Abstract
AIM: To explore the relationship of clinicopathological features and the distribution of neutrophils in the tumor microenvironment with the prognosis of cholangiocarcinoma.
METHODS: Two hundred and fifty-four formalin-fixed and paraffin embedded tissue blocks were analyzed, including tissues from cholangiocarcinoma (n = 254), and tumor adjacent tissues (n = 238). Tissue sections were stained for CD15 using immunohistochemical staining. CD15 expression was detected to identify the distribution of neutrophils in the local tumor microenvironment. The neutrophil density of the tumor tissues and the adjacent tumor tissues was detected to reflect their inflammatory status. Clinical data and follow-up information of cholangiocarcinoma patients who underwent surgery from January 2004 to December 2010 were analyzed retrospectively. The relationship between clinicopathological features and the distribution of neutrophils with prognosis of the patients were analyzed.
RESULTS: The positive expression level of CD15 was only significantly related to the TNM stage. CD15 expression was higher in tumor tissues than in adjacent tissues (73.6% vs 54.6%), with significant differences. Patients with high expression of CD15 had significantly shorter overall survival (OS) than those with low expression of CD15 (median overall survival time 39.77 mo vs 16.87 mo, P = 0.008). Patients with high CD15 expression had significantly shorter disease free survival time (DFS) than those with low expression of CD15 (median DFS 38.27 mo vs 16.83 mo, P = 0.029). COX multivariate analysis indicated that high CD15 expression in tumor tissues was an independent risk factor for predicting OS for patients with cholangiocarcinoma [P = 0.012, relative risk (RR) = 1.601], but it was not an independent risk factor for predicting DFS (P = 0.073, RR = 1.462).
CONCLUSION: Patients with high CD15 expression in cancer tissues had shorter DFS and OS. High expression of CD15 is an independent risk factor for OS.
Keywords: Cholangiocarcinoma, Surgery, Neutrophils, CD15, Prognosis, Tumor microenvironment
Core tip: Inflammation is the 7th important feature to promote tumor development. However, whether inflammation promotes tumor growth in cholangiocarcinoma remains unclear. This study analyzed the inflammation in the tumor microenvironment of the cholangiocarcinoma tissues, and further elaborated the mechanisms of inflammation in cholangiocarcinoma occurrence, development and metastasis, and provided new insights into the treatment of cholangiocarcinoma.
INTRODUCTION
Cholangiocarcinoma originates from epithelial tumors. Diagnosis is often delayed, and prognosis is poor. Clinicians and scientists around the world are committed to determining the etiology of cholangiocarcinoma. Most cholangiocarcinomas are primary. Research from the United States and Europe[1-4], found that hepatitis C is the most important risk factor for cholangiocarcinoma (intrahepatic cholangiocarcinoma in particular), but research from South Korea and China[5-7] found that hepatitis B is a risk factor in intrahepatic cholangiocarcinoma. A study from Japan confirmed the results from Europe and the United States[8]. These studies also confirmed that cirrhosis is a risk factor of cholangiocarcinoma. Primary sclerosing cholangitis may develop into cholangiocarcinoma (especially hilar cholangiocarcinoma), which is characterized by chronic inflammation complicated with liver damage and, possibly, the proliferation of progenitor cells. The incidence rate of cholangiocarcinoma among these patients is 5%-10%[9-12]. Approximately 50% of the patients with primary sclerosing cholangitis were later diagnosed with cholangiocarcinoma within 24 mo[9-13]. The average age of these patients is about 40 years old, while the average age of the general population who develop cholangiocarcinoma is about 70 years old[4,14]. Although there are many risk factors that might promote the development of primary sclerosing cholangitis into cholangiocarcinoma, they are not sufficient to guide risk stratification for disease surveillance[11,15,16]. A full understanding of the biological characteristics of cholangiocarcinoma and important factors that may affect treatment strategies, and implementation of standardized and individualized treatment are the keys to the treatment of cholangiocarcinoma. The relationship between inflammation and cancer development has been the concern of many scholars, and this relationship is also the focus of the present study. In 2011, Hanahan et al[17] in a review published in Cell proposed ten