Abstract
AIM: To investigate the outcome of palliative chemotherapy in old patients with gastroesophageal cancer at the National Center for Tumor Diseases, Heidelberg.
METHODS: Using a prospectively generated database, we retrospectively analyzed 55 patients ≥ 70 years under palliative chemotherapy for advanced gastroesophageal cancer at the outpatient clinic of the National Center for Tumor Diseases Heidelberg, Germany between January 2006 and December 2013. Further requirements for inclusion were (1) histologically proven diagnosis of gastroesophageal cancer; (2) advanced (metastatic or inoperable) disease; and (3) no history of radiation or radiochemotherapy. The clinical information included Eastern Cooperative Oncology Group performance status (ECOG PS), presence and site of metastases at diagnosis, date of previous surgery and perioperative chemotherapy, start and stop date of first-line treatment, toxicities and consecutive dosage reductions of first-line treatment, response to first-line therapy, date of progression, usage of second-line therapies and date and cause of death. Survival times [progression-free survival (PFS), overall survival (OS) and residual survival (RS)] were calculated. Toxicity and safety were examined. Prognostic factors including ECOG PS, age and previous perioperative treatment were analyzed.
RESULTS: Median age of our cohort was 76 years. 86% of patients received a combination of two cytotoxic drugs. 76 percent of patients had an oxaliplatin-based first-line therapy with the oxaliplatin and 5-fluorouracil regimen being the predominantely chosen regimen (69%). Drug modifications due to toxicity were necessary in 56% of patients, and 11% of patients stopped treatment due to toxicities. Survival times of our cohort are in good accordance with the major phase III trials that included mostly younger patients: PFS and OS were 5.8 and 9.5 mo, respectively. Survival differed significantly between patient groups with low (≤ 1) and high (≥ 2) ECOG PS (12.7 mo vs 3.8 mo, P < 0.001). Very old patients (≥ 75 years) did not show a worse outcome in terms of survival. Patients receiving second-line treatment (51%) had a significantly longer RS than patients with best supportive care (6.8 vs 1.4 mo, P = 0.001). Initial ECOG PS was a strong prognostic factor for PFS, OS and RS.
CONCLUSION: Old patients with non-curable gastroesophageal cancer should be offered chemotherapy, and ECOG PS is a tool for balancing benefit and harm upfront. Second-line treatment is reasonable.
Keywords: Gastroesophageal cancer, Old patients, Palliative chemotherapy, Toxicity, Eastern Cooperative Oncology Group performance status
Core tip: Data concerning efficacy and safety of palliative chemotherapy for gastroesophageal cancer in patients ≥ 70 years are scarce. Concerns about poor tolerability due to reduced functional status are common, and older patients are at risk for undertreatment. In our analysis of 55 patients ≥ 70 years, the survival times were in good accordance to the results of the landmark phase III trials including younger patients. Except for increased polyneuropathy, toxicity rates were also comparable. Initial Eastern Cooperative Oncology Group performance status was a strong prognostic factor for PFS, OS and RS.
INTRODUCTION
For older adults, cancer incidence rates are higher than for younger persons[1]. The current epidemiologic development in the western world will therefore result in a rising incidence of cancer, and an increasing proportion of old and very old patients among all cancer cases[2]. Despite this development for most malignancies, older cancer patients remain underrepresented in clinical studies[3], and age above 70 years often is an exclusion criterion. There is widespread critique concerning this issue, but to date, extrapolation of trial results to the older population remains questionable[4]. When treating elderly cancer patients, medical comorbidities, impaired organ function and reduced functional status are frequently found[5]. Ensuring an adequate antitumor treatment while avoiding toxicity is a pivotal question of geriatric oncology in daily routine.
