Abstract
Background
Non-Hodgkin lymphoma (NHL) is the sixth most common cancer in the UK; approximately 35 people are diagnosed and 13 die from the disease daily.
Aim
To identify the primary care clinical features of NHL and quantify their risk in symptomatic patients.
Design and setting
Matched case–control study using Clinical Practice Research Datalink patient records.
Method
Putative clinical features of NHL were identified in the year before diagnosis. Results were analysed using conditional logistic regression and positive predictive values (PPVs).
Results
A total of 4362 patients aged ≥40 years, diagnosed with NHL between 2000 and 2009, and 19 468 age, sex, and general practice-matched controls were studied. Twenty features were independently associated with NHL. The five highest risk symptoms were lymphadenopathy, odds ratio (OR) 263 (95% CI = 133 to 519), head and neck mass not described as lymphadenopathy OR 49 (95% CI = 32 to 74), other mass OR 12 (95% CI = 10 to 16), weight loss OR 3.2 (95% CI = 2.3 to 4.4), and abdominal pain OR 2.5 (95% CI = 2.1 to 2.9). Lymphadenopathy has a PPV of 13% for NHL in patients ≥60 years. Weight loss in conjunction with repeated back pain or raised gamma globulin had PPVs >2%.
Conclusion
Unexplained lymphadenopathy in patients aged ≥60 years produces a very high risk of NHL in primary care. These patients warrant urgent investigation, potentially sooner than 6 weeks from initial presentation where the GP is particularly concerned.
Keywords: cancer, clinical features, diagnosis, lymphadenopathy, non-Hodgkin lymphoma, primary health care
INTRODUCTION
Lymphoma is lymphocyte cancer, with two main types: non-Hodgkin lymphoma (NHL), which makes up approximately 90% of lymphomas and Hodgkin lymphoma (around 10%). NHL has over 60 subtypes.1 It is the sixth most common UK cancer,2 with approximately 12 800 new cases and 4600 deaths annually.2 The only way to diagnose NHL is by biopsy. It has a male:female ratio of 12:10, and is more common with increasing age: over 70% of UK cases occur in those aged ≥60 years.3 Five-year UK survival across all subtypes is 61% for males and 66% for females; follicular lymphoma has the highest survival at 87% and mantle cell lymphoma the lowest at 27%.1
Despite recent improvements, the UK still lags behind Europe for NHL survival. Between 1995 and 1999 there were an estimated 632 ‘avoidable’ NHL deaths (meaning if relative survival matched the best in Europe).4,5 Patient delay in presentation to medical care and diagnostic or treatment delays are possible causes.6–9 Mean diagnostic delay is estimated as 103 days,9 while greater delays are found in younger patients.10 Currently, over 30% of patients with NHL visit a GP three or more times before referral.11 Government initiatives have focused on improving cancer survival through publishing referral guidance for GPs and reducing waiting times for specialist treatment.12 Current UK guidelines make recommendations for haematological cancer as a whole. For unexplained lymphadenopathy or fatigue, a full blood count, blood film, and inflammatory markers are recommended. Specialist referral is recommended for patients with persistent lymphadenopathy of over 6 weeks, lymph nodes bigger than 2 cm, increasing in size or widespread, or with accompanying weight loss, splenomegaly or night sweats.12 Four general symptoms — intermittent fever, weight loss, pruritus, and night sweats (sometimes called B symptoms) — are also associated with lymphomas, typically at a later stage.13
Diagnosing NHL currently relies on symptomatic presentation to a health professional, usually a GP, although the main features of NHL in primary care have not been reported. One primary care case–control study investigated a period of 15 years before a diagnosis of lymphoma, finding increased consultations over the whole period.14 Within the secondary care literature, lymphadenopathy is the main reported feature of NHL.6,15,16 Abdominal pain, fatigue, stomach/bowel problems, infection, back pain, and pain on drinking alcohol have also been reported.6,17
This study aimed to identify and quantify the clinical features of NHL in primary care, to guide GPs when to consider referral for investigation, and to inform health policy regarding referral and investigation pathways.
How this fits in
The number of avoidable deaths in the UK from non-Hodgkin lymphoma (NHL) has risen. One-third of patients with NHL visit their GP three or more times before being referred to a specialist. Lymphadenopathy is a known feature of NHL and a primary indicator for referral in guidance for haematological cancer. A comprehensive investigation of primary care features has never been studied. Patients aged ≥60 years with lymphadenopathy have a 13% risk of having NHL. When the findings of this study are added to those of Hodgkin lymphoma, the positive predictive values for lymphadenopathy and head and neck mass rise to 18.6% and 4.6%, respectively. Any mass symptoms in combination with illness symptoms such as weight loss or abdominal pain elicit an elevated risk of having NHL. The findings should aid doctors’ clinical decision making in selecting relevant patients for referral and further investigation, thereby reducing diagnostic delay.