important features of malignant tumors, including self-sufficiency in growth signals, insensitivity to antigrowth signals, resisting cell death, limitless replicative potential, sustained angiogenesis, tissue invasion and metastasis, tumor promotion inflammation, avoiding immune destruction, deregulating cellular energetics, genome instability and mutation. Among these, the tumor-promoting inflammation represents the seventh most important feature. In the course of infection or injury repair, a large number of inflammatory cells in vivo aggregate and release large amounts of cytokines, chemokines, growth factors and cytotoxic mediators, forming a local inflammatory microenvironment. In the process of tumor formation, development and metastasis, cell death and appreciation would occur in a large number of cells, which would produce a large amount of inflammatory mediators. Tumor cells can also produce the factors released through accumulation of inflammatory cells, and these factors can lead to the formation of blood vessels and inflammatory responses. The persistent interactions of inflammatory mediators and cytokines in inflammatory cells lead to the long-term presence of a tumor inflammatory microenvironment, thereby promoting tumorigenesis, accelerating tumor development, and playing an important role in the various stages of tumor metastasis. Neutrophils are major inflammatory cells in the tumor microenvironment. CD15 is one of the markers of mature granulocytes. We used CD15 staining to evaluate the distribution of neutrophils in tumor microenvironment, and to explore the relationship of clinicopathological features and the neutrophil distribution in the tumor microenvironment with prognosis.
MATERIALS AND METHODS
Tumor tissues
Two hundred and ninety one resected specimens from patients who underwent operations for cholangiocarcinoma from January 2004 to December 2010 at the Department of General Surgery, General Hospital of Air Force, China, were analyzed retrospectively. Clinical data (including age, gender and tumor pathological features) were collected. The patients had a clear diagnosis of cholangiocarcinoma and had neither received radiotherapy or chemotherapy before surgery, nor preoperative liver transplantation. Those with a pathological diagnosis of sarcoma, mixed cell carcinoma or hepatocellular carcinoma were excluded. After surgery, all tissue samples were fixed in 4% formalin for 24 h, and then embedded in paraffin for hematoxylin and eosin (HE) and immunohistochemical (IHC) staining. Sections were cut at 4 μm. HE-stained samples were individually examined microscopically by two independent pathologists (Ren L and Mao ZY). The clinicopathological characteristics of the tumors are shown in Table 1.
Table 1.
Relationship between CD15 expression and clinicopathological characteristics in tumor tissues n (%)
| Clinicopathological parameter | Group | n |
CD15 expression in tumor tissues |
|||
| High | Low | χ2 | P value | |||
| Age (yr) | < 60 | 119 | 92 (77.3) | 27 (22.7) | 1.569 | 0.210 |
| ≥ 60 | 135 | 95 (70.4) | 40 (29.6) | |||
| Gender | Male | 170 | 128 (75.3) | 42 (24.7) | 0.740 | 0.390 |
| Female | 84 | 59 (70.2) | 25 (29.8) | |||
| Differentiation | Well differentiated | 17 | 11 (64.7) | 6 (35.3) | 1.093 | 0.579 |
| Moderately differentiated | 140 | 102 (72.9) | 38 (27.1) | |||
| Poorly differentiated | 97 | 74 (76.3) | 23 (23.7) | |||
| TNM stage | I | 136 | 92 (67.6) | 44 (32.4) | 8.589 | 0.032 |
| II | 71 | 54 (76.1) | 17 (23.9) | |||
| III | 34 | 31 (91.2) | 3 (8.8) | |||
| IV | 13 | 10 (76.9) | 3 (23.1) | |||
| Lymphatic metastasis | Metastasis | 39 | 33 (84.6) | 6 (15.4) | 2.867 | 0.090 |
| No metastasis | 215 | 154 (71.6) | 61 (28.4) | |||
| Neural invasion | Yes | 82 | 56 (68.3) | 26 (31.7) | 1.771 | 0.183 |
| No | 172 | 131 (76.2) | 41 (23.8) | |||
| Vascular invasion | Yes | 14 | 10 (71.4) | 4 (28.6) | 0.037 | 0.765 |
| No | 240 | 177 (73.8) | 63 (26.2) | |||
| Infiltration | Non- infiltrative | 148 | 106 (71.6) | 42 (28.4) | 0.731 | 0.393 |
| Infiltrative | 106 | 81 (76.4) | 25 (23.6) | |||
| Size | < 3 cm | 138 | 95 (68.8) | 43 (31.2) | 3.557 | 0.059 |
| ≥ 3 cm | 116 | 92 (79.3) | 24 (20.7) | |||
| Edge | Positive | 63 | 46 (73.0) | 17 (27.0) | 0.016 | 0.900 |
| Negative | 191 | 141 (73.8) | 50 (26.2) | |||
| Position | Intrahepatic | 69 | 52 (75.4) | 17 (24.6) | 0.148 | 0.701 |
| Extrahepatic | 185 | 135 (73.0) | 50 (27.0) | |||
| Surgical approach | Radical | 243 | 179 (73.7) | 64 (26.3) | 0.000 | 1.000 |
| Palliative | 11 | 8 (72.7) | 3 (27.3) | |||
TNM stage: Tumor node metastasis stage.