Gastric cancer and cancer of the gastroesophageal junction belong to the most common cancer forms worldwide[6]. In the United States, there were diagnosed approximately 40000 new cases of esophageal and gastric cancer with estimated 26000 deaths in 2013[7]. While incidence rates for distal gastric cancer keep declining continuously over the past decades[8], the incidence for cancer of the esophagogastric junction remains increasing[9]. In the United States, the 5-year relative survival for all stages in patients aged 65 or more is 26%[8], and for metastasized disease it is less than 5% for all patients. To date, palliative chemotherapy is the accepted standard of care for patients with metastasized disease since benefits in terms of survival times and quality of life have been widely shown[10,11]. Still, there is no internationally accepted standard therapy for the first-line situation[12]. Combination therapies are considered to be more active than single-agent approaches[11]. For cytotoxic double combinations, infusional or oral fluoropyrimidines in combination with platin-derivates have been shown to be active[13-15]. The addition of docetaxel as a cytotoxic triplet combination showed improved efficacy in terms of response rate and overall survival but is associated with increased toxicity[16]. For HER2-positive patients, trastuzumab is known to significantly improve overall survival in combination with a fluoropyrimidine and cisplatin[17]. Although gastroesophageal carcinomas predominantely occur in elder patients[8], the knowledge and recommendations about an adequate first-line treatment for older persons are sparse. There is some evidence that for patients older than 65 years survival times are comparable to those of younger ones, but grade 3 or higher toxicities might be more frequent for currently used regimens[18]. Oxaliplatin-based combinations might have a better toxicity profile and seem to be associated with an improved efficacy in contrast to cisplatin based regimens[14], whereas the benefit of using three-drug cytostatic combinations in elderly patients is questionable[19].
Since most patients develop disease progression under first-line therapy, options for second-line treatment are frequently needed. Recently, the value of a second-line therapy with either docetaxel or irinotecan was shown[20]. But again, specific data for old patients are rarely found.
In our study, we retrospectively analyzed palliative chemotherapy and outcomes for old and very old patients (≥ 70 years) with advanced gastroesophageal cancer at the outpatient clinic of the National Center for Tumor Diseases (NCT) at Heidelberg University Hospital. In particular, we investigated the characteristics, toxicities and outcomes of cytotoxic treatment strategies in the first-line situation and evaluated the frequency and efficacy of second-line chemotherapy.
MATERIALS AND METHODS
Using a prospectively maintained database (the NCT Clinical Cancer Registry), we retrospectively identified patients aged 70 years or over who started palliative first-line chemotherapy for advanced gastroesophageal cancer at the outpatient clinic of the NCT Heidelberg, Germany between January 2006 and December 2013. Further requirements for inclusion were (1) histologically proven diagnosis of gastroesophageal cancer; (2) advanced (metastatic or inoperable) disease; and (3) no history of radiation or radiochemotherapy. The observation period for each patient started with initiation of palliative first-line treatment (i.e., first systemic chemotherapy after primary diagnosis of metastatic or inoperable disease or, in resected patients, after diagnosis of recurrence). The follow-up period for this analysis ended on July 1, 2014. Survival data were available for all patients.
Via an electronic medical record, the responsible oncologists and medical staff routinely documented the clinical data. The retrieved information included Eastern Cooperative Oncology Group performance status (ECOG PS)[21], presence and site of metastases at diagnosis, date of previous surgery and perioperative chemotherapy, start and stop date of first-line treatment, toxicities and consecutive dosage reductions of first-line treatment, response to first-line therapy, date of progression, usage of second-line therapies and date and cause of death. Toxic effects were registered according to the National Cancer Institute´s common terminology criteria for adverse events[22]. Tumor response was routinely evaluated according to the response evaluation criteria in solid tumors[23]. The attending oncologist decided about the cytotoxic regimen and the start and end of antitumor treatment.
The study was approved by the local Ethics Committee (Ethics Committee University of Heidelberg; S-335/2014, 28.07.2014). According to local ethics policy for retrospective analysis of own anonymized clinical data, IC was not obtained.
Statistical analysis
Man Whitney U-Test and Fisher’s exact test were used for comparing independent samples of quantitative and binary data, respectively. Progression-free survival (PFS) was defined as time from start of palliative first-line treatment to documented tumor progression or death. Overall survival (OS) was defined as the time from start of palliative first-line treatment to death. Residual survival (RS) was defined as the time from the day of documented disease progression to death. Time-to event data were analyzed using standard methods, including Kaplan-Meier product-limit estimates. Multivariate analysis was performed using cox regression analysis. All analyses of prognostic factors were of an exploratory nature. Statistical analysis was performed using the SPSS statistical software, Version 21. The statistical methods of this study were reviewed by Georg Martin Haag, NCT Heidelberg.
