Abstract
AIM: To compare ultrasound-based acoustic structure quantification (ASQ) with established non-invasive techniques for grading and staging fatty liver disease.
METHODS: Type 2 diabetic patients at risk of non-alcoholic fatty liver disease (n = 50) and healthy volunteers (n = 20) were evaluated using laboratory analysis and anthropometric measurements, transient elastography (TE), controlled attenuation parameter (CAP), proton magnetic resonance spectroscopy (1H-MRS; only available for the diabetic cohort), and ASQ. ASQ parameters mode, average and focal disturbance (FD) ratio were compared with: (1) the extent of liver fibrosis estimated from TE and non-alcoholic fatty liver disease (NAFLD) fibrosis scores; and (2) the amount of steatosis, which was classified according to CAP values.
RESULTS: Forty-seven diabetic patients (age 67.0 ± 8.6 years; body mass index 29.4 ± 4.5 kg/m²) with reliable CAP measurements and all controls (age 26.5 ± 3.2 years; body mass index 22.0 ± 2.7 kg/m²) were included in the analysis. All ASQ parameters showed differences between healthy controls and diabetic patients (P < 0.001, respectively). The ASQ FD ratio (logarithmic) correlated with the CAP (r = -0.81, P < 0.001) and 1H-MRS (r = -0.43, P = 0.004) results. The FD ratio [CAP < 250 dB/m: 107 (102-109), CAP between 250 and 300 dB/m: 106 (102-114); CAP between 300 and 350 dB/m: 105 (100-112), CAP ≥ 350 dB/m: 102 (99-108)] as well as mode and average parameters, were reduced in cases with advanced steatosis (ANOVA P < 0.05). However, none of the ASQ parameters showed a significant difference in patients with advanced fibrosis, as determined by TE and the NAFLD fibrosis score (P > 0.08, respectively).
CONCLUSION: ASQ parameters correlate with steatosis, but not with fibrosis in fatty liver disease. Steatosis estimation with ASQ should be further evaluated in biopsy-controlled studies.
Keywords: Transient elastography, Non-alcoholic fatty liver disease, Liver stiffness, Non-alcoholic fatty liver disease, Fibrosis score, Controlled attenuation parameter
Core tip: Non-invasive characterization of hepatic steatosis and fibrosis is becoming important for the screening, diagnosis, and monitoring of patients with chronic liver diseases. This work compared acoustic structure quantification (ASQ) and established non-invasive methods to characterize fatty liver disease. ASQ parameters differed between healthy controls and diabetic patients with fatty liver disease independent of the extent of fibrosis. The focal disturbance ratio and further ASQ parameters correlated with the severity of steatosis. Therefore, ASQ could be used to evaluate steatosis and merits further investigation; however, ASQ seems to be impractical to characterize fibrosis in patients with fatty liver disease.
INTRODUCTION
Non-invasive characterization of hepatic steatosis and fibrosis are attracting growing scientific and medical interest for the screening, diagnosis, and monitoring of patients with chronic liver diseases[1-3]. In particular, fibrosis staging by means of elastography is highly accurate for detecting advanced liver injury and has, therefore, been progressively implemented in clinical practice[1].
In addition to fibrosis characterization, non-invasive quantification of hepatic fat content has attracted increasing attention in terms of both risk assessment and monitoring of patients with non-alcoholic fatty liver disease (NAFLD) and liver diseases from other etiologies[1,4]. The controlled attenuation parameter (CAP) - computed by commercial software that comes with the transient elastography device (Fibroscan) - correlates steatosis with the signal attenuation during liver stiffness measurement (LSM), and is very accurate to detect advanced hepatic steatosis[5-9]. Moreover, magnetic resonance based techniques (e.g., proton magnetic resonance spectroscopy, 1H-MRS) allow quantification of the hepatic lipid fraction, an additional parameter of the severity of steatosis[10,11]. However, these approaches are affected by high costs and limited availability (1H-MRS) or by anthropometry (CAP)[4,8].
Analysis of B-Mode ultrasound may represent a further option for the non-invasive grading and staging of liver damage, as conventional sonography is highly sensitive to subtle changes in tissue texture[12,13]. However, comparison of gray scale images is operator-dependent and also varies with some technical parameters[12]. Computerized analysis of acoustic tissue properties may overcome these limitations: acoustic structure quantification (ASQ) software analyzes the characteristic intensity pattern (“speckles”) of conventional B-Mode ultrasound and compares its distribution histogram with a theoretical probability density function (PDF, Rayleigh distribution) of the echo amplitude[13,14]. Histology-based studies have reported that ASQ parameters correlate with the degree of fibrosis in patients with liver diseases of different etiologies[13-15]. Moreover, a pilot study revealed strong agreement between ASQ values and hepatic fat accumulation in an animal model[16].