METHOD
This was a matched case–control study using electronic UK primary care patient records from the Clinical Practice Research Datalink (CPRD). The methods follow that of previous papers by the same authors.18–20 This large computerised database contains anonymised patient data from over 680 general practices, covering 8.8% of the UK population. Patient registration data and primary care clinical events are recorded. The CPRD has stringent quality standards for data entry.
Cases and controls
Cases in the CPRD with NHL were collated using a list of 106 NHL codes (available from authors). Patients were aged ≥40 years (thus capturing almost 95% of adult NHL)2 and were diagnosed with NHL between January 2000 and December 2009. Up to five age, sex, and practice controls were matched to each case. The first NHL code was taken as the date of diagnosis. The index date for controls matched the diagnosis date for cases. Exclusion criteria were patients with Hodgkin lymphoma, mycosis fungoides, or Sézary syndrome, and their matched controls; any case or control with <1 year of records before the index date; cases without controls; controls with NHL; and controls who had not sought medical care after registration.
Selection of putative clinical variables
Potential clinical features of NHL (abnormal investigation results, signs, and symptoms) reported in existing literature and from online patient support groups were used. This allowed for known and new symptoms to be studied. PubMed, EBSCO, and Google were searched using the search terms ‘non-Hodgkin lymphoma symptoms’, ‘non-Hodgkin lymphoma reported to GP’, and ‘early signs/indications/symptoms of non-Hodgkin lymphoma’.
The CPRD contains over 100 000 medical codes, several of which can pertain to one feature. Accordingly, a symptom library of codes was compiled for each feature. Occurrences were identified in the year before the index date. Only those features present in at least 2% of cases were retained. The possibility of recording bias was tested on a condition thought to have no association with NHL — varicose veins. Abnormal investigation results were defined as the patient having a test value falling outside their local laboratory’s normal range. Patients with a normal laboratory result were grouped with those who had not been tested.
Composite variables
Some investigations were grouped together. The raised inflammatory markers variable was a composite of any of abnormal erythrocyte sedimentation rate, plasma viscosity, or C-reactive protein. Similarly, abnormal liver function investigations reflected a raised value of any of the hepatic enzymes reported by each laboratory. Low full blood count was also any of low haemoglobin, low white cell count, or thrombocytopaenia. Three categories of masses were compiled. First was masses in the head or neck called ‘head and neck mass’ (this variable incorporated cervical lymphadenopathy); second, those called ‘lymphadenopathy’, incorporating generalised lymphadenopathy and lymphadenopathy with no site mentioned; with the final category being mass elsewhere in the body, called ‘mass’. There was some overlap between these three categories. No reliable information could be extracted relating to the size of masses or their specific sites when multiple. To estimate the duration of masses indirectly (direct measurement was impossible because duration is poorly recorded) the first and last report of any of the three mass variables in the year before diagnosis were identified; masses that have an apparent duration at least of 42 days were reported separately, this being the duration of lymphadenopathy recommended for investigation in current NICE guidance.12
Analysis and statistical methods
The main analysis was conditional logistic regression. First, univariable analysis was performed with retention of variables for later stages using a P-value threshold of ≤0.1. These features were then grouped into small clinically coherent groups (such as malaise, fatigue, and nausea) for multivariable analyses, with retention requiring a P-value of ≤0.05. A final multivariable model used the surviving variables from the group stages, using a P-value threshold of 0.01. Excluded variables were checked against the final model. Clinically plausible interaction terms were added to the final model and retained if their P-value was also ≤0.01.
