Abstract
Gastric adenocarcinoma is one of the most common malignancies worldwide. Histochemical and immunohistologic analyses classify the phenotypes of gastric adenocarcinoma into several groups based on the variable clinical and pathologic features. A new and rare variant of gastric adenocarcinoma with chief cell differentiation (GA-CCD) has recently been recognized. Studies reporting the distinct clinicopathologic characteristics proposed the term oxyntic gland polyp/adenoma because of the benign nature of the GA-CCD. Typically, GA-CCD is a solitary mucosal lesion that develops either in the gastric cardia or fundus. Histologically, this lesion is characterized by tightly clustered glands and anastomosing cords of chief cells. Immunohistochemically, GA-CCD is diffusely positive for mucin (MUC) 6 and negative for MUC2 and MUC5AC. However, other gastric tumors such as a gastric neuroendocrine tumor or fundic gland polyp have been difficult to exclude. Because GA-CCD tends to be endoscopically misdiagnosed as a neuroendocrine tumor or fundic gland polyp, comprehensive assessment and observation by an endoscopist are strongly recommended. Herein, we report a rare case of oxyntic gland adenoma endoscopically mimicking a gastric neuroendocrine tumor that was successfully removed by endoscopic mucosal resection.
Keywords: Chief cell differentiation, Gastric carcinoma, Mucin 6, Neuroendocrine tumor, Oxyntic gland adenoma
Core tip: Gastric adenocarcinoma with chief cell differentiation is a new and rare variant of gastric adenocarcinoma. Due to difficulty in ruling out other gastric tumors, such as gastric neuroendocrine tumor, fundic gland polyp, or gastric adenoma, comprehensive examination and observation by an endoscopist are strongly recommended. We report a rare case of oxyntic gland adenoma endoscopically mimicking a gastric neuroendocrine tumor that was successfully removed by endoscopic mucosal resection.
INTRODUCTION
First reported in 2007, fundic gland type with chief cell differentiation represents a novel, rare variant of gastric adenocarcinoma[1]. Singhi et al[2] recently reported ten cases of gastric adenocarcinoma with chief cell differentiation (GA-CCD) in patients from multiple ethnic backgrounds. The term oxyntic gland polyp/adenoma was proposed instead of GA-CCD, as GA-CCD is regarded as benign based on the natural course of the disease described in the previous studies. GA-CCD is very rare, with unknown clinicopathologic features and natural course[1-3]. We herein report a rare case of oxyntic gland adenoma endoscopically mimicking a gastric neuroendocrine tumor that was successfully removed by endoscopic mucosal resection (EMR).
CASE REPORT
A 73-year-old man with a history of type 2 diabetes mellitus, dyslipidemia and benign prostate hypertrophy was admitted for evaluation of a suspected gastric neuroendocrine tumor following an esophagogastroduodenoscopy (EGD) screening of a gastric lesion. The patient had no history of alcohol use or smoking. Upon physical examination, the patient exhibited no specific symptoms or abnormalities. Blood test results were as follows: white blood cell count, 5.9 × 103/μL (normal range: 4.0-10.0 × 103/μL); hemoglobin, 15.9 g/dL (normal range: 12.0-16.0 g/dL); platelet count, 162 × 103/μL (normal range: 150-350 × 103/μL). All other laboratory findings were within normal limits. The serum gastrin level was 61.5 pg/mL (normal range: 0.0-180.0 pg/mL). EGD showed a 6 mm, slightly elevated, yellowish lesion with irregular surface vessels at the greater curvature of the upper body (Figure 1). The rapid urease test (CLO test; Delta West Pty Ltd., Bentley, Australia) was negative. Endoscopic ultrasonography revealed a 6.7 mm, homogeneous, hypoechoic mass invading the submucosal layer (Figure 2) with a thick mucosa. No specific gastric wall abnormalities or lymph node enlargements were noted on the abdominal CT scan.
Figure 1.