ASQ results have not yet been compared with those of established non-invasive techniques. Therefore, we prospectively compared ASQ measurements in healthy controls and diabetic patients at risk for NAFLD or associated liver injury with TE and CAP values (reference standards) as well as 1H-MRS results and serum based NAFLD fibrosis scores.
MATERIALS AND METHODS
Patients and controls
The study was reviewed and approved by the local ethics committee (University of Leipzig, register no. 358/08-B-ff and No. 419-12-17122012) and performed in accordance with the ethical guidelines of the Helsinki Declaration. Written informed consent was obtained from all participants before study enrollment.
Between April 2013 and March 2014, outpatients with type 2 diabetes mellitus at risk of NAFLD and without other liver diseases were recruited. For the control group, healthy volunteers with no history of any chronic liver disease, diabetes mellitus, or metabolic syndrome, were recruited. Increased ultrasound echogenicity of the liver parenchyma (compared with the right renal cortex) or CAP > 252 dB/m were regarded as exclusion criteria for the control group[5]. Significant alcohol intake (weekly consumption > 210 g for men and 140 g for woman, respectively) was ruled out for the total study cohort before inclusion[17].
For all participants, anthropometric examination, ultrasound, LSM (transient elastography, M probe), CAP assessment and laboratory parameters (patients only) were performed on the same day after a fasting period of at least three hours. 1H-MRS was performed either on the same day or after 6 mo during follow-up visits.
Laboratory assessment and NAFLD fibrosis score
Albumin, blood count, and serum levels of aminotransferases (ALT and AST) were determined for all diabetic patients. Individuals with highly elevated aminotransferases (at least five times the upper normal limit) were excluded from further examinations because of the risk of inaccurate LSM.
The NAFLD fibrosis score was calculated according to Angulo et al[18]: Score = -1.675 + 0.037 × age (years) + 0.094 × body mass index (kg/m2) + 1.13 × diabetes (yes 1, no 0) + 0.99 × AST/ALT ratio - 0.013 × platelet (Gpt/l) - 0.66 × albumin (g/dL). A score of at least 0.676 indicated high risk of advanced liver fibrosis[18].
Ultrasound, elastography, and controlled attenuation parameter
All subjects underwent conventional ultrasound to rule out mechanical cholestasis. LSM was performed using the M probe of transient elastography (TE; Fibroscan, Echosens, Paris, France). According to the manufacturer’s recommendation, examinations with fewer than 10 valid measurements or an interquartile range > 30% of the median LSM value (only in cases with liver stiffness ≥ 7.1 kPa) were excluded from further analysis[19]. TE values ≥ 7.9 kPa indicated the presence of advanced fibrosis[20].
The CAP gives additional information about the attenuation of ultrasonic signals during TE examination. CAP computation is included in the TE software, and the results (CAP, LSM) are shown together[21]. CAP was only considered when TE measurements were valid and reliable[5,21]. CAP served as reference method; therefore, only cases with valid CAP results were included in the final analysis. CAP values ≥ 252 dB/m indicated fatty liver disease[5], and values ≥ 300 dB/m were regarded as advanced steatosis[8]. No patient had values between 250 and 252 dB/m; therefore, the diabetic subjects were classified into four groups to better present the data: (1) CAP < 250 dB/m; (2) CAP ≥ 250 and < 300 dB/m; (3) CAP ≥ 300 and < 350 dB/m; and (4) CAP ≥ 350 dB/m.
Acoustic structure quantification
Analytical method: The ASQ software analyzes the linear raw data from ultrasound B-mode images. It provides a number of tissue parameters that are related to the scattering of echoes in user-defined region of interests (ROI). The histogram of the measured intensity distribution in the B-mode image ROI is essentially compared with the PDF of an ideal, homogenous scatterer (Rayleigh distribution)[22]. ASQ subdivides the primary ROI into a large number of (up to 1000) secondary ROIs and displays the so-called C2-histogram of the distribution of frequency ratios given by frequency of the ratio
C2 = σ2/σR2, where σ and σR stand for the standard deviations of measured and estimated (Rayleigh) PDFs, respectively[13]. In the liver, ASQ uses an empirical cut-off parameter α and reports slightly modified parameters σ m and Cm2 instead to minimize the influence of individual strong scatterers[13].
Mode (value with highest appearance, “peak value”), average and SD are then derived from the C2 or Cm2 histogram. A more recent ASQ implementation computes two curves (displayed in red and blue), depending on whether the variation in α quantitatively changes the σ parameter by less (depicted in red) or more (depicted in blue) than an empirical percentage (20%), respectively. The focal disturbance (FD) ratio is then defined as the ratio of the areas under the curve between the red and blue curve[16].