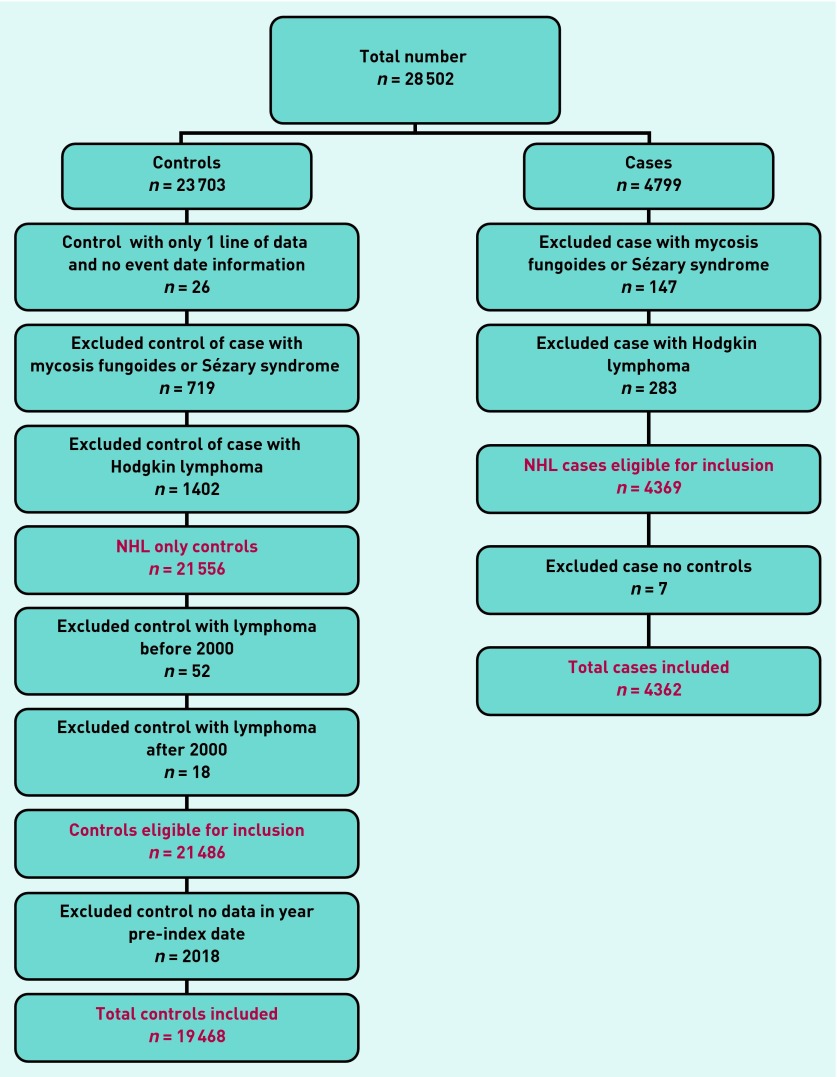
Risk estimates in the form of positive predictive values (PPVs) were calculated using Bayes’ theorem (prior odds × likelihood ratio = posterior odds). Prior odds were calculated from the age-specific national incidence of NHL for 2008, expressed as odds. PPVs were estimated for consulting patients only, thus, the posterior odds were divided by 0.906 because 2018 (10%) of 21 486 eligible controls were non-consulters (Figure 1). No sub-analyses by histological subtype were performed. This was a pre hoc decision, because the investigation aimed to identify features of any NHL, as opposed to specific subtypes. In any case, many of the NHL codes did not specify the precise subtype.
Figure 1.
Non-Hodgkin lymphoma exclusion data.
Power calculation
The CPRD provided estimates of 5000 cases and 22 500 controls. Because this number was effectively fixed, power calculations were performed instead of sample size calculations. This number provided more than 99% power (5% two-sided alpha) to detect a difference in a rare variable from 2% of cases to 1% of controls. For a more common variable, the study had more than 86% power to detect a change in prevalence of 20% in cases to 18% in controls. Data analysis was conducted using Stata software (version 13.1).
RESULTS
The CPRD provided 28 502 patients (4799 cases; 23 703 controls). Application of the exclusion criteria (Figure 1) led to a final number of 23 830 (4362 cases; 19 468 controls).
Patient demographic and consultation information is given in Table 1. Cases consulted significantly more frequently than did controls in the year before diagnosis (P<0.001; Wilcoxon rank-sum test).
Table 1.
Patient demographics and consultation rates in the year before diagnosis
| Cases | Controls | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Male (n = 2300) | Female (n = 2062) | Total (n = 4362) | Male (n = 9932) | Female (n = 9536) | Total (n = 19 468) | |
| Median age at diagnosis, years (IQR) | 69 (60–77) | 71 (62–79) | 70 (61–78) | 70 (61–77) | 71 (62–79) | 71 (61–78) |
| Median number of consultations, n (IQR) | 15 (9–23) | 16 (10–25) | 16a (10–24) | 7 (3–14) | 8 (4–15) | 8a (4–14) |
Cases consulted significantly more frequently than controls in the year before diagnosis (P <0.001). IQR = interquartile range.