RESULTS
Patients’ demographics
We identified 55 patients meeting the inclusion criteria. Median duration of observation was 9.1 mo (range 0.9-45.8). The median age at diagnosis of advanced disease was 76 years (range 70-86), 32 patients (58.2%) were 75 years or older. Fifteen patients (27.3%) with secondary metastases or local recurrent tumor had undergone prior tumor resection. Twelve of these had received perioperative chemotherapies. Thirty-seven patients (67.3%) had an ECOG PS of 0 or 1. Four patients started treatment with an ECOG PS of 3. Complete patient characteristics are outlined in Table 1. At time of analysis, 50 deaths (90.9%) had occurred. Of those, 18 patients had died during first-line treatment before documented tumor progression. In 8 of those cases, tumor-associated factors (e.g., tumor bleeding) or tumor progression were clinically assumed and documented as causes of death. One patient died from pleural empyema and one further patient died from cholangitis. In 8 cases, no further information on the exact mode of death was available.
Table 1.
Patients demographics
| Patient characteristics | n (%) |
| Number of patients | 55 |
| Median age (range), yr | 76 (70-86) |
| Gender | |
| Female | 16 (29.1) |
| Male | 39 (70.9) |
| ECOG PS | |
| 0 | 10 (18.2) |
| 1 | 27 (49.1) |
| 2 | 14 (25.5) |
| 3 | 4 (7.3) |
| Metastatic disease | 52 (94.5) |
| Locally advanced tumor | 3 (5.5) |
| Primary palliative treatment | 40 (72.7) |
| Secondary palliative treatment | 15 (27.3) |
| Prior (neo) adjuvant CTX | 12 (21.8) |
| Histology | |
| Adenocarcinoma | 52 (94.5) |
| Sqaumous cell carcinoma | 3 (5.5) |
| Site of Tumor | |
| Stomach, distal | 25 (45.5) |
| Cardia | 15 (27.3) |
| Esophagus, distal | 9 (16.4) |
| Other | 2 (3.6) |
| NA | 4 (7.3) |
| Number of deaths | 50 (90.9) |
| Cause of death | |
| Tumor | 40 (80) |
| Infection | 2 (4) |
| Unknown | 8 (16) |
ECOG PS: Eastern Cooperative Oncology Group performance status.
First-line chemotherapy and toxicities
Median duration of first-line therapy was 105 d (range 6-568). Patients with an ECOG PS ≥ 2 had a median duration of therapy of 70 d compared to 146 d for ECOG PS ≤ 1 (P = 0.006). Forty-seven patients (85.5%) received a combination of two cytotoxic agents (doublet). Three patients (5.5%) were treated with a single-agent therapy (oral or infusional fluoropyrimidine), and five patients (9.1%) underwent therapy with three cytotoxic agents (triplet). Seven of our patients (12.7%) had a cisplatin containing regimen for first-line treatment. In 42 cases (76.4%), oxaliplatin-based regimens were used. Three patients were treated in a clinical trial (Cisplatin-based chemotherapy +/- an anti EGFR antibody).
For this analysis, we included all toxicities that led to treatment modifications (Grade 3 or worse). In total, 31 patients (56.4%) developed such side effects. In 14 of those cases (45.2% of all relevant side effects or 25.5% of all patients) polyneuropathy (PNP) and in 6 cases (19.4% or 10.9%, respectively) hematotoxicity caused dosage reductions. Six patients (10.9%) discontinued therapy due to toxicity. PNP leading to treatment modifications was significantly more common in patients receiving prolonged firstline therapy (13 of 31 patients with a first-line ≥ 80 d vs 1 of 24 patients with a duration < 80 d (P = 0.002). No significant differences in toxicities requiring discontinuation of therapy were found for patients with an ECOG PS ≥ 2 or for patients ≥ 75 years, and we also did not find significant differences for combination regimens (doublet vs triplet vs monotherapies). A summary of therapies and toxicities is given in Table 2.
Table 2.