IHC for CD15
Mouse anti-CD15 Monoclonal Antibody (ZM-0037, Working Solution, Zhongshan Golden Bridge Biotechnology Inc., Beijing, China) was used and heat-induced epitope retrieval (pressure cooker preheat for 5 min in citrate buffer, pH = 6) was used before to CD15 staining. Primary antibodies were then added and incubated for 2 h at 37 °C. Slides were processed on an immunostainer (LabVision Autostainer 360, Fujian, China). The primary antibody was replaced by phosphate-buffered saline as a negative control to assess the specificity of the antibodies. Hematoxylin-counterstained sections were mounted in aqueous mounting medium and observed under a light microscope.
The IHC staining (percentage of stained cells × staining intensity) for CD15 was scored for each case after semi-quantitative evaluation by two independent pathologists (Ren L and Mao ZY). The percentage of stained cells observed in every 100 positive cells was used as the percentage of positive cells. Staining intensity was scored as negative (0), weak (1), moderate (2), or strong (3). The final score of the specimen area were: percentage of positive cells × staining intensity × 100. The final score for each antibody in immunohistochemical staining ranged from 0 to 300 points. With the median score as a borderline, antibodies were assigned to a high expression group and a low expression group, and the median value was assigned to the high expression group.
Statistical analysis
SPSS19.0 statistical software was used for statistical analysis. A χ2 test was used to compare rates, and Spearman’s test was used for correlation analysis. Kaplan-Meier survival curves were used to calculate survival, the log-rank test was used for univariate analysis, and COX regression was adopted for multivariate survival analysis. A P value < 0.05 was considered statistically significant.
RESULTS
Expression of CD15 in tumor tissues and adjacent tissues
The expression of CD15 in the tumor tissues and adjacent tissues is presented in Table 2. CD15 staining in membrane and perinuclear granules is shown in Figure 1. The CD15 expression rate in tumor tissues was 73.6% (187/254), and was 54.6% in adjacent tissues (130/238). CD15 expression in tumor tissues was higher than in adjacent tissues (P = 0.000).
Table 2.
CD15 expression in tumor tissues and adjacent tissues
| Group | n1 | High CD15 expression | Low CD15 expression |
| Tumor tissues | 254 | 187 | 67 |
| Adjacent tissues | 238 | 130 | 108 |
1The number of cases is different from the previous because of off-chip effects during the dyeing process or no tumor tissue.
Figure 1.
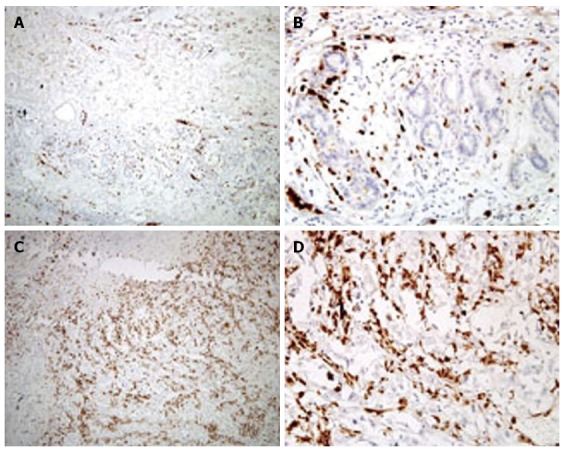
Immunophenotypes of the investigated antigens in tumor tissues. CD15 staining in membrane and perinuclear granules. A: Low CD15 expression, magnification × 100; B: Low CD15 expression, magnification × 400; C: High CD15 expression, magnification × 100; D: High CD15 expression, magnification × 400.