Clinical features
Forty-three symptoms and 22 abnormal investigation results were considered initially. Twenty remained significant in the final model. Their frequencies, univariable likelihood ratios, and multivariable odds ratios are shown in Table 2. Of the B symptoms reported with lymphoma, fever, sweating, and weight loss, only weight loss was frequent enough to proceed to multivariable analysis. There were 53 (1.2%) cases with excessive sweating and 51 (0.3%) controls; for fever the respective figures were 53 (1.2%) and 83 (0.4%). Raised cholesterol was excluded from the final model because it was apparently protective, with an OR of 0.7 (95% CI = 0.6 to 0.8). The proportion of patients with varicose veins did not differ between cases and controls (P = 0.68). Some overlap between the mass variables occurred when multiple recordings were made, with the second occurrence sometimes using a different mass label for what was presumably the same feature. In total, 229 (5.2%) cases and 10 (0.05%) controls had multiple consultations with one of the mass variables at least 42 days apart, a univariable OR of 103 (95% CI = 55 to 194); P<0.001. No interaction terms, including with sex, were found. Of the 4362 cases, 3438 (79%) had at least one of the final model features from Table 2.
Table 2.
Features of non-Hodgkin lymphoma in patients aged ≥40 years
| Feature | Cases, n (%) n = 4362 | Controls, n (%) n = 19 468 | Likelihood ratio (95% CI) | Odds ratio in multivariable analysis (95% CI) |
|---|---|---|---|---|
| Symptoms | ||||
| Infection (UTI/URTI/skin/chest) | 902 (21) | 2897 (15) | 1.4 (1.3 to 1.5) | 1.3 (1.1 to 1.4) |
| Lymphadenopathy | 632 (14) | 13 (0.1) | 217 (125 to 375) | 263 (133 to 519) |
| Abdominal pain | 610 (14) | 833 (4) | 3.3 (3.0 to 3.6) | 2.5 (2.1 to 2.9) |
| Mass | 473 (11) | 199 (1) | 11 (9 to 12) | 12 (10 to 16) |
| Shortness of breath | 413 (9) | 976 (5) | 1.9 (1.7 to 2.1) | 1.5 (1.3 to 1.8) |
| Head and neck mass | 355 (8) | 36 (0.2) | 44 (31 to 62) | 49 (32 to 74) |
| Fatigue | 300 (7) | 547 (3) | 2.5 (2.1 to 2.8) | 1.4 (1.2 to 1.7) |
| Constipation | 261 (6) | 526 (3) | 2.2 (1.9 to 2.6) | 1.4 (1.2 to 1.8) |
| Vomiting and nausea | 247 (6) | 391 (2) | 2.8 (2.4 to 3.3) | 1.4 (1.1 to 1.7) |
| Indigestion | 203 (5) | 491 (3) | 1.9 (1.6 to 2.2) | 1.5 (1.2 to 1.9) |
| Weight loss | 164 (4) | 115 (1) | 6.4 (5.0 to 8.1) | 3.2 (2.3 to 4.4) |
| Back pain: second occurrence | 163 (4) | 308 (2) | 2.4 (2.0 to 2.9) | 1.7 (1.3 to 2.3) |
| Malaise | 159 (4) | 240 (1) | 3.0 (2.4 to 3.6) | 1.7 (1.2 to 2.3) |
|
| ||||
| Investigations | ||||
| Low full blood count | 1369 (32) | 1645 (8) | 3.7 (3.5 to 4.0) | 3.3 (2.9 to 3.7) |
| Raised inflammatory markers | 1184 (27) | 1202 (6) | 4.4 (4.1 to 4.7) | 2.5 (2.2 to 2.9) |
| Raised liver function tests | 863 (20) | 1878 (10) | 2.1 (1.9 to 2.2) | 1.3 (1.1 to 1.5) |
| Leucocytosis | 521 (12) | 478 (2) | 4.9 (4.3 to 5.5) | 3.0 (2.5 to 3.6) |
| Microcytosis | 227 (5) | 246 (1) | 4.1 (3.5 to 4.9) | 1.5 (1.1 to 1.9) |
| Macrocytosis | 207 (5) | 405 (2) | 2.3 (1.9 to 2.7) | 1.4 (1.1 to 1.8) |
| Raised gamma globulin | 174 (4) | 228 (1) | 3.4 (2.8 to 4.1) | 1.8 (1.3 to 2.4) |
URTI = upper respiratory tract infections. UTI = urinary tract infections.