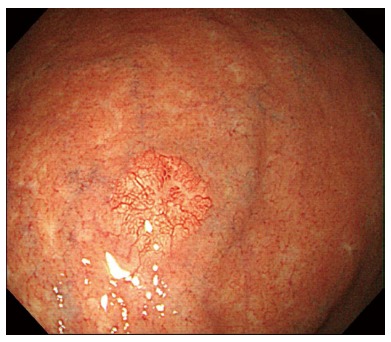
Esophagogastroduodenoscopy findings. Esophagogastroduodenoscopy revealed a 6 mm, slightly elevated, yellowish lesion with irregular surface vessels at the greater curvature of the upper body.
Figure 2.
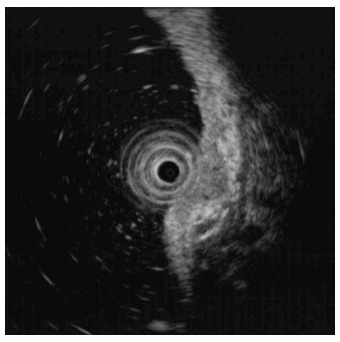
Endoscopic ultrasonography findings. Endoscopic ultrasonography revealed a 6.7 mm, homogeneous, hypoechoic mass that appeared to invade the submucosal layer.
EMR was performed under conscious sedation for the duration of the treatment in order to establish the definitive diagnosis of the lesion. The inject-lift-and-cut technique was employed through two separate channels of a double-channel endoscope; the grasping forceps were used to lift the lesion, and an electrocautery snare was used to remove the lesion (Figure 3). No complications, such as bleeding or perforation, were observed after the procedure. The patient started eating two days after EMR, and was discharged on the third hospital day.
Figure 3.
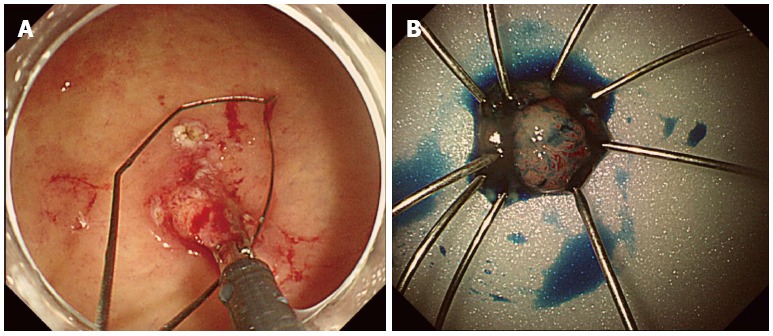
Endoscopic mucosal resection findings. A: The inject-lift-and-cut technique was performed; B: Pathologic specimen.
Sections from the stomach showed proliferation of glands with complex architecture confined to the mucosa and no submucosal invasion (Figure 4A). A high-power view of the lesion showed a complex growth of glands lined with one to two cell layers of oxyntic epithelium containing abundant chief cells (Figure 4B). The lining cells showed moderate nuclear pleomorphism with no identifiable mitotic figures. Immunohistochemical analysis showed most of the glandular cells were positive for mucin (MUC) 6 (Figure 5) and negative for MUC2, MUC5AC and α-fetoprotein. Ki-67 index was < 1% (Figure 5E). Consequently, the patient was diagnosed with oxyntic gland adenoma with high-grade dysplasia that correlated with immunohistochemical staining. A follow-up EGD, performed two months after the EMR, showed a whitish scar with no recurrence.
Figure 4.
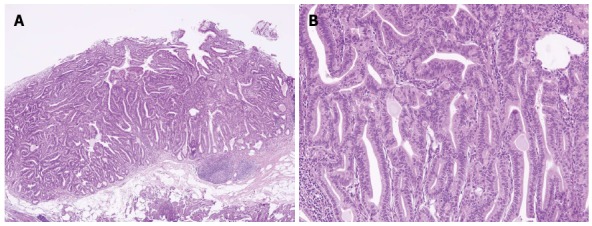
Pathologic examinations. A: Hematoxylin and eosin staining showed proliferation of glands with complex architecture, situated deep in the mucosal layer with surface involvement (× 40); B: A higher magnification showed complex growth of glands lined with one to two layers of oxyntic epithelium with abundant chief cells. The lining cells showed moderate nuclear pleomorphism, but no mitotic figures were identified (× 200).