Correlations between CD15 expression levels and clinicopathological characteristics of tumors
The correlations between CD15 expression and clinicopathological features are shown in Table 3. The positive expression rate of CD15 was significantly related to the TNM stage (Figure 2).
Table 3.
Multivariate survival analysis of CD15 expression in cancer tissues and clinicopathological features
| Parameters |
Risk of recurrence |
Risk of death |
||||||
| P value | Exp(B) |
95%CI for Exp(B) |
P value | Exp(B) |
95%CI for Exp(B) |
|||
| Lower | Upper | Lower | Upper | |||||
| Differentiation | 0.004 | 1.545 | 1.148 | 2.079 | 0.007 | 1.43 | 1.103 | 1.853 |
| TNM stage | 0.001 | 1.535 | 1.185 | 1.989 | 0.013 | 1.324 | 1.061 | 1.652 |
| Lymphatic metastasis | 0.854 | 0.952 | 0.562 | 1.611 | 0.114 | 1.427 | 0.918 | 2.217 |
| Neural invasion | 0.532 | 1.134 | 0.764 | 1.684 | 0.827 | 1.039 | 0.736 | 1.468 |
| Vascular invasion | 0.227 | 0.594 | 0.255 | 1.382 | 0.965 | 0.986 | 0.523 | 1.859 |
| Infiltration | 0.742 | 0.928 | 0.595 | 1.448 | 0.585 | 1.112 | 0.759 | 1.63 |
| Size | 0.943 | 1.014 | 0.692 | 1.487 | 0.758 | 0.949 | 0.679 | 1.326 |
| Edge | 0.014 | 1.014 | 0.692 | 1.487 | 0.007 | 1.641 | 1.148 | 2.347 |
| Position | 0.551 | 0.859 | 0.521 | 1.416 | 0.466 | 0.849 | 0.546 | 1.319 |
| Surgical approach | 0.575 | 1.258 | 0.564 | 2.804 | 0.161 | 1.62 | 0.826 | 3.178 |
| CD15 | 0.073 | 1.462 | 0.965 | 2.215 | 0.012 | 1.601 | 1.107 | 2.314 |
TNM: Tumor node metastasis.
Figure 2.
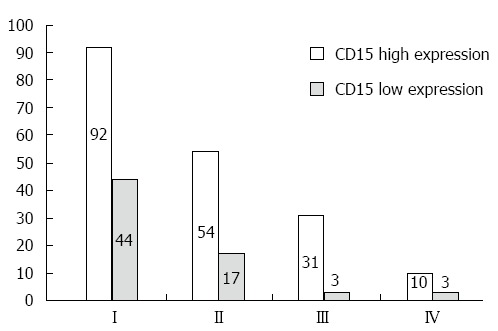
Histogram of the correlation between CD15 expression in tumor tissues and tumor node metastasis staging. TNM: Tumor node metastasis.
Correlation between CD15 expression in tumor tissues and prognosis
Patients with high expression of CD15 had significantly shorter overall survival (OS) than those with low expression, and the difference was statistically significant (P = 0.008). Patients with high CD15 expression had significantly shorter disease free survival time (DFS) than those with low CD15 expression (P = 0.029). Based on COX multivariate analysis, high CD15 expression in cancer tissues was an independent risk factor in predicting OS for patients with cholangiocarcinoma [P = 0.012, relative risk (RR) =1.601]. Univariate analysis showed that patients with high CD15 expression in tumor tissues had significantly shorter progression-free survival and overall survival than those with low expression, and the difference was statistically significant (median DFS: 38.37 mo vs 16.83 mo; median OS: 39.77 mo vs 16.87 mo, P = 0.029 and 0.008, respectively), as shown in Figures 3 and 4.
Figure 3.