ASQ imaging: In the present study, B-mode images of the right liver lobe were recorded in the region of LSM and CAP acquisition using a curved array transducer (PVT-375 BT 3.5 MHz) and an Aplio 500 ultrasound scanner (Toshiba Medical Systems, Otawara, Japan). Display depth and focus were fixed at 10 and 5.5 cm, respectively. The ultrasound signal gain was set to 90%. A raw data loop of 2-3 s was recorded using the ASQ preset mode and files were exported to a PC workstation in DICOM format. ASQ was analyzed with the vendor’s software (PC-ASQR, Aplio500, Version 1.01R000). Mode, average and FD ratio were computed in four ROIs covering most of the liver parenchyma, but avoiding larger vessels and bile ducts (Figure 1). The respective mean ASQ values of five separate frames were computed to obtain more reliable measures for each subject.
Figure 1.
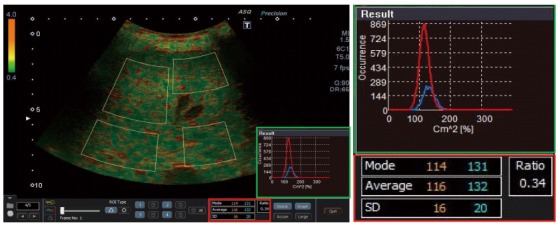
Acoustic structure quantification analysis of liver tissue. Four separate regions of interest were drawn to cover most of the parenchyma, but avoiding large vessels. The acoustic structure quantification software automatically calculated the parameters mode, average and standard deviation for both curves of the histogram (green frame), as well as the ratio of the areas under both curves (focal disturbance ratio, red frame).
Magnetic resonance spectroscopy
The majority of patients (79%) underwent 1H-MRS on the same or the following day of ASQ and TE examinations. In 21% of the cases, the time interval between ultrasound examination and 1H-MRS was 6.5 mo (range: 6.2-7.3 mo) for technical reasons.
1H-MRS was performed as described previously with some technical modifications[8]. In brief, T2-corrected, single-voxel MR spectra were acquired on a 1.5-T scanner (Achieva XR, Philips Healthcare, Best, Netherlands) using local shimming and a stimulated-echo acquisition method (STEAM). Voxels sized 20 mm × 20 mm × 20 mm were placed in liver segment VII, avoiding larger bile ducts and vessels. Spectroscopic data were acquired without water suppression, using the following sequence parameters: repetition time, TR = 3.000 ms; number of echoes, 5; echo times 10-50 ms; 2048 data points; bandwidth 1.000 Hz/pixel; 40 averages; total acquisition time 270 s. MR spectra were analyzed using a commercial tool (LCModel 6.3, Oakville, Canada) that determines the relative hepatic lipid concentrations. Calculated areas of water and fat peaks were corrected for T2 relaxation (using MR spectra at different echo times) and were used to calculate the hepatic fat fraction[8].
Data processing and statistical methods
All parameters were recorded in a spreadsheet file (Microsoft Excel, Microsoft). Statistical testing was carried out using commercial software (MedCalc 14.12, MedCalc Software, Ostend, Belgium). Data were expressed either as mean ± SD or median and range, as appropriate.
Fisher’s exact test and χ2 tests were used to test for the association of variables. Nonparametric tests (Mann-Whitney U test, Kruskal-Wallis test) were used to compare median values of independent samples, where post-hoc pair-wise comparisons were performed according to Conover[23]. For mean values, the t-test was applied. The Pearson correlation coefficient was calculated to analyze the degree of association between two variables. P values < 0.05 indicated a significant difference. Diagnostic performance of ASQ parameters was analyzed using receiver operating characteristic (ROC) curves.
The statistical methods of this study were reviewed by PD Dr. David Petroff (IFB Adiposity Diseases, Leipzig University Medical Center/Clinical Trial Center, University of Leipzig, Leipzig, Germany).
RESULTS
Clinical characteristics of the study cohort
Fifty patients with type 2 diabetes mellitus and 20 healthy volunteers were recruited. TE and CAP were available in 47 of the diabetic patients (94%): three subjects had an invalid measurement (two males, all cases with fewer than ten valid shots) and where excluded from further analysis. 1H-MRS was available for n = 43 diabetic patients because of contraindications and technical reasons in four cases. The characteristics of the analyzed cohort are displayed in Table 1.
Table 1.