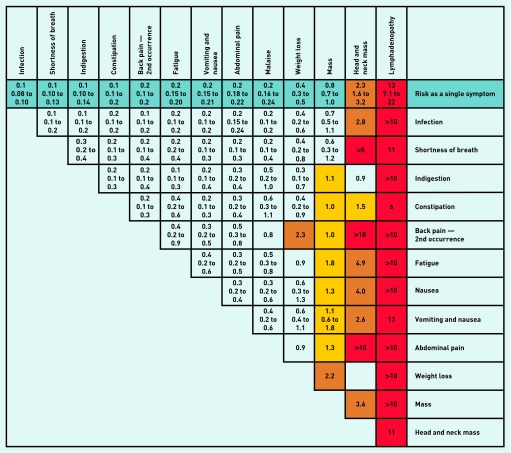
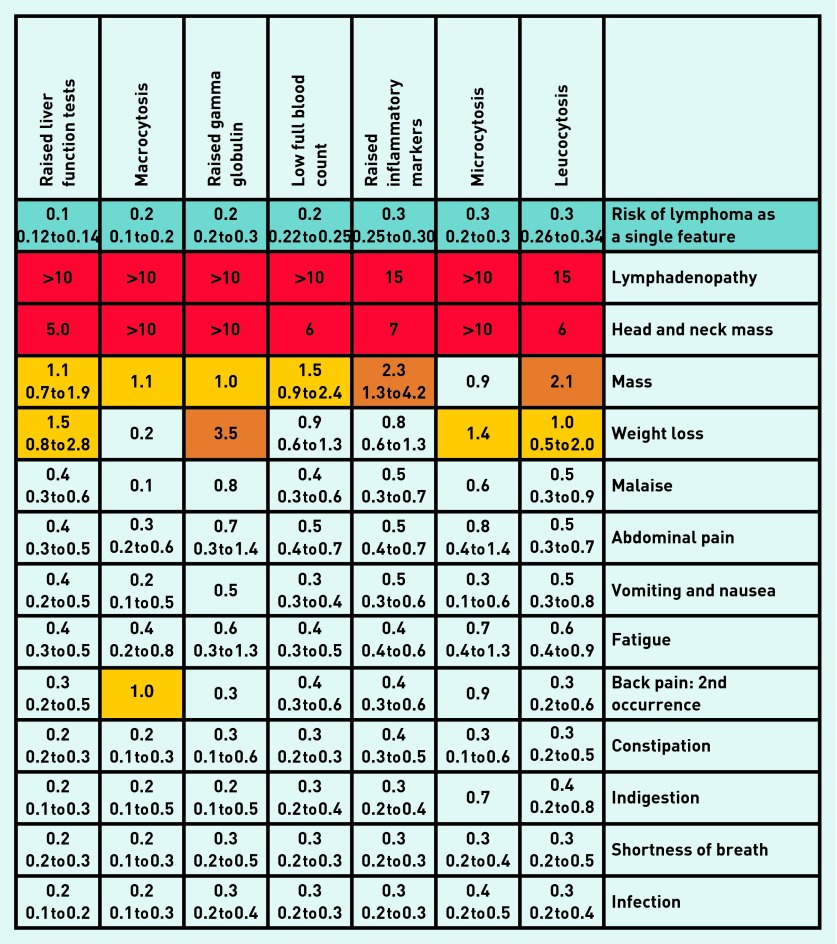
Positive predictive values
PPVs for the final model features are shown in a risk assessment tool (see Figures 2 and 3) and calculated for those aged ≥60 years. Choosing those aged ≥60 years targets patients near to the average age of NHL diagnosis; this accounted for 78.5% of overall cases.
Figure 2.
Positive predictive values for non-Hodgkin lymphoma symptoms in patients aged ≥ 60 years, for single and paired features.
Notes: The positive predictive value (PPV) is shown on the first line of each cell with the 95% CIs shown underneath. PPVs were not calculated if < 5 cases had the feature. Where < 10 cases or controls had the combined features, CIs were omitted. Where no control had the combination of paired symptoms a label of > 5 or > 10 was given; although strictly undefined, these PPVs are likely very high. The yellow shaded cells indicate a PPV of 1.0–1.9%; orange cells 2.0–4.9%; and red cells ≥ 5%. The cells showing the same feature vertically and horizontally represent a second attendance with the same investigation.
Figure 3.
Positive predictive values for non-Hodgkin lymphoma blood tests with symptoms in patients aged ≥ 60 years: risk estimate for single investigations and paired with symptoms. Notes: The positive predictive value (PPV) is shown on the first line of each cell with the 95% CIs shown underneath. PPVs were not calculated if < 5 cases had the feature. Where < 10 cases or controls had the combined features, CIs were omitted. Where no control had the combination of paired symptoms a label of > 5 or > 10 was given; although strictly undefined, these PPVs are likely very high. The yellow shaded cells indicate a PPV of 1.0–1.9%; orange cells 2.0–4.9%; and red cells ≥ 5%. The cells showing the same feature vertically and horizontally represent a second attendance with the same investigation.