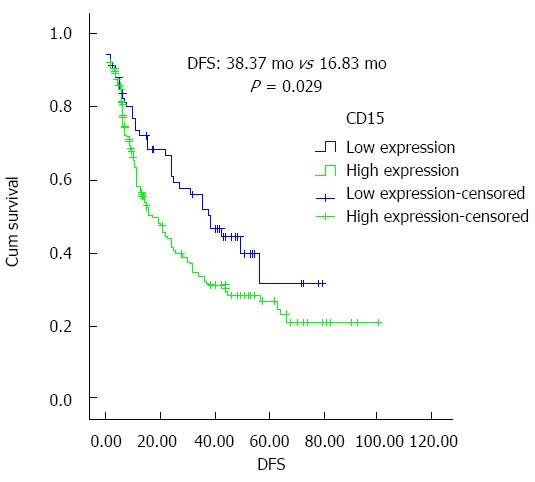
CD15 expression in tumor tissues and disease free survival time. DFS: Disease free survival time.
Figure 4.
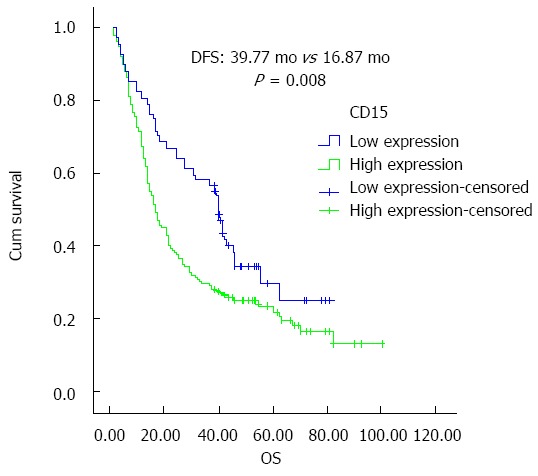
CD15 expression in tumor tissues and overall survival. OS: overall survival.
Multivariate analysis in CD15 expression and prognosis of patients with cholangiocarcinoma
The clinical and pathological features of patients with cholangiocarcinoma were analyzed using univariate analysis, and the statistically significant results obtained and CD15 levels were then included in COX multivariate analysis to observe the effects of the indicators on prognosis. As shown in Table 3, high CD15 expression in tumor tissues is an independent risk factor for OS of the patients with cholangiocarcinoma, and patients with high CD15 expression in tumor tissues had a 1.601 times higher risk of death compared with patients with low CD15 expression.
DISCUSSION
Although inflammation is a risk factor for the onset of cholangiocarcinoma, the mechanism is not fully understood. Many experiments found that the relationship between a tumor and inflammation can be studied based on the local inflammatory tumor microenvironment, and a systemic inflammatory response. For example, C-reactive protein and IL-6 are considered to reflect the level of markers of systemic inflammation. Increased levels can predict the prognosis of various tumors, and the higher the level of the inflammation, the worse the prognosis[18,19]. Coincident with our results, Jensen et al[20] reported that the presence of intratumoral neutrophils was an independent prognostic factor for poor OS following nephrectomy in localized clear cell tumors, such as renal cell carcinoma. Li et al[21] confirmed recently that intratumoral neutrophils were a poor prognostic factor for hepatocellular carcinoma following resection. Furthermore, elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma[22,23], small-cell lung carcinoma[24], gastric cancer[25-27] and other tumors[28]. All these studies indicated that patients with tumors infiltrating neutrophils have a poor prognosis, which may offer some help in a preliminary clinical prognosis of cancer patients, and provide clues for treatment.