Clinical characteristics of the study cohort n (%)
| Parameter | Healthy controls | Type 2 diabetes mellitus |
| Sex (F/M) | 10/10 | 23/24 |
| Age (yr) | 26.5 ± 3.2 | 67.0 ± 8.6b |
| Body mass index (kg/m²) | 22.0 ± 2.7 | 29.4 ± 4.5b |
| < 25 kg/m² | 19 (95) | 7 (15) |
| 25-30 kg/m² | 1 (5) | 21 (45) |
| > 30 kg/m² | - | 19 (40) |
| Waist-to-hip ratio | 0.85 ± 0.08 | 0.97 ± 0.08b |
| < 1.0 | 19 (95) | 27 (57%) |
| ≥ 1.0 | 1 (5) | 20 (43%) |
| CAP (dB/m) | 192 (151-237) | 329 (100-396)d |
| < 250 dB/m | 20 (100) | 6 (13) |
| 250-300 dB/m | - | 10 (21) |
| 300-350 dB/m | - | 19 (40) |
| > 350 dB/m | - | 12 (26) |
P < 0.01 vs controls;
P < 0.01 vs controls by design. Controls were excluded if CAP > 252 dB/m. CAP: Controlled attenuation parameter.
Non-invasive fibrosis estimation with ASQ vs TE and NAFLD score
LSM values (logarithmic) showed good correlation with NAFLD fibrosis scores: r = 0.46 (0.20; 0.66), P = 0.0012. None of the healthy controls had elevated TE, whereas advanced hepatic fibrosis was considerable in the diabetic cohort: ten patients (21%) had LSM > 7.9 kPa and 12 patients (26%) had NAFLD scores > 0.676.
The association of ASQ parameters mode, average, and FD ratio was analyzed according to the risk of hepatic fibrosis. All parameters showed differences between healthy controls and diabetic patients (Figures 1 and 2). No significant association of mode and average for both (blue and red) C2-histogram curves was observed in diabetic patients, independent of the presence of fibrosis as defined by TE (Figure 2) or NAFLD score (P-values >0.08, respectively). In addition, FD ratios did not differ between both groups of diabetic patients: 0.060 (0.044; 0.076) vs 0.053 (0.037; 0.089) (TE cut-off, P = 0.640) and 0.059 (0.030; 0.125) vs 0.057 (0.047; 0.071) (NAFLD fibrosis score cut-off, P = 0.946), respectively.
Figure 2.
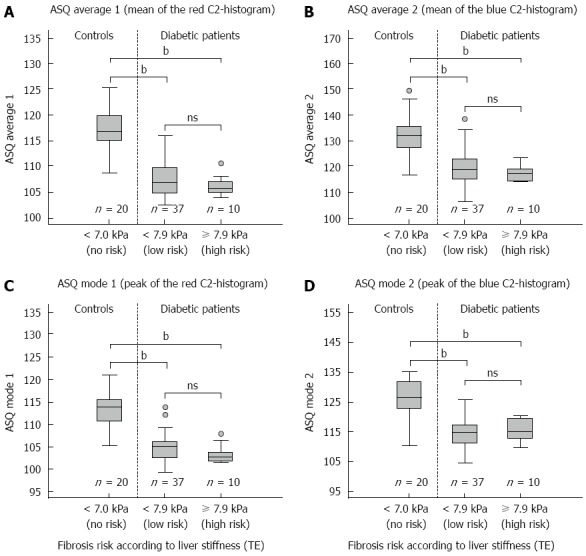
Correlation of acoustic structure quantification parameters with liver stiffness. Acoustic structure quantification (ASQ) parameters mode and the average of the "red" (A, C) and "blue" C2-histogram (B, D) show no significant differences between diabetic patients with and without increased liver stiffness (transient elastography, TE). The differences of all parameters between both patient groups and healthy controls (bP < 0.01 vs control, Mann-Whitney U test) suggest an influence of diabetes-related anthropometric and metabolic factors, e.g., steatosis, on ASQ analysis.
Non-invasive steatosis characterization with ASQ vs CAP and 1H-MRS
Diabetic patients were classified according to the degree of fatty liver disease, defined by CAP values: only n = 6 cases were at low risk for fatty liver (CAP < 250 dB m), whereas n = 33 were above the highly specific cut-off level (CAP > 300 dB/m) for advanced hepatic steatosis[8]. Gender distribution did not differ between healthy controls and the four diabetes subgroups, whereas BMI was increased in patients with advanced hepatic steatosis (Table 2). Furthermore, 1H-MRS revealed a positive correlation between CAP and the hepatic fat fraction [n = 43; r = 0.5 (0.31; 0.73), P < 0.001] (cf. also Table 2).
Table 2.