Characteristics of chemotherapy
| Treatment characteristics | n (%) |
| Median duration of first-line therapy (range), d | 105 (6-568) |
| First-line therapy | |
| Single-agent (Fluoropyrimidine) | 3 (5.5) |
| Doublet | 47 (85.5) |
| FLO | 38 (69.1) |
| FLP | 2 (3.6) |
| XP | 2 (3.6) |
| FOLFIRI | 2 (3.6) |
| Other | 3 (5.5) |
| Triplet | 5 (9.1) |
| EOX | 2 (3.6) |
| FLOT | 2 (3.6) |
| TFLP | 1 (1.8) |
| Oxaliplatin-based therapy | 42 (76.4) |
| Cisplatin-based therapy | 7 (12.7) |
| Other therapy | 6 (10.9) |
| Trastuzumab containing therapy | 5 (9.1) |
| Participation in clinical trial | 3 (5.5) |
| Toxicities with dosage reduction | 31 (56.4) |
| Polyneuropathy HematotoxicityLeucopeniaThrombopenia | 14 (25.5) 6 (10.9)3 (5.5)3 (5.5) |
| Fatigue | 4 (7.3) |
| Nausea | 4 (7.3) |
| Other | 3 (5.5) |
| Toxicities with interruption of treatment | 6 (10.9) |
| Polyneuropathy | 2 (3.6) |
| Fatigue | 2 (3.6) |
| Anaphylaxia | 1 (1.8) |
| Infection | 1 (1.8) |
| Second-line treatment | 28 (50.9) |
FLO: 5-Fluorouracil (5-FU), leucovorin, oxaliplatin; FLP: 5-FU, leucovorin, cisplatin; XP: Capecitabine, cisplatin; FOLFIRI: 5-FU, leucovorin, irinotecan; EOX: Epirubicin, oxaliplatin, capecitabine; FLOT: 5-FU, leucovorin, oxaliplatin, docetaxel; TFLP: Docetaxel, 5-FU, leucovorin, cisplatin.
Progression and survival
Progression-free survival: Median PFS was 5.8 mo (95%CI: 4.1-7.6 mo). Median PFS for patients with an ECOG PS 0 or 1 was 6.8 mo (95%CI: 5.4-8.1) compared to 2.5 mo (95%CI: 1.5-3.5) for patients with ECOG PS ≥ 2; (P < 0.001; Figure 1A). Age ≥ 75 years was not associated with significant different PFS, neither there were significant differences for metastasized compared to locally advanced tumors, previous tumor resection or not.
Figure 1.
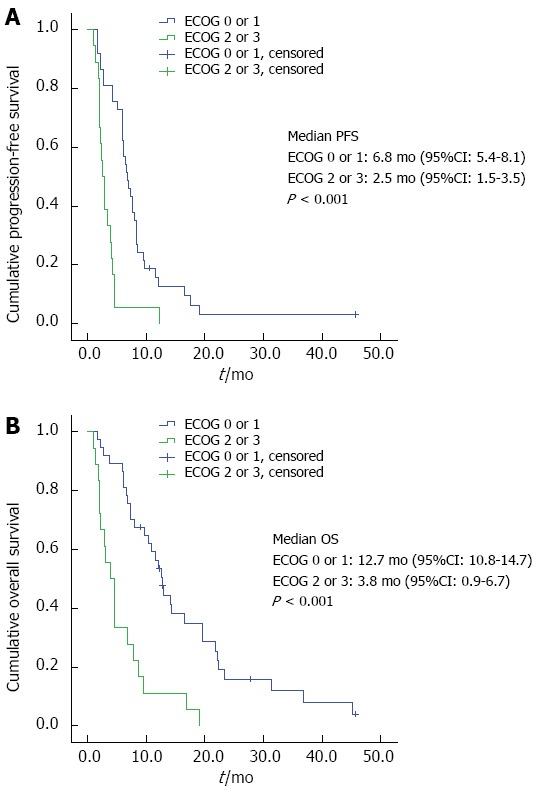
Progression-free survival (A), overall survival (B) by Eastern Cooperative Oncology Group performance status.
Overall survival: Median overall survival in our patients was 9.5 mo (95%CI: 5.8-13.2). Median OS for patients with ECOG PS 0 or 1 was 12.7 mo (95%CI: 10.8-14.7) vs 3.8 mo (95%CI: 0.9-6.7) for patients with ECOG 2 or higher, (P < 0.001, Figure 1B). There was no apparent association between age at diagnosis and OS. Patients receiving a monotherapy had a median OS of 2.1 (95%CI: 0.2-4.0) mo vs 9.5 mo for doublets (95%CI: 6.2-12.7) and 19.0 mo for triplets (95%CI: 5.5-32.5, P < 0.001). OS did not significantly differ between the groups of patients with metastasized compared to irresectable locally advanced tumors or those with or without previous tumor resection.