Figure 2 shows the PPVs for single and combined symptoms, for patients aged ≥60 years. Lymphadenopathy as a single symptom had a PPV of 13%. All three mass variables produced risk estimates of between 0.6% and over 10% when combined with other symptoms. The PPV for those aged ≥60 years with two mass codes at least 42 days apart was 6.4% (95% CI = 3.1 to 13), and for those aged 40–59 years it was 1.8% (95% CI = 0.6 to 5.7).
Figure 3 shows the PPVs for symptoms combined with blood tests, again in patients aged ≥60 years. For patients aged 40–59 years, the PPVs for lymphadenopathy, head and neck mass, and mass were 3.7% (95% CI = 1.4 to 10), 3.7% (95% CI = 0.9 to 14), and 0.1% (95% CI = 0.1 to 0.2), respectively.
DISCUSSION
Summary
This is the first study to identify and quantify the clinical features of NHL in primary care. Thirteen symptoms and seven abnormal investigations were associated with NHL. Lymphadenopathy had high PPVs; masses elsewhere in the body also had high PPVs but they were lower than lymphadenopathy. This remained the case, even when combined with abnormal blood test results or symptoms. Weight loss was the only other symptom to have a moderately high PPV, although this was only when additional features were present, such as recurrent back pain, or with abnormalities in blood tests. These findings come from the UK, but are likely to be generalisable to other healthcare systems with the patient first seeing a generalist.
Strengths and limitations
This is a large study of over 4000 primary care patients with NHL and is the first to study symptoms recorded before diagnosis. The CPRD is the largest longitudinal primary care database and is recognised for its data quality. Therefore, the results are likely to be representative of UK patients and thus generalisable. The study’s large sample size allowed for sub-analyses by age, while still providing enough power to identify rare but relevant symptoms of NHL. The study’s comprehensive strategy for identifying putative features of NHL, including using online support forums to search for patient-reported symptoms, makes it unlikely that relevant features were omitted. Last, the study’s primary care setting is important. The clinical problem of which patients to select for cancer investigation resides in primary care, so requires primary care research.
The use of primary care records has some limitations. Information is not well recorded for the duration or severity of a complaint, or for cancer staging. The study also relied on accurate data recording. Individual GPs have personal recording styles, although the framework of the CPRD codes provides some uniformity. This was particularly important for the mass variables chosen. There was some overlap between these terms, although most of this overlap was with lymphadenopathy — the highest risk of the three masses — as one of the variables. GPs can record information in a hidden ‘free text’ section, which can affect the strength of associations if it preferentially occurs in either cases or controls.21 In theory, patients included as cases had a greater opportunity to report symptoms because of their more frequent attendances; however, the test using varicose veins did not suggest this was occurring. Another aspect is that of matching. Once cases and controls are matched, the matching variable cannot be studied directly. However, using stratified analyses (by age, primarily) and seeking internal interaction terms (used for sex here) it is possible, to a large extent, to sidestep this apparent limitation. Finally, as in the authors’ previous studies, the problem of estimating PPVs from a case–control study design was overcome by calculating the prior odds of NHL from registry data.18,22
Comparison with existing literature
Consistent with previous research, cases consult their GP significantly more than controls in the year before the cancer diagnosis.23 The study’s main finding was the strong association between lymphadenopathy and NHL; similarly, head and neck masses also were strongly associated. This was no surprise, because these are the main features in the secondary care literature.6,24,25 Other masses (some of which may have been lymphadenopathy) were also associated with NHL, but with a much lower risk. Cervical lymphadenopathy in primary care has been investigated in three old studies.26–28 In the first, no malignancies were found from a group of 80 primary care patients with lymphadenopathy, 44% of whom had isolated cervical node enlargement.28 A second study of 249 patients also found no malignancies, and no final diagnosis was established for most.26 A third study examined referrals for lymph node biopsy. Twenty-nine malignancies were found in 82 referred patients, with a prior probability for lymphadenopathy presenting to primary care of 1.1% calculated from these results.27 All three studies emphasised the ability of GPs to identify lymphadenopathy of malignant origin.