Distribution of anthropometry, hepatic lipid content, fibrosis risk and acoustic structure quantification parameters in different grades of fatty liver n (%)
| Parameter | Controls |
Type 2 diabetes mellitus |
|||
| CAP < 250 dB/m | CAP 250-< 300 dB/m | CAP 300-< 350 dB/m | CAP ≥ 350 dB/m | ||
| Sex (F/M) | 10/10 | 2/4 | 6/4 | 8/11 | 7/5 |
| Body mass index (kg/m²)b | 22.0 ± 2.7 | 26.7 ± 4.6 | 28.3 ± 5.1 | 29.2 ± 4.3 | 32.1 ± 3 |
| Waist-to-hip ratiob | 0.85 ± 0.08 | 1.00 ± 0.03 | 0.91 ± 0.13 | 0.98 ± 0.06 | 1.01 ± 0.05 |
| Liver stiffness (kpa)a | 4.9 (2.9-6.8) | 3.5 (3-6.1) | 5.1 (3.5-11.7) | 5.7 (3.4-12.3) | 6.9 (4.1-70.6) |
| ≥ 7.9 kpac | 0 (0) | 0 (0) | 1 (10) | 5 (26) | 4 (33) |
| NAFLD fibrosis score | - | -0.68 (-2.45-1.25) | 0.29 (-0.3-1.26) | -0.4 (-3.04-1.1) | 0.11 (-1.68-2.28) |
| ≥ 0.676 | 1 (17) | 3 (30) | 2 (11) | 4 (33) | |
| 1H-MR spectroscopy | - | 4.19 (2.69-16.62) | 9.77 (1.56-15.71) | 18.99 (5.66-35.55) | 18.38 (9.42-41.11) |
| (relative lipid signal, %) | |||||
| Asq | |||||
| Mode 1 (red histogram)b | 114 (105-121) | 107 (102-109) | 106 (102-114) | 105 (100-112) | 102 (99-108) |
| Average 1 (red histogram)a | 117 (109-125) | 110 (104-116) | 108 (106-114) | 106 (103-112) | 105 (102-112) |
| Mode 2 (blue histogram)b | 126 (110-135) | 117 (114-126) | 119 (105-125) | 114 (107-119) | 114 (105-121) |
| Average 2 (blue histogram)b | 132 (116-149) | 122 (116-139) | 123 (114-130) | 118 (109-134) | 115 (106-123) |
| Focal disturbance (FD) ratiob | 0.34 (0.12-0.71) | 0.16 (0.03-0.36) | 0.07 (0.03-0.18) | 0.06 (0.02-0.09) | 0.04 (0.02-0.13) |
P < 0.05 (Kruskal-Wallis test),
P < 0.01 (Kruskal-Wallis test) vs healthy controls;
P < 0.05 (exact test for count data), vs healthy controls. ASQ: Acoustic structure quantification; CAP: Controlled attenuation parameter.
ASQ parameters mode and average of both C2-histogram curves and FD ratio differed between healthy controls and diabetic patients (P-values < 0.001). They also showed a stepwise decrease according to the CAP-defined classification of steatosis in diabetic patients (Table 2, Figure 3). ROC analysis for the detection of advanced steatosis (defined by CAP > 300 dB/m) in diabetic patients by the FD ratio revealed an area under the curve (accuracy) of 0.76 (0.61; 0.87) (sensitivity 97%, specificity 50%) at a cut-off value of 0.092. With the addition of control subjects, the accuracy increased to 0.89 (sensitivity 97%, specificity 78%) with the same cut-off.
Figure 3.
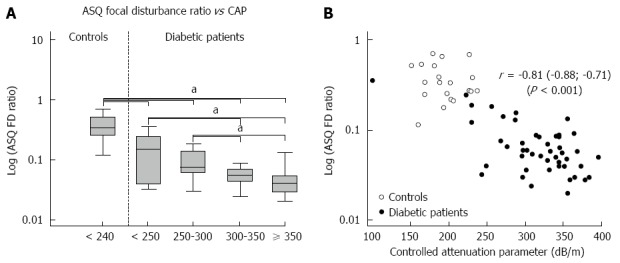
Correlation of acoustic structure quantification focal disturbance ratio and controlled attenuation parameter values in healthy controls and diabetic patients. Acoustic structure quantification (ASQ) focal disturbance (FD) ratio decreased in patients with advanced steatosis compared with healthy controls and patients with mild steatosis (aP < 0.05, vs control, Kruskal-Wallis test) (A). Logarithmic ASQ values correlated strongly with controlled attenuation parameter (CAP) values (B).
As with the CAP results (Figure 3B), ASQ parameters were negatively correlated with 1H-MRS in the diabetic cohort (Table 2), e.g., r = -0.43 (-0.65; -0.15), P = 0.004 for the FD ratio. Agreement between both methods increased when only cases with a hepatic fat fraction < 25% were considered (n = 36): r = -0.64 (-0.80; -0.40), P < 0.001.