Figure 5.
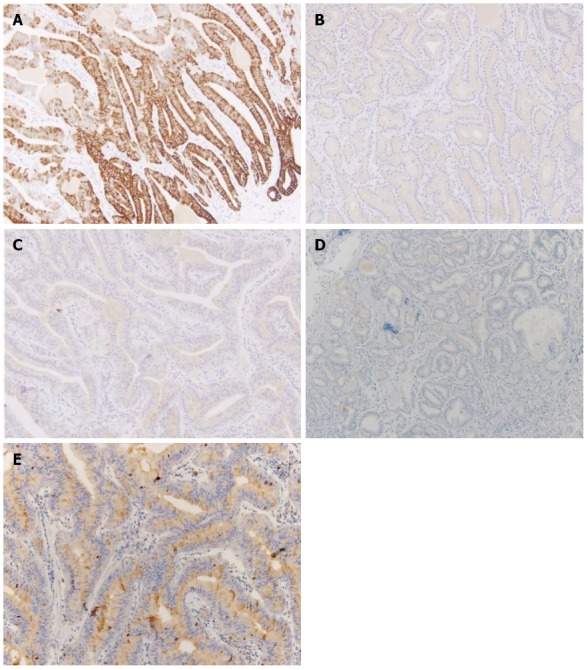
Immunohistochemical staining for mucin, α-fetoprotein, and Ki-67. A: Most of the glandular cells were positive for mucin (MUC) 6; B: Most of the glandular cells were negative for MUC2; C: Most of the glandular cells were negative for MUC5AC; D: Most of the glandular cells were negative for alpha fetal protein; E: Ki-67 immunolabeling demonstrates a low proliferation index (< 1%).
DISCUSSION
Gastric adenocarcinoma is defined as a malignant epithelial tumor, originating from glandular epithelium of the gastric mucosa[2]. Using previous histochemical and immunohistologic analyses for MUC, the phenotype of gastric adenocarcinoma can be classified into various subtypes[1]. The gastric phenotypes include the foveolar, pyloric gland, and fundic gland types[4]. Currently, little information is available concerning gastric adenocarcinoma featuring fundic gland cell types[1]. GA-CCD is a recently reported rare variant of gastric adenocarcinoma[2]. After the initial report on GA-CCD[1], the subsequent follow-up study of ten GA-CCD cases expanded on the clinicopathologic features of this disease[3]. Park et al[4] reported three cases of GA-CCD among Koreans (Table 1). Interestingly, Singhi et al[2] noted that the use of the term GA-CCD seemed to overemphasize the biologic potential of these lesions, which have not been reported to metastasize and display a lower proliferative index compared to fundic gland polyps. Considering the benign feature of GA-CCD, these authors proposed the term oxyntic gland polyp/adenoma to be used until further studies could clarify the pathogenesis of these lesions and their natural history[2]. In our case, a lesion endoscopically mimicking a gastric neuroendocrine tumor was diagnosed as oxyntic gland adenoma. Initial biopsy findings were chronic gastritis with intestinal metaplasia. However, EMR allowed for a definitive diagnosis and treatment of an oxyntic gland adenoma with high grade-dysplasia. The previously reported cases of oxyntic gland adenoma have had favorable prognoses[1-4]. Similarly, the present case was expected to have a good prognosis despite the presence of high-grade dysplasia. However, a more prolonged follow-up investigation of the patient is needed. Some studies have reported the presence of histologic variants of gastric adenocarcinoma featuring specific cell types based on MUC immunohistochemistry or electron microscopy, including choriocarcinoma, hepatoid carcinoma, carcinoma with lymphoid stroma, Paneth cell carcinoma, small cell carcinoma, and parietal cell carcinoma[5]. Depending on the variant, the prognosis was either better or worse than the typical gastric adenocarcinoma[5].
Table 1.