CD15 is a specific marker of neutrophils[29]. The white blood cell count is an important index in the detection of inflammation in vivo. Inflammation can promote tumor angiogenesis, growth and invasion[30-32]. Neutrophils account for 50%-70% of total circulating leukocytes[33,34]. Neutrophils are the most abundant white blood cells, which help the body against invading microorganisms. Therefore, the number of neutrophils is an important inflammatory marker. Currently the neutrophil-to-lymphocyte ratio is studied extensively, and the ratio is used to assess the overall survival of cancer patients as a simple and effective indicator. A higher ratio indicates a poor overall survival, and a risk of early relapse[35-37]; thus, an increased neutrophil count and CD15 expression. This study analyzed the effects of CD15 expression in local inflammatory status on DFS and OS, and found that CD15 expression in cancer tissues was significantly higher than in the adjacent tissues. By univariate analysis, CD15 expression in cancer tissues affected significantly the DFS and OS, and high CD15 expression in cancer tissues predicted higher risk of tumor recurrence and mortality. CD15 is a primary marker for neutrophils, high CD15 expression indicates increased neutrophil count and reduced lymphocyte count. The release of large amounts of cytokines and other inflammatory cytokines may contribute to an increase in tumor metastasis and tumor angiogenesis[38-43]. Therefore, the CD15 expression in cancer tissues suggested poor prognosis in this study, which is consistent with the findings mentioned above. CD15 positive neutrophils may predict poor prognosis, especially for the prediction of OS, but not for DFS. However, prediction of OS could help in the treatment of postoperative cholangiocarcinoma, and CD15 immunohistochemical staining results could predict the survival of patients earlier, such that intervention can be started earlier, which would extend the survival of patients.
This study used inflammation-related prognostic indicators to predict the prognosis of patients with cholangiocarcinoma. The local inflammatory status in the tumor microenvironment was evaluated by CD15 immunohistochemical staining. The results were scored to detect the distribution of cholangiocarcinoma inflammatory cells in the tumor microenvironment and to analyze the correlation between the distribution of cholangiocarcinoma inflammatory cells in the tumor microenvironment and local inflammation and prognosis, to reflect the relationship between inflammation and cholangiocarcinoma.
There are currently no approved targeted agents for cholangiocarcinoma[44]. However, drug-related genes have been detected, including KRAS, PIK3CA, MET, EGFR, BRAF and NRAS[45]. In some cholangiocarcinomas, gene mutations occur, but targeted therapy is still not effective, possibly because cholangiocarcinoma gene mutation is relatively complex, involving mutations of two or more genes[46]. Yoshikawa et al[47] used Vandetanib to treat cholangiocarcinoma and inhibit both VEGFR and EGFR signalling appears a promising therapeutic approach for cholangiocarcinoma. The absence of KRAS mutation and the presence of EGFR amplification may be potential predictive molecular markers of sensitivity to EGFR-targeted therapy in cholangiocarcinoma.
El-Khoueiry et al[48] used Sorafenib in two clinical studies for unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma treatment, but did not achieve objective responses. Patients with biliary cancers receiving this drug showed some therapeutic benefit. Additional studies with Sorafenib in combination with chemotherapy or other targeted agents may be warranted. Takahashi et al[49] suggested that Axitinib is a promising therapy for vascular endothelial growth factor-expressing cholangiocarcinoma, irrespective of tumor origin and Gemcitabine sensitivity. El-Khoueiry et al[50] reported that Sorafenib and Erlotinib did not have promising clinical activities in an unselected population of patients with biliary cancers. Improved patient selection, based on tumor biology and molecular markers, is critical for future evaluation of targeted therapies in this disease. Based on several promising phase II clinical trials currently underway, the most promising targeted agents for cholangiocarcinoma thus far include anti-EGFR agents, MEK inhibitors and anti-angiogenic agents[44].
In summary, distribution of local inflammatory cells in patients with cholangiocarcinoma in the tumor microenvironment is valuable to assess prognosis. The local inflammatory state, in varying degrees, reflects the in vivo state of chronic inflammation in patients with malignant tumors. The chronic inflammatory state and the resulting immune status deserve further study and are meaningful for subsequent treatment of cancers. These results indicate that neutrophil distribution in various cancers might indicate poor prognosis and may serve as a target for clinical treatment.
COMMENTS
Background
Cholangiocarcinoma accounts for 3% of all gastrointestinal tumors and is the second most common tumor in hepatobiliary cancers. A full understanding of the biological characteristics of cholangiocarcinoma and the adoption standardized, individualized treatment are the key to cholangiocarcinoma treatment. Clinicians and scientists around the world are committed to determining the etiology of cholangiocarcinoma to permit early diagnosis and treatment of cholangiocarcinoma. This study investigated the inflammatory cell distribution in the tumor microenvironment and further elaborated the mechanisms of inflammation in cholangiocarcinoma occurrence, development and metastasis, to provide new insights into the treatment of cholangiocarcinoma.