DISCUSSION
The present study provided a comprehensive comparison of the ASQ technique with established non-invasive methods for the grading and staging of fatty liver disease. Our data underline the value of ASQ for the quantification of liver fat: the FD ratio showed a strong correlation with the CAP method, which in turn achieved high diagnostic accuracy for the grading of liver steatosis compared with liver histology[1,4-9]. Furthermore, the FD ratio also correlated with the hepatic lipid fraction, as quantified by 1H-MRS, especially in cases with low to moderate hepatic fat content. These results confirm data from a mouse model, which demonstrated the potential value of the refined ASQ algorithm for non-invasive liver fat quantification[16]. Our findings are also in line with preliminary observations on the regression of FD ratios in NAFLD patients treated for morbid obesity[24]. Therefore, ASQ may become a novel tool for the estimation of hepatic steatosis that is equally as accurate as other non-invasive methods[4].
ASQ was originally developed as an alternative to liver biopsy for fibrosis staging. Three biopsy-controlled, cross-sectional studies have shown a correlation of ASQ parameters (mode and average) with the extent of liver fibrosis[13-15]. However, these ASQ parameters correlated with CAP results and thus with the degree of liver steatosis in our cohort (Table 2). All ASQ parameters also differed between healthy controls and patients at risk of fatty liver disease, independent of the extent of fibrosis. By contrast, none of these parameters were associated with either the NAFLD fibrosis score or liver tissue stiffness, although both approaches represent well-established surrogates of liver fibrosis[3,4]. We therefore assumed that anthropometric factors and advanced steatosis, which were associated with a higher frequency of increased liver stiffness in our cohort, interfered with fibrosis in the B-mode speckle pattern and thus limited the prognostic value of ASQ for fibrosis in such cases. The three previous studies where ASQ correlated with fibrosis were performed either in lean cohorts without advanced steatosis and cirrhosis[13], in patients with homogeneous steatosis for all fibrosis stages[14], or did not analyze the impact of steatosis on ASQ results in detail[15]. Furthermore, the study of Ricci et al[15] reported low diagnostic accuracy of ASQ for fibrosis detection (AUROC 0.71 for any grade) in patients with viral hepatitis, which may be associated with steatosis especially for cases with progressive fibrosis[25]. Thus, our results may demonstrate a limited value of ASQ for characterizing fibrosis in the presence of steatosis. This finding is supported by recent data from a histology-controlled cohort where ASQ proved imprecise in assessing liver fibrosis[26].
Our study had some limitations: quantitative analysis of ASQ parameters is still an experimental approach and the method has not yet been standardized (e.g., position and size of B-Mode ROIs, number of measurements, technical ultrasound parameters). Most previous ASQ studies did not adequately describe their data acquisition[13,15], which prevented a proper comparison with our results. Another source of discrepancy in fibrosis detection is a vendor’s update to the ASQ data processing[16] that came with the introduction of the FD ratio[13-15]. Furthermore, we only correlated ASQ results with other non-invasive detection techniques because liver histology was ethically prohibited in this pilot study and only provides a semi-quantitative estimate of steatosis[8]. However, an impact of the non-invasive reference methods on our study findings cannot be ruled out. As has been discussed previously[8], anthropometric factors, as well as the extent of liver fat, can alter liver stiffness, CAP and MR spectroscopic results[27-31]. In particular, MR techniques are susceptible to fibrosis-related iron deposits and may not properly discriminate between advanced grades of hepatic steatosis[27,28]. This could explain the moderate correlation between 1H-MRS and ASQ in our cohort, where advanced steatosis and fibrosis was highly prevalent. Accordingly, the findings of our pilot study should be further verified in histology-controlled studies.
In conclusion, our results provided the first evidence that ASQ FD ratio could be used for non-invasive evaluation of hepatic steatosis in patients at risk of fatty liver disease, and merits further investigation. In its current implementation, the ASQ algorithm seems to be impractical to characterize fibrosis in patients with fatty liver disease. There is also a need for biopsy-controlled studies to further validate ASQ parameters and to evaluate critical factors, such as data acquisition or patient anthropometry.
ACKNOWLEDGMENTS
We thank Mrs. Sieglinde Erdmann and Mrs. Katrin Moritz for their support in coordinating the study participants. We further thank Dr. Tobias Wiesner and Dr. Magdalena Brodowski for their assistance in recruiting the study participants. We thank Prof. Dr. Michael Gebel (Hannover Medical School, Hannover, Germany) for critical discussion of ASQ methodology and suggestions for the standardization of the measurement procedure.
COMMENTS
Background
Non-invasive characterization of hepatic fat content and fibrosis is becoming important for the care of patients with chronic liver diseases. Among different approaches, liver stiffness measurement combined with ultrasound attenuation analysis represent the current non-invasive references standards to estimate fibrosis and steatosis.