Second-line chemotherapy and residual survival: Regarding the group of patients being alive at the time point of documented tumor progression, second-line chemotherapy was offered to 28 patients, whereas 7 patients received best supportive care (BSC) only. Patients receiving second-line treatment had a significantly longer RS than the BSC group (6.8 mo vs 1.4. mo, P = 0.001; Figure 2). Patients with an ECOG PS of 0 or 1 before first-line therapy had a significantly longer RS (7.4 mo vs 4.0 mo, P = 0.047; Figure 3). No significant influence on RS was found for the variables age or duration of first-line treatment.
Figure 2.
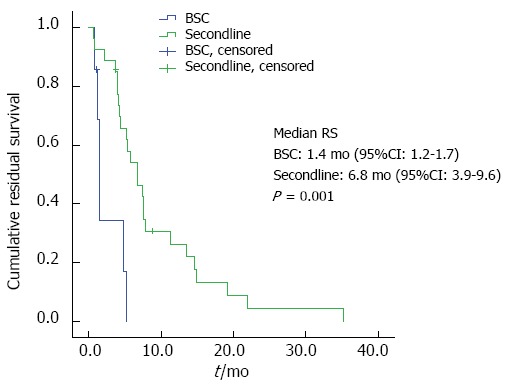
Residual survival by second-line therapy.
Figure 3.
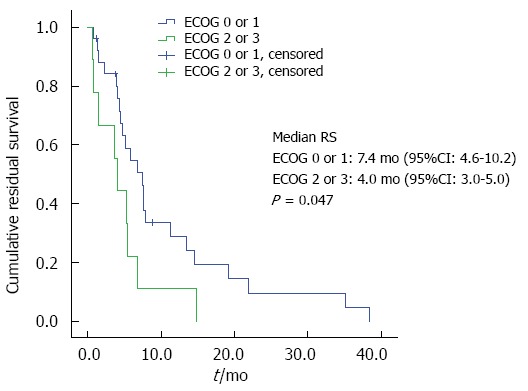
Residual survival by Eastern Cooperative Oncology Group performance status.
Multivariate analysis of overall survival confirmed the prognostic role of the initial ECOG PS (Hazard Ratio for patients with an ECOG PS of 2/3 3.9) and the application of secondline therapy (Hazard Ratio for patients receiving a secondline therapy 0.44, Table 3).
Table 3.
Multivariate analysis of overall survival
| Prognostic factor | Hazard ratio | 95%CI | Significance |
| Initial ECOG (2/3 vs 0/11) | 3.9 | 2.0-7.5 | P < 0.001 |
| Secondline (Yes vs No1) | 0.44 | 0.2-0.8 | P = 0.006 |
| Very old (≥ 75 yr vs < 75 yr1) | 1.36 | 0.7-2.5 | P = 0.314 |
Reference. ECOG: Eastern Cooperative Oncology Group.
DISCUSSION
Although gastroesophageal cancer predominantely occurs in patients older than 65 years[24], the landmark phase III trials establishing the standard protocols for palliative chemotherapy usually included much younger patients. Recently, some effort has been made to evaluate cytotoxic treatment strategies for patients ≥ 65 years in the perioperative and palliative setting[19,25,26]. In our study, we analyzed a remarkably old cohort of palliative patients with a median age of 76 years (range 70-86) with the majority of patients (60%) being even older than 75 years. We did not find significant differences for survival times between the patient groups aged 70-74 years and ≥ 75 years. Our median times for PFS (5.8 mo) and for OS (9.5 mo) are in very good accordance with the results of the major phase III trial collectives including mainly younger patients. For a study collective with a median age of 56 years, Kang et al[13] found a PFS of 5.6 mo and an OS of 10.5 mo for a doublet treatment with capecitabine and cisplatin. Concordant data for PFS (5.6 mo) and OS (10.7 mo) are recently reported for the combination of cisplatin and capecitabine[27]. Median age in the latter trial was 59 years with 30% of patients being older than 65 years. Similar results (PFS 5.8 mo, OS 10.7 mo) were described for a doublet treatment with oxaliplatin and 5-fluorouracil (FLO) in a study collective with a median age of 64 years[14]. In a subgroup analysis of this trial’s patients older than 65 years, FLO seemed to be associated with improved survival times compared to a cisplatin-containing doublet. Comparable results for a modified FOLFOX regimen (PFS 6.8 and OS 10.5 mo, respectively) are recently reported in a phase II study with 43 patients older than 69 years[25]. In a feasibility study, a triple combination (FLOT) failed to improve survival times for patients older than 69 years[19] compared to the FLO regimen.