Weight loss was the non-mass feature with the highest risk estimates in this study. Abdominal pain, fatigue, indigestion, infection, anaemia, and back pain, each of which has been reported in secondary care, were also found in this study to be features in primary care, albeit low-risk ones.6,17,29
Implications for practice
The clearest message from this study is the importance of lymphadenopathy and head and neck masses. There is some overlap between these, with many of the head and neck masses probably being of lymphatic tissue. The risks of NHL with these are generally >5%, and warrant serious consideration of lymphoma. The abnormal blood tests and other symptoms helped to refine the risk a little, generally increasing the overall risk when they accompanied head and neck masses, although making little practical change to the overall likely management. Conversely, many patients with NHL had normal inflammatory markers, so this test cannot be used to exclude the disease. It was not possible to examine persistence of the mass directly, because duration of symptoms is poorly recorded.30 However, the proxy used for duration, two mass codes at least 6 weeks apart, had a relatively high PPV of 6.4% (95% CI = 3.1 to 13) in those aged ≥60 years, a figure high enough to justify investigation. The word ‘unexplained’ is used in referral guidance.12 Although this word does not feature in the CPRD medcodes for mass or lymphadenopathy, it is likely that many of the masses were unexplained, because GPs prefer to document diagnoses where possible. When should GPs refer unexplained lymphadenopathy or neck mass? The reports that GPs appear able to distinguish malignant from benign lymphadenopathy are helpful.26–28 When the results of this study are added to those for Hodgkin lymphoma in the associated article,31 the PPVs rise to 18.6% for either lymphoma or lymphadenopathy, 4.6% for head and neck mass, and 1.1% for mass elsewhere. Therefore the default decision should be referral of patients ≥60 years with these features, unless there is a clear reason not to.
Most B symptoms were too infrequent to be analysed, other than weight loss, which had a modest association with NHL. Thus B symptoms are of limited value in diagnosis, although they do have prognostic implications, should a lymphoma be diagnosed.32 Weight loss is a difficult symptom in cancer diagnosis. It is rare in isolation, and can be caused by several malignancies, although often has a benign cause. When of malignant origin, there is often a pointer towards which malignancy is likely, simplifying referral decisions. NHL is probably fairly low in the list, so it is unlikely that patients with weight loss should be considered for NHL as a first choice.
Lymphadenopathy and head and neck masses in adults are the strongest predictors of NHL and Hodgkin lymphoma, and warrant urgent investigation, particularly if they have been present for ≥6 weeks. No blood test or other symptoms change that statement. This largely accords with current guidance, although it could be argued that the need to wait 6 weeks — to allow resolution or an alternative diagnosis to emerge — is unnecessarily long. Implementation of this study’s recommendations can be by education, dissemination of the risk assessment tool, by incorporation into practice software, or a combination of these.
Acknowledgments
The authors acknowledge the contribution to the research presented in this paper made by the Discovery Programme Steering Committee comprising Roger Jones (chair), Jonathan Banks, Alison Clutterbuck, Jon Emery, Joanne Hartland, Sandra Hollinghurst, Maire Justice, Jenny Knowles, Helen Morris, Tim Peters, and Greg Rubin.
Funding
This article presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Grant Reference Number RP-PG-0608-10045). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. Fiona M Walter is part-funded by a NIHR Clinician Scientist award. Richard D Neal is part-funded by Public Health Wales and Betsi Cadwaladr University Health Board.
Ethical approval
Independent Scientific Advisory Committee — protocol 09-110.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
William T Hamilton is clinical lead on the ongoing revision of the NICE guidance on investigation of suspected cancer. His contribution to this article is in a personal capacity, and is not to be interpreted as representing the view of the Guideline Development Group, or of NICE itself. Peter W Rose reports personal fees from GP Update Ltd, outside the submitted work. Other than this, the authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Cancer Research UK Different types of non Hodgkin lymphoma. 2014. http://www.cancerresearchuk.org/cancer-help/type/non-hodgkins-lymphoma/about/types/the-most-common-types-of-non-hodgkins-lymphoma (accessed 11 Feb 2015)
- 2.Cancer Research UK Non-Hodgkin lymphoma (NHL) key stats. 2014 http://www.cancerresearchuk.org/cancer-info/cancerstats/keyfacts/non-hodgkin-lymphoma/ (accessed 11 Feb 2015) [Google Scholar]
- 3.National Institute for Health and Care Excellence . Non-Hodgkin’s lymphoma: draft scope. London: NICE; 2013. [Google Scholar]
- 4.Cancer Research UK Non-Hodgkin lymphoma survival statistics. 2014. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/nhl/survival/nonhodgkin-lymphoma-survival-statistics (accessed 31 Jul 2014)