Research frontiers
Computerized analysis of acoustic tissue properties may represent an alternative for grading and staging of liver damage. To this end, acoustic structure quantification (ASQ) software analyzes the intensity pattern of B-Mode ultrasound. Several ASQ parameters correlate with the extent of fibrosis in histology-controlled studies. However, ASQ has not yet been compared with established non-invasive techniques, and the impact of hepatic steatosis on ASQ has not yet been studied.
Innovations and breakthroughs
These results provide the first evidence that ASQ parameters correlate with the extent of hepatic steatosis. However, ASQ may be impractical to characterize fibrosis in patients with fatty liver disease.
Applications
If further proved in biopsy-controlled studies, ASQ may complement ultrasound assessment of patients with chronic liver diseases, especially for the non-invasive estimation of hepatic fat content.
Terminology
Non-alcoholic fatty liver disease (NAFLD): obesity, nutrition and further factors can lead to fat deposition in hepatocytes, which is called NAFLD. This process may lead to hepatic inflammation and ultimately to fibrosis and cirrhosis. Elastography: measurement of tissue stiffness by means of mechanical and ultrasound impulses. Liver stiffness correlates with the extent of fibrosis.
Peer-review
The authors compared ultrasound-based ASQ with established non-invasive techniques for grading and staging fatty liver disease. The topic is relevant because NAFLD is perhaps the commonest liver disease. The authors claim that this study provides the first evidence that the ASQ FD ratio could be used for non-invasive evaluation of hepatic steatosis in patients at risk for fatty liver disease. Overall, this is a well written manuscript.
Footnotes
Supported by Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1001 (Project No. K7-40); the German Research Foundation (DFG); and the University of Leipzig within the program of Open Access Publishing.
Ethics approval: The study was reviewed and approved by the local ethics committee (University of Leipzig, register no. 358/08-B-ff and no. 419-12-17122012).
Informed consent: All participants provided written informed consent prior to study enrollment.
Conflict-of-interest: TK received travel grants from Echosens (Paris/France). All other authors have nothing to disclose.
Data sharing: Technical appendix, statistical code, and dataset are available from the corresponding author at thomas.karlas@medizin.uni-leipzig.de. No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 30, 2014
First decision: December 11, 2014
Article in press: February 11, 2015
P- Reviewer: Chen LZ, Sanal MG S- Editor: Ma YJ L- Editor: Stewart G E- Editor: Wang CH
References
- 1.Wong GL. Transient elastography: Kill two birds with one stone? World J Hepatol. 2013;5:264–274. doi: 10.4254/wjh.v5.i5.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238–253. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 3.Dyson JK, McPherson S, Anstee QM. Non-alcoholic fatty liver disease: non-invasive investigation and risk stratification. J Clin Pathol. 2013;66:1033–1045. doi: 10.1136/jclinpath-2013-201620. [DOI] [PubMed] [Google Scholar]
- 4.Berzigotti A. Getting closer to a point-of-care diagnostic assessment in patients with chronic liver disease: controlled attenuation parameter for steatosis. J Hepatol. 2014;60:910–912. doi: 10.1016/j.jhep.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 5.de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918. doi: 10.1111/j.1478-3231.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 6.Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, Duarte-Rojo A, Wong D, Crotty P, Elkashab M. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902–910. doi: 10.1111/j.1478-3231.2012.02781.x. [DOI] [PubMed] [Google Scholar]
- 7.de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, Merrouche W, Foucher J, Brigitte le B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–1031. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Karlas T, Petroff D, Garnov N, Böhm S, Tenckhoff H, Wittekind C, Wiese M, Schiefke I, Linder N, Schaudinn A, et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS One. 2014;9:e91987. doi: 10.1371/journal.pone.0091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mi YQ, Shi QY, Xu L, Shi RF, Liu YG, Li P, Shen F, Lu W, Fan JG. Controlled attenuation parameter for noninvasive assessment of hepatic steatosis using Fibroscan®: validation in chronic hepatitis B. Dig Dis Sci. 2015;60:243–251. doi: 10.1007/s10620-014-3341-x. [DOI] [PubMed] [Google Scholar]
