubMed] [Google Scholar]
- 49.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Ren G, Su J, Zhang L, Zhao X, Ling W, L’huillie A, Zhang J, Lu Y, Roberts AI, Ji W, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 51.Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90:1312–1320. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- 52.Lin Y, Lin L, Wang Q, Jin Y, Zhang Y, Cao Y, Zheng C. Transplantation of human umbilical mesenchymal stem cells attenuates dextran sulfate sodium-induced colitis in mice. Clin Exp Pharmacol Physiol. 2015;42:76–86. doi: 10.1111/1440-1681.12321. [DOI] [PubMed] [Google Scholar]
- 53.Robinson AM, Sakkal S, Park A, Jovanovska V, Payne N, Carbone SE, Miller S, Bornstein JC, Bernard C, Boyd R, et al. Mesenchymal stem cells and conditioned medium avert enteric neuropathy and colon dysfunction in guinea pig TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1115–G1129. doi: 10.1152/ajpgi.00174.2014. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Liu S, Xu Y, Zhang A, Jiang J, Tan W, Xing J, Feng G, Liu H, Huo F, et al. Human umbilical cord-derived mesenchymal stem cells downregulate inflammatory responses by shifting the Treg/Th17 profile in experimental colitis. Pharmacology. 2013;92:257–264. doi: 10.1159/000354883. [DOI] [PubMed] [Google Scholar]
- 55.Kim HS, Shin TH, Lee BC, Yu KR, Seo Y, Lee S, Seo MS, Hong IS, Choi SW, Seo KW, et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013;145:1392–403.e1-8. doi: 10.1053/j.gastro.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 56.Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, et al. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549–1558. doi: 10.1002/stem.698. [DOI] [PubMed] [Google Scholar]
- 57.Liang L, Dong C, Chen X, Fang Z, Xu J, Liu M, Zhang X, Gu DS, Wang D, Du W, et al. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transplant. 2011;20:1395–1408. doi: 10.3727/096368910X557245. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 59.Papadaki HA, Kritikos HD, Gemetzi C, Koutala H, Marsh JC, Boumpas DT, Eliopoulos GD. Bone marrow progenitor cell reserve and function and stromal cell function are defective in rheumatoid arthritis: evidence for a tumor necrosis factor alpha-mediated effect. Blood. 2002;99:1610–1619. doi: 10.1182/blood.v99.5.1610. [DOI] [PubMed] [Google Scholar]
- 60.Ma J, Ning YN, Xu M, Hou Y, Wang N, Hou XY, Yu YY, Li H, He WD, Shao LL, et al. Thalidomide corrects impaired mesenchymal stem cell function in inducing tolerogenic DCs in patients with immune thrombocytopenia. Blood. 2013;122:2074–2082. doi: 10.1182/blood-2013-03-491555. [DOI] [PubMed] [Google Scholar]
- 61.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120:3142–3151. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavlaki K, Pontikoglou CG, Demetriadou A, Batsali AK, Damianaki A, Simantirakis E, Kontakis M, Galanopoulos A, Kotsianidis I, Kastrinaki MC, et al. Impaired proliferative potential of bone marrow mesenchymal stromal cells in patients with myelodysplastic syndromes is associated with abnormal WNT signaling pathway. Stem Cells Dev. 2014;23:1568–1581. doi: 10.1089/scd.2013.0283. [DOI] [PubMed] [Google Scholar]
- 63.Tang Y, Ma X, Zhang H, Gu Z, Hou Y, Gilkeson GS, Lu L, Zeng X, Sun L. Gene expression profile reveals abnormalities of multiple signaling pathways in mesenchymal stem cell derived from patients with systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:826182. doi: 10.1155/2012/826182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernardo ME, Avanzini MA, Ciccocioppo R, Perotti C, Cometa AM, Moretta A, Marconi M, Valli M, Novara F, Bonetti F, et al. Phenotypical/functional characterization of in vitro-expanded mesenchymal stromal cells from patients with Crohn’s disease. Cytotherapy. 2009;11:825–836. doi: 10.3109/14653240903121260. [DOI] [PubMed] [Google Scholar]
- 65.Scharl M, Rogler G. Inflammatory bowel disease: dysfunction of autophagy? Dig Dis. 2012;30 Suppl 3:12–19. doi: 10.1159/000342588. [DOI] [PubMed] [Google Scholar]
- 66.Dang S, Xu H, Xu C, Cai W, Li Q, Cheng Y, Jin M, Wang RX, Peng Y, Zhang Y, et al. Autophagy regulates the therapeutic potential of mesenchymal stem cells in experimental autoimmune encephalomyelitis. Autophagy. 2014;10:1301–1315. doi: 10.4161/auto.28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473–481. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rafei M, Birman E, Forner K, Galipeau J. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 2009;17:1799–1803. doi: 10.1038/mt.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 70.Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N, et al. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523–531. doi: 10.1124/jpet.108.137083. [DOI] [PubMed] [Google Scholar]
- 71.Castelo-Branco MT, Soares ID, Lopes DV, Buongusto F, Martinusso CA, do Rosario A, Souza SA, Gutfilen B, Fonseca LM, Elia C, et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. doi: 10.1371/journal.pone.0033360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galipeau J. The mesenchymal stromal cells dilemma--does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, Rabson AB, Roberts AI, Wang Y, Shi Y. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 2014;74:1576–1587. doi: 10.1158/0008-5472.CAN-13-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.François M, Birman E, Forner KA, Gaboury L, Galipeau J. Adoptive transfer of mesenchymal stromal cells accelerates intestinal epithelium recovery of irradiated mice in an interleukin-6-dependent manner. Cytotherapy. 2012;14:1164–1170. doi: 10.3109/14653249.2012.684378. [DOI] [PubMed] [Google Scholar]