- 5.Abdel-Rahman M, Stockton D, Rachet B, et al. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable? Br J Cancer. 2009;101(Suppl 2):S115–S124. doi: 10.1038/sj.bjc.6605401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howell DA, Smith AG, Jack A, et al. Time-to-diagnosis and symptoms of myeloma, lymphomas and leukaemias: a report from the Haematological Malignancy Research Network. BMC Hematol. 2013;13(1):9. doi: 10.1186/2052-1839-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howell DA, Smith AG, Roman E. Referral pathways and diagnosis: UK government actions fail to recognize complexity of lymphoma. Eur J Cancer Care. 2007;16(6):529–532. doi: 10.1111/j.1365-2354.2007.00789.x. [DOI] [PubMed] [Google Scholar]
- 8.Vedsted P, Olesen F. Are the serious problems in cancer survival partly rooted in gatekeeper principles? An ecologic study. Br J Gen Pract. 2011 doi: 10.3399/bjgp11X588484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allgar V, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: Cancer. Br J Cancer. 2005;92(11):1959–1970. doi: 10.1038/sj.bjc.6602587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neal RD, Allgar VL. Sociodemographic factors and delays in the diagnosis of six cancers: analysis of data from the ‘National Survey of NHS Patients: Cancer’. Br J Cancer. 2005;92(11):1971–1975. doi: 10.1038/sj.bjc.6602623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13(4):353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence . Referral guidelines for suspected cancer. London: NICE; 2005. [Google Scholar]
- 13.Cancer Research UK Treatment for high grade non Hodgkin lymphoma. 2014 http://www.cancerresearchuk.org/about-cancer/type/non-hodgkins-lymphoma/treatment/types/treatment-for-high-grade-non-hodgkins-lymphoma (accessed 11 Feb 2015) [Google Scholar]
- 14.Crouch S, Simpson J, Ansell P, et al. Illness patterns prior to diagnosis of lymphoma: analysis of UK medical records. Cancer Epidemiol. 2011;35(2):145–150. doi: 10.1016/j.canep.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Howell DA, Smith AG, Roman E. Help-seeking behaviour in patients with lymphoma. Eur J Cancer Care. 2008;17(4):394–403. doi: 10.1111/j.1365-2354.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- 16.Dommett RM, Redaniel MT, Stevens MC, et al. Features of cancer in teenagers and young adults in primary care: a population-based nested case-control study. Br J Cancer. 2013;108(11):2329–2333. doi: 10.1038/bjc.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3(3):230–238. doi: 10.1007/BF02596337. [DOI] [PubMed] [Google Scholar]
- 18.Shephard E, Neal R, Rose P, et al. Clinical features of kidney cancer in primary care: a case-control study using primary care records. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X665215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stapley S, Peters TJ, Neal RD, et al. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2013;108(1):25–31. doi: 10.1038/bjc.2012.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker S, Hyde C, Hamilton W. Risk of uterine cancer in symptomatic women in primary care: case-control study using electronic records. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X671632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price SJ, Shephard EA, Stapley SA, et al. Non-visible versus visible haematuria and bladder cancer risk: a study of electronic records in primary care. Br J Gen Pract. 2014 doi: 10.3399/bjgp14X681409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stapley S, Peters TJ, Neal RD, et al. The risk of pancreatic cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2012;106(12):1940–1944. doi: 10.1038/bjc.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton W, Lancashire R, Sharp D, et al. The risk of colorectal cancer with symptoms at different ages and between the sexes: a case-control study. BMC Med. 2009;7:17. doi: 10.1186/1741-7015-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friese CR, Abel GA, Magazu LS, et al. Diagnostic delay and complications for older adults with multiple myeloma. Leuk Lymphoma. 2009;50(3):392–400. doi: 10.1080/10428190902741471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88(3):354–361. doi: 10.1038/sj.bjc.6600738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson HA., Jr Lymphadenopathy in a family practice: a descriptive study of 249 cases. J Fam Pract. 1985;20(5):449–452. [PubMed] [Google Scholar]
- 27.Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27(4):373–376. doi: 10.1080/09503158808416945. [DOI] [PubMed] [Google Scholar]
- 28.Allhiser JN, McKnight TA, Shank JC. Lymphadenopathy in a family practice. J Fam Pract. 1981;12(1):27–32. [PubMed] [Google Scholar]
- 29.Tørring ML, Frydenberg M, Hamilton W, et al. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J Clin Epidemiol. 2012;65(6):669–678. doi: 10.1016/j.jclinepi.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton WT, Round AP, Sharp D, Peters TJ. The quality of record keeping in primary care: a comparison of computerised, paper and hybrid systems. Br J Gen Pract. 2003;53(497):929–933. [PMC free article] [PubMed] [Google Scholar]
- 31.Shephard EA, Neal RD, Rose PW, et al. Quantifying the risk of Hodgkin lymphoma in symptomatic primary care patients aged ≥40 years: a case-control study using electronic records. Br J Gen Pract. 2015 doi: 10.3399/bjgp15X684805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol. 2003;21(1):20–27. doi: 10.1200/JCO.2003.11.141. [DOI] [PubMed] [Google Scholar]