- 10.Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–445. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Raptis DA, Fischer MA, Graf R, Nanz D, Weber A, Moritz W, Tian Y, Oberkofler CE, Clavien PA. MRI: the new reference standard in quantifying hepatic steatosis? Gut. 2012;61:117–127. doi: 10.1136/gutjnl-2011-300155. [DOI] [PubMed] [Google Scholar]
- 12.Berzigotti A, Castera L. Update on ultrasound imaging of liver fibrosis. J Hepatol. 2013;59:180–182. doi: 10.1016/j.jhep.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Toyoda H, Kumada T, Kamiyama N, Shiraki K, Takase K, Yamaguchi T, Hachiya H. B-mode ultrasound with algorithm based on statistical analysis of signals: evaluation of liver fibrosis in patients with chronic hepatitis C. AJR Am J Roentgenol. 2009;193:1037–1043. doi: 10.2214/AJR.07.4047. [DOI] [PubMed] [Google Scholar]
- 14.Yamada H, Ebara M, Yamaguchi T, Okabe S, Fukuda H, Yoshikawa M, Kishimoto T, Matsubara H, Hachiya H, Ishikura H, et al. A pilot approach for quantitative assessment of liver fibrosis using ultrasound: preliminary results in 79 cases. J Hepatol. 2006;44:68–75. doi: 10.1016/j.jhep.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Ricci P, Marigliano C, Cantisani V, Porfiri A, Marcantonio A, Lodise P, D’Ambrosio U, Labbadia G, Maggini E, Mancuso E, et al. Ultrasound evaluation of liver fibrosis: preliminary experience with acoustic structure quantification (ASQ) software. Radiol Med. 2013;118:995–1010. doi: 10.1007/s11547-013-0940-0. [DOI] [PubMed] [Google Scholar]
- 16.Kuroda H, Kakisaka K, Kamiyama N, Oikawa T, Onodera M, Sawara K, Oikawa K, Endo R, Takikawa Y, Suzuki K. Non-invasive determination of hepatic steatosis by acoustic structure quantification from ultrasound echo amplitude. World J Gastroenterol. 2012;18:3889–3895. doi: 10.3748/wjg.v18.i29.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 18.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 19.Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, Fouchard-Hubert I, Gallois Y, Oberti F, Bertrais S, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182–1191. doi: 10.1002/hep.25993. [DOI] [PubMed] [Google Scholar]
- 20.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 21.Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13–20. doi: 10.1016/j.clinre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Tuthill TA, Sperry RH, Parker KJ. Deviations from Rayleigh statistics in ultrasonic speckle. Ultrason Imaging. 1988;10:81–89. doi: 10.1177/016173468801000201. [DOI] [PubMed] [Google Scholar]
- 23.Conover WJ. Practical nonparametric statistics. 3rd ed. New York: John Wiley & Sons; 1999. [Google Scholar]
- 24.Onodera M. The New Non-Invasive Quantification of Hepatic Steatosis With Morbid Obesity by Acoustic Structure Quantification (ASQ) From Ultrasound Echo Amplitude [Abstract] Ultrasound Med Biol. 2013;39 Suppl:S2. [Google Scholar]
- 25.Konerman MA, Yapali S, Lok AS. Systematic review: identifying patients with chronic hepatitis C in need of early treatment and intensive monitoring--predictors and predictive models of disease progression. Aliment Pharmacol Ther. 2014;40:863–879. doi: 10.1111/apt.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krämer C, Jaspers N, Nierhoff D, Kuhr K, Bowe A, Goeser T, Michels G. Acoustic structure quantification ultrasound software proves imprecise in assessing liver fibrosis or cirrhosis in parenchymal liver diseases. Ultrasound Med Biol. 2014;40:2811–2818. doi: 10.1016/j.ultrasmedbio.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Guiu B, Cercueil JP. MRI as the new reference standard in quantifying liver steatosis: the need for international guidelines. Gut. 2012;61:1369–1370; author reply 1369-1370. doi: 10.1136/gutjnl-2011-301780. [DOI] [PubMed] [Google Scholar]
- 28.Guiu B, Loffroy R, Hillon P, Petit JM. Magnetic resonance imaging and spectroscopy for quantification of hepatic steatosis: urgent need for standardization! J Hepatol. 2009;51:1082–1083; author reply 1082-1083. doi: 10.1016/j.jhep.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Macaluso FS, Maida M, Cammà C, Cabibbo G, Cabibi D, Alduino R, Di Marco V, Craxì A, Petta S. Steatosis affects the performance of liver stiffness measurement for fibrosis assessment in patients with genotype 1 chronic hepatitis C. J Hepatol. 2014;61:523–529. doi: 10.1016/j.jhep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 30.Cournane S, Browne JE, Fagan AJ. The effects of fatty deposits on the accuracy of the Fibroscan® liver transient elastography ultrasound system. Phys Med Biol. 2012;57:3901–3914. doi: 10.1088/0031-9155/57/12/3901. [DOI] [PubMed] [Google Scholar]
- 31.Petta S, Amato MC, Di Marco V, Cammà C, Pizzolanti G, Barcellona MR, Cabibi D, Galluzzo A, Sinagra D, Giordano C, et al. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;35:238–247. doi: 10.1111/j.1365-2036.2011.04929.x. [DOI] [PubMed] [Google Scholar]