Surprisingly, only 3 of our patients (5.5%) received single-agent therapy. OS was significantly shorter than for patients under combination therapy. Considering the small number, we do not have enough data to come to firm conclusions regarding the role of less intensive chemotherapies in older patients. Nevertheless, Sun et al[28] recently reported significantly reduced toxicities for single-agent treatment in a Korean geriatric collective while survival times did not differ significantly but were generally striking low (OS 6.6 mo for single-agent treatment vs 7.6 for doublet combinations). Median age for single-agent treatment was 77 years, and 73 years for patients receiving doublet combinations in this analysis[28]. 90% of our patients initially received cytotoxic doublet combinations. Platin-derivates were used in 90% of all regimens with the FLO regimen being predominant (oxaliplatin usage 76%, FLO regimen 69%). Notably, more than half of our patients needed dose adjustments due to toxicities. One third of the oxaliplatin group developed relevant PNP leading to treatment modifications. This is clearly more than expected from the trials so far that described grade 3/4 PNP in 14%[14], 2%[25] or 20%[19]. Relevant PNP in our cohort was found significantly more frequent when treatment duration exceeded 80 d. Cessation of therapy was necessary in 11% of our patients with 4% caused by PNP. Rates for grade 3/4 hematotoxicity (11%), nausea (7%) and fatigue (7%) were found to be comparable to the results from phase III trials.
Thus, in terms of survival, we did not find any evidence that old and even very old patients that have been deemed fit enough for palliative chemotherapy do have an inferior outcome compared to younger patients in clinical studies. These findings are consistent with current own data concerning palliative chemotherapy in old patients with pancreatic cancer[29] and with previous analysis of other solid malignancies[30,31]. Based on the convincing survival times, in our opinion doublet combinations should be considered for first-line treatment whenever possible in old and even very old patients. However, oncologists should be aware that toxicities might appear more frequent. Symptoms of PNP should be monitored closely, and patients and their relatives should be informed in advance. In case of successful and prolonged treatment with oxaliplatin, planning dose reductions upfront might be reasonable.
In our cohort, residual survival after first progression was significantly longer for patients who received second-line therapy than for the BSC group. One possible explanation for this advantage is that only patients in good condition were offered second-line therapy. However, given that our results are comparable to the recent randomized phase III trial on second-line therapy[20], we strongly believe that second-line strategies should be offered to old and very old patients in good performance status after first progression.
Fear of increased toxicities and uncertainty concerning both clinical value and physiological resources may cause withholding or limitation of tumor-specific therapies by the attending oncologist. Therefore, old cancer patients are at risk for therapeutic disparity and undertreatment. In our analysis of unselected old patients, higher age was not significantly associated with impaired survival times. Thus, the feasibility and efficacy of systemic treatment in advanced gastroesophageal cancer in the old population seems to be independent of chronological age. The evaluation of the “functional age” rather than biological age is known as more appropriate for assessing the eligibility for chemotherapy, and the usefulness of comprehensive geriatric assessments (CGA) in this setting has been demonstrated[32-34]. CGA has not been established in our outpatient setting yet, and rather in our patients we use the ECOG PS as the initial classification system. In our study population, all survival times were significantly depending on the initial ECOG PS. An ECOG PS ≥ 2 was strongly associated with a poor clinical outcome with a median OS of only 3.8 mo. Thus, systemic cytotoxic treatment for old patients in reduced performance status should only be offered cautiously. In contrast, treatment decisions based on chronological age only would reflect a form of “ageism” and should be avoided.