Recently published literature on oxyntic gland polyp/adenoma cases
| Ref. | Journal | Year | Number of cases | Title |
| Tsukamoto et al[1] | Pathol Int | 2007 | 1 | Gastric adenocarcinoma with chief cell differentiation |
| Ueyama et al[3] | Am J Surg Pathol | 2010 | 10 | Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma |
| Park et al[4] | Korean J Pathol | 2012 | 3 | Gastric adenocarcinoma of fundic gland type: report of three cases |
| Singhi et al[2] | Am J Surg Pathol | 2012 | 10 | Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma |
| Ueyama et al[9] | Endosc | 2014 | 10 | Gastric adenocarcinoma of the fundic gland type (chief cell predominant type) |
Histologically, GA-CCD is a well-differentiated adenocarcinoma composed primarily of cells resembling chief cells[1-4]. GA-CCD is typically centered in the deep mucosa and consists of clustered glands and irregular anastomosing cords of predominantly chief cells[1]. In our present case, no lymphovascular or submucosal invasion, geographic necrosis or mitotic figures were identified, which helped exclude malignancy despite the striking architectural disturbance. MUCs are high-molecular-weight glycoproteins synthesized by secretory epithelial cells as membrane-bound or secreted products[6]. Pinto-de-Sousa et al[7] showed the MUC phenotype associated with the tumor site. Normal gastric mucosa express MUC1, MUC4, MUC5AC, and MUC6[6]. According to the previous reports, GA-CCD is diffusely positive for MUC6, but negative for MUC2 and MUC5AC[1-4,8]. In our present case, immunohistochemical analysis revealed the lesion positive for MUC6 and negative for MUC2, MUC5AC, and α-fetoprotein with a low Ki-67 proliferative index, excluding a neuroendocrine tumor and confirming the diagnosis.
In several studies, GA-CCD was located in the upper third of the stomach and arose from a deeper area of the gastric mucosa[1-3]. For this reason, endoscopic findings of GA-CCD may resemble those of fundic gland polyp or gastric neuroendocrine tumor[4]. Recently, Ueyama et al[9] analyzed the endoscopic findings of ten GA-CCD cases including the shape, color, vessels and background mucosa using the Sydney system to elucidate the endoscopic characteristics[10]. In terms of gross appearance, all of the lesions were classified into two subtypes: the submucosal tumor-shape type (60%) and a flat and depressed type (40%)[9]. Therefore, it was suggested that the submucosal tumor-shape type is needed to rule out other submucosal tumors. Consequently, the authors proposed that the discriminating characteristics for gastric neuroendocrine tumor were related to color tone, vessels, and hardness. Gastric neuroendocrine tumors have a yellow tone, few vessels on the surface, and a hard appearance, whereas GA-CCD are faded or whitish, have a soft appearance, and have more branched vessels on the surface[9]. Endoscopically, the four most frequent features of GA-CCD were submucosal tumor shape, whitish color, dilated vessels with branch architecture, and background mucosa without atrophic change[9]. In our present case, endoscopic findings showed that the lesion was hard, movable, and a submucosal tumor shape, with irregular and dilated vessels on the surface, consistent with two of the four most frequent features of GA-CCD. Further research is necessary to better our understanding of the endoscopic characteristics of GA-CCD.
Helicobacter pylori (H. pylori) plays an important role in gastric carcinogenesis, as the majority of non-cardia gastric cancers develop from H. pylori-infected mucosa[11]. Literature research did not yield any references describing a relationship between H. pylori infection and oxyntic gland adenoma. In the case of our patient, the rapid urease test was negative. Further research is necessary to elucidate the relationship between H. pylori infection and oxyntic gland adenoma.
In conclusion, the lesion was diagnosed as GA-CCD based on the fact that the lesion endoscopically mimicked a gastric neuroendocrine tumor with shared characteristics of chief cells, was positive for unique histopathologic features, and expressed MUC6. Because GA-CCD tends to be endoscopically misdiagnosed as a neuroendocrine tumor or fundic gland polyp, a tailored examination and observation by an endoscopist are strongly recommended.
COMMENTS
Case characteristics
A 73-year-old man with a history of type 2 diabetes mellitus, dyslipidemia and benign prostate hypertrophy was admitted for evaluation of a suspected gastric neuroendocrine tumor following esophagogastroduodenoscopy screening of a gastric lesion.