Research frontiers
In this study, the authors investigated the expression of CD15 by immunohistochemical (IHC) staining to examine inflammatory state of cholangiocarcinoma. The results are helpful to understand the occurrence, development and metastasis of cholangiocarcinoma.
Innovations and breakthroughs
In this study, the authors explored the expression of CD15 to reflect inflammatory state of cholangiocarcinoma: the expression of CD15 had been seldom detected in cholangiocarcinoma. The results could help explain the occurrence, development and metastasis of cholangiocarcinoma.
Applications
IHC staining of CD15 to mark neutrophils and to reflect the inflammation caused by cholangiocarcinoma is a fast, convenient and economical method in clinical applications.
Terminology
Cholangiocarcinoma originates from epithelial tumors. Diagnosis is often delayed and prognosis is poor. Cholangiocarcinoma accounts for 3% of all gastrointestinal cancers, and is the second most common tumor in hepatobiliary cancers.
Peer-review
The authors conducted an IHC analysis of CD15 to assess the neutrophil distribution and to further reflect the inflammatory state of cholangiocarcinoma. The study is very interesting.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 20, 2014
First decision: November 14, 2014
Article in press: January 21, 2015
P- Reviewer: Schmelzle M S- Editor: Yu J L- Editor: Stewart GJ E- Editor: Ma S
References
- 1.Welzel TM, Mellemkjaer L, Gloria G, Sakoda LC, Hsing AW, El Ghormli L, Olsen JH, McGlynn KA. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer. 2007;120:638–641. doi: 10.1002/ijc.22283. [DOI] [PubMed] [Google Scholar]
- 2.Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959–964. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116–123. doi: 10.1002/hep.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 5.Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, Kwon S, Lee SK, Seo DW, Kim MH, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008;103:1716–1720. doi: 10.1111/j.1572-0241.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14:632–635. doi: 10.3748/wjg.14.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, Takemura S, Tanaka H, Yamazaki O, Hirohashi K, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95:592–595. doi: 10.1111/j.1349-7006.2004.tb02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:1051–1058. doi: 10.1097/MEG.0b013e3283554bbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 11.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 12.Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158–164. doi: 10.1016/j.jhep.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Boberg KM, Bergquist A, Mitchell S, Pares A, Rosina F, Broomé U, Chapman R, Fausa O, Egeland T, Rocca G, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37:1205–1211. doi: 10.1080/003655202760373434. [DOI] [PubMed] [Google Scholar]
- 14.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54:1842–1852. doi: 10.1002/hep.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto K, Ikeda Y, Korenaga D, Tanoue K, Hamatake M, Kawasaki K, Yamaoka T, Iwatani Y, Akazawa K, Takenaka K. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103:1856–1864. doi: 10.1002/cncr.20976. [DOI] [PubMed] [Google Scholar]
- 19.Porta C, De Amici M, Quaglini S, Paglino C, Tagliani F, Boncimino A, Moratti R, Corazza GR. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19:353–358. doi: 10.1093/annonc/mdm448. [DOI] [PubMed] [Google Scholar]
- 20.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 21.Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, Xu YF. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 22.Hughes-Benzie RM, Hunter AG, Allanson JE, Mackenzie AE. Simpson-Golabi-Behmel syndrome associated with renal dysplasia and embryonal tumor: localization of the gene to Xqcen-q21. Am J Med Genet. 1992;43:428–435. doi: 10.1002/ajmg.1320430165. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt H, Suciu S, Punt CJ, Gore M, Kruit W, Patel P, Lienard D, von der Maase H, Eggermont AM, Keilholz U. Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American Joint Committee on Cancer Stage IV Melanoma: results of the EORTC 18951 Biochemotherapy Trial. J Clin Oncol. 2007;25:1562–1569. doi: 10.1200/JCO.2006.09.0274. [DOI] [PubMed] [Google Scholar]