COMMENTS
Background
Palliative chemotherapy is the preferred treatment for metastatic esophagogastric cancer. Superiority regarding overall survival and quality of life compared to best supportive care alone has been shown in several trials. Despite the fact that esophagogastric cancer occurs more often in old patients, this group of patients is underrepresented in clinical trials. When treating old and very old cancer patients, medical comorbidities, impaired organ function and reduced functional status are frequently found. Concerns about poor tolerability might prevent physicians from initiating a tumorspecific therapy.
Research frontiers
Within clinical trials representing old patients in a good general state, there is some evidence that for patients older than 65 years survival times are comparable to those of younger ones, but grade 3 or higher toxicities might be more frequent for currently used regimens. Oxaliplatin-based combinations seem to have a better toxicity profile and seem to be associated with an improved efficacy in contrast to cisplatin-based regimens, whereas the additional benefit of using three-drug cytostatic combinations in elderly patients is questionable. Secondline therapy might be an option in selected patients with a good general condition after progression on first-line therapy. In general, data from clinical trials represent a selected cohort of old patients, whereas real-life data of elderly patients are scarce.
Innovations and breakthroughs
In this study, the authors analyzed efficacy and toxicity in old patients with metastatic esophagogastric cancer. The applied chemotherapeutical regimens were documented, patterns of toxicity were analyzed. Eastern Cooperative Oncology Group (ECOG)-Score and age were analyzed as potential prognostic factors. Within this real-life setting, efficacy in terms of PFS, OS and residual survival after tumorprogression (RS), was comparable to major phase III trials with younger patients. Toxicity was within the expected range. Initial ECOG-Score is a strong prognostic marker regarding overall survival and RS.
Applications
Tumorspecific treatment should be offered to old and very old patients in a good general condition regardless of chronological age. Initial ECOG-Score is a major prognostic marker which could help selecting patients for chemotherapeutical treatment.
Terminology
PFS: Progression-free survival is defined as the time between initiation of systemic therapy and tumor progression or death from any course. Overall Survival is defined as the time between initiation of systemic therapy and death from any cause. The ECOG Scale of Performance Status is widely used to quantify the functional status of cancer patients, and is an important factor determining prognosis in a number of malignant conditions.
Peer-review
The manuscript has a high significance and novelty. Generally speaking, the presentation and organization of the manuscripts good, the quality of language is good. This manuscript describes a single-center clinical study on the effects of palliative chemotherapy in old patients with gastroesophageal cancer. The technique is not novel but the subject of research is ignored by academic world.
Footnotes
Supported by Zentrum für Geriatrische Onkologie und Biologie in der Metropolregion Rhein Neckar (ZOBEL).
Ethics approval: The study was approved by the local Ethics Committee (Ethics Committee University of Heidelberg; S-335/2014, 28.07.2014).
Biostatistics Statement: The statistical methods of this study were reviewed by Georg Martin Haag from the NCT Heidelberg.
Informed consent: According to local ethics policy for retrospective analysis of own anonymized clinical data, IC was not obtained.