Clinical diagnosis
On physical examination, the patient exhibited no specific symptoms or abnormalities.
Differential diagnosis
Gastric adenocarcinoma; gastric neuroendocrine tumor; gastric polyp.
Laboratory diagnosis
White blood cell, 5.85 × 103/μL; hemoglobin, 15.90 gm/dL; platelet, 162 × 103/μL; serum gastrin level, 61.5 pg/mL; metabolic panel and liver function test were within normal limits.
Imaging diagnosis
Endoscopic gastroduodenoscopy revealed a 6 mm, slightly elevated, yellowish lesion with irregular surface vessels at the greater curvature of the gastric upper body.
Pathological diagnosis
Biopsy revealed oxyntic gland adenoma with high-grade dysplasia, positive for mucin (MUC) 6, and negative for MUC2, MUC5AC, and α-fetoprotein.
Treatment
The patient was treated with endoscopic mucosal resection without complications.
Related reports
There was a clear margin of endoscopically resected lesion with no lymphovascular invasion.
Experiences and lessons
Because gastric adenocarcinoma with chief cell differentiation tends to be endoscopically misdiagnosed as a neuroendocrine tumor or fundic gland polyp, a comprehensive examination and observation by an endoscopist are strongly recommended.
Peer-review
This case report is of great interest as the authors describe a rare case of oxyntic gland adenoma endoscopically mimicking gastric neuroendocrine tumor that was successfully removed by endoscopic mucosal resection.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 23, 2014
First decision: October 14, 2014
Article in press: January 16, 2015
P- Reviewer: Aoyagi K, Ji JF S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Tsukamoto T, Yokoi T, Maruta S, Kitamura M, Yamamoto T, Ban H, Tatematsu M. Gastric adenocarcinoma with chief cell differentiation. Pathol Int. 2007;57:517–522. doi: 10.1111/j.1440-1827.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- 2.Singhi AD, Lazenby AJ, Montgomery EA. Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma. Am J Surg Pathol. 2012;36:1030–1035. doi: 10.1097/PAS.0b013e31825033e7. [DOI] [PubMed] [Google Scholar]
- 3.Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609–619. doi: 10.1097/PAS.0b013e3181d94d53. [DOI] [PubMed] [Google Scholar]
- 4.Park ES, Kim YE, Park CK, Yao T, Kushima R, Kim KM. Gastric adenocarcinoma of fundic gland type: report of three cases. Korean J Pathol. 2012;46:287–291. doi: 10.4132/KoreanJPathol.2012.46.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takubo K, Honma N, Sawabe M, Arai T, Izumiyama-Shimomura N, Kammori M, Sasajima K, Esaki Y. Oncocytic adenocarcinoma of the stomach: parietal cell carcinoma. Am J Surg Pathol. 2002;26:458–465. doi: 10.1097/00000478-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Toki F, Takahashi A, Aihara R, Ogata K, Ando H, Ohno T, Mochiki E, Kuwano H. Relationship between clinicopathological features and mucin phenotypes of advanced gastric adenocarcinoma. World J Gastroenterol. 2010;16:2764–2770. doi: 10.3748/wjg.v16.i22.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto-de-Sousa J, David L, Reis CA, Gomes R, Silva L, Pimenta A. Mucins MUC1, MUC2, MUC5AC and MUC6 expression in the evaluation of differentiation and clinico-biological behaviour of gastric carcinoma. Virchows Arch. 2002;440:304–310. doi: 10.1007/s00428-001-0548-y. [DOI] [PubMed] [Google Scholar]
- 8.Müller-Höcker J, Rellecke P. Chief cell proliferation of the gastric mucosa mimicking early gastric cancer: an unusual variant of fundic gland polyp. Virchows Arch. 2003;442:496–500. doi: 10.1007/s00428-003-0780-8. [DOI] [PubMed] [Google Scholar]
- 9.Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type) Endoscopy. 2014;46:153–157. doi: 10.1055/s-0033-1359042. [DOI] [PubMed] [Google Scholar]
- 10.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]