- 24.Paesmans M, Sculier JP, Lecomte J, Thiriaux J, Libert P, Sergysels R, Bureau G, Dabouis G, Van Cutsem O, Mommen P, Ninane V, Klastersky J. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer. 2000;89:523–533. doi: 10.1002/1097-0142(20000801)89:3<523::aid-cncr7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Jung MR, Park YK, Jeong O, Seon JW, Ryu SY, Kim DY, Kim YJ. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104:504–510. doi: 10.1002/jso.21986. [DOI] [PubMed] [Google Scholar]
- 26.Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–220. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 28.Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23:200–207. doi: 10.1016/j.semcancer.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Kerr MA, Stocks SC. The role of CD15-(Le(X))-related carbohydrates in neutrophil adhesion. Histochem J. 1992;24:811–826. doi: 10.1007/BF01046353. [DOI] [PubMed] [Google Scholar]
- 30.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniel D, Chiu C, Giraudo E, Inoue M, Mizzen LA, Chu NR, Hanahan D. CD4+ T cell-mediated antigen-specific immunotherapy in a mouse model of cervical cancer. Cancer Res. 2005;65:2018–2025. doi: 10.1158/0008-5472.CAN-04-3444. [DOI] [PubMed] [Google Scholar]
- 33.Strell C, Lang K, Niggemann B, Zaenker KS, Entschladen F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp Cell Res. 2010;316:138–148. doi: 10.1016/j.yexcr.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 35.Bertuzzo VR, Cescon M, Ravaioli M, Grazi GL, Ercolani G, Del Gaudio M, Cucchetti A, D’Errico-Grigioni A, Golfieri R, Pinna AD. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation. 2011;91:1279–1285. doi: 10.1097/TP.0b013e3182187cf0. [DOI] [PubMed] [Google Scholar]
- 36.Huang ZL, Luo J, Chen MS, Li JQ, Shi M. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2011;22:702–709. doi: 10.1016/j.jvir.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 37.Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27:553–561. doi: 10.1111/j.1440-1746.2011.06910.x. [DOI] [PubMed] [Google Scholar]
- 38.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci USA. 2008;105:2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 42.Benelli R, Albini A, Noonan D. Neutrophils and angiogenesis: potential initiators of the angiogenic cascade. Chem Immunol Allergy. 2003;83:167–181. doi: 10.1159/000071560. [DOI] [PubMed] [Google Scholar]
- 43.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faris JE, Zhu AX. Targeted therapy for biliary tract cancers. J Hepatobiliary Pancreat Sci. 2012;19:326–336. doi: 10.1007/s00534-011-0496-0. [DOI] [PubMed] [Google Scholar]
- 45.Voss JS, Holtegaard LM, Kerr SE, Fritcher EG, Roberts LR, Gores GJ, Zhang J, Highsmith WE, Halling KC, Kipp BR. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum Pathol. 2013;44:1216–1222. doi: 10.1016/j.humpath.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts LR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031.e15. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshikawa D, Ojima H, Kokubu A, Ochiya T, Kasai S, Hirohashi S, Shibata T. Vandetanib (ZD6474), an inhibitor of VEGFR and EGFR signalling, as a novel molecular-targeted therapy against cholangiocarcinoma. Br J Cancer. 2009;100:1257–1266. doi: 10.1038/sj.bjc.6604988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Khoueiry AB, Rankin CJ, Ben-Josef E, Lenz HJ, Gold PJ, Hamilton RD, Govindarajan R, Eng C, Blanke CD. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs. 2012;30:1646–1651. doi: 10.1007/s10637-011-9719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi H, Ojima H, Shimizu H, Furuse J, Furukawa H, Shibata T. Axitinib (AG-013736), an oral specific VEGFR TKI, shows potential therapeutic utility against cholangiocarcinoma. Jpn J Clin Oncol. 2014;44:570–578. doi: 10.1093/jjco/hyu045. [DOI] [PubMed] [Google Scholar]
- 50.El-Khoueiry AB, Rankin C, Siegel AB, Iqbal S, Gong IY, Micetich KC, Kayaleh OR, Lenz HJ, Blanke CD. S0941: a phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br J Cancer. 2014;110:882–887. doi: 10.1038/bjc.2013.801. [DOI] [PMC free article] [PubMed] [Google Scholar]