Conflict-of-interest: The authors state that there is no conflict of interest to disclose.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 9, 2014
First decision: November 14, 2014
Article in press: December 22, 2014
P- Reviewer: Gong JP, Plaza MA S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer. 1997;80:1273–1283. [PubMed] [Google Scholar]
- 2.Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11:437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Soares HP, Balducci L, Djulbegovic B. Treatment tolerance and efficacy in geriatric oncology: a systematic review of phase III randomized trials conducted by five National Cancer Institute-sponsored cooperative groups. J Clin Oncol. 2007;25:1272–1276. doi: 10.1200/JCO.2006.09.2759. [DOI] [PubMed] [Google Scholar]
- 4.Pallis AG, Fortpied C, Wedding U, Van Nes MC, Penninckx B, Ring A, Lacombe D, Monfardini S, Scalliet P, Wildiers H. EORTC elderly task force position paper: approach to the older cancer patient. Eur J Cancer. 2010;46:1502–1513. doi: 10.1016/j.ejca.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Repetto L, Venturino A, Fratino L, Serraino D, Troisi G, Gianni W, Pietropaolo M. Geriatric oncology: a clinical approach to the older patient with cancer. Eur J Cancer. 2003;39:870–880. doi: 10.1016/s0959-8049(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 8.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, based on November, 2013 SEER data submission, posted to the SEER web site, April 2014. Available from: http://seer.cancer.gov/csr/1975_2011/
- 9.Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998-2003. Int J Cancer. 2008;123:1422–1428. doi: 10.1002/ijc.23691. [DOI] [PubMed] [Google Scholar]
- 10.Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 11.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 12.Lordick F, Lorenzen S, Yamada Y, Ilson D. Optimal chemotherapy for advanced gastric cancer: is there a global consensus? Gastric Cancer. 2014;17:213–225. doi: 10.1007/s10120-013-0297-z. [DOI] [PubMed] [Google Scholar]
- 13.Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 14.Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G, Homann N, Wilhelm G, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 17.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 18.Jatoi A, Foster NR, Egner JR, Burch PA, Stella PJ, Rubin J, Dakhil SR, Sargent DJ, Murphy BR, Alberts SR. Older versus younger patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction, and stomach: a pooled analysis of eight consecutive North Central Cancer Treatment Group (NCCTG) trials. Int J Oncol. 2010;36:601–606. doi: 10.3892/ijo_00000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Batran SE, Pauligk C, Homann N, Hartmann JT, Moehler M, Probst S, Rethwisch V, Stoehlmacher-Williams J, Prasnikar N, Hollerbach S, et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+) Eur J Cancer. 2013;49:835–842. doi: 10.1016/j.ejca.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Kang JH, Lee SI, Lim do H, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 21.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services. National Institutes of Health. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). 28 May 2009 (v4.03: 14 June 2010) Available from: http://evs.nci.nih.gov/ftp1/CTCAE/About.html.
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am. 2002;31:421–440, viii. doi: 10.1016/s0889-8553(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 25.Catalano V, Bisonni R, Graziano F, Giordani P, Alessandroni P, Baldelli AM, Casadei V, Rossi D, Fedeli SL, D’Emidio S, et al. A phase II study of modified FOLFOX as first-line chemotherapy for metastatic gastric cancer in elderly patients with associated diseases. Gastric Cancer. 2013;16:411–419. doi: 10.1007/s10120-012-0204-z. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzen S, Pauligk C, Homann N, Schmalenberg H, Jäger E, Al-Batran SE. Feasibility of perioperative chemotherapy with infusional 5-FU, leucovorin, and oxaliplatin with (FLOT) or without (FLO) docetaxel in elderly patients with locally advanced esophagogastric cancer. Br J Cancer. 2013;108:519–526. doi: 10.1038/bjc.2012.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 28.Sun DS, Jeon EK, Won HS, Park JC, Shim BY, Park SY, Hong YS, Kim HK, Ko YH. Outcomes in elderly patients treated with a single-agent or combination regimen as first-line chemotherapy for recurrent or metastatic gastric cancer. Gastric Cancer. 2014:Epub ahead of print. doi: 10.1007/s10120-014-0405-8. [DOI] [PubMed] [Google Scholar]
- 29.Berger AK, Abel U, Komander C, Harig S, Jäger D, Springfeld C. Chemotherapy for advanced pancreatic adenocarcinoma in elderly patients (≥70 years of age): a retrospective cohort study at the National Center for Tumor Diseases Heidelberg. Pancreatology. 2014;14:211–215. doi: 10.1016/j.pan.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Hutson TE, Bukowski RM, Rini BI, Gore ME, Larkin JM, Figlin RA, Barrios CH, Escudier B, Lin X, Fly K, et al. Efficacy and safety of sunitinib in elderly patients with metastatic renal cell carcinoma. Br J Cancer. 2014;110:1125–1132. doi: 10.1038/bjc.2013.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folprecht G, Seymour MT, Saltz L, Douillard JY, Hecker H, Stephens RJ, Maughan TS, Van Cutsem E, Rougier P, Mitry E, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26:1443–1451. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]
- 32.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maas HA, Janssen-Heijnen ML, Olde Rikkert MG, Machteld Wymenga AN. Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer. 2007;43:2161–2169. doi: 10.1016/j.ejca.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, Parodi S, Dal Lago D, Gioia F, Monfardini S, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]


