Abstract
Chemokines are a family of chemotactic cytokines that play an essential role in leukocyte trafficking. Upregulation of both CC and CXC chemokines is a hallmark of the inflammatory and reparative response following myocardial infarction. Release of danger signals from dying cells and damaged extracellular matrix activates innate immune pathways that stimulate chemokine synthesis. Cytokine- and chemokine-driven adhesive interactions between endothelial cells and leukocytes mediate extravasation of immune cells into the infarct. CXC chemokines (such as interleukin-8) are bound to glycosaminoglycans on the endothelial surface and activate captured neutrophils, inducing expression of integrins. CC chemokines (such as monocyte chemoattractant protein (MCP)-1) mediate recruitment of pro-inflammatory and phagocytotic mononuclear cells into the infarct. CC Chemokines may also regulate late infiltration of the healing infarct with inhibitory leukocytes that suppress inflammation and restrain the post-infarction immune response. Non-hematopoietic cells are also targeted by chemokines; in healing infarcts, the CXC chemokine Interferon-γ inducible Protein (IP)-10 exerts antifibrotic actions, inhibiting fibroblast migration. Another member of the CXC subfamily, Stromal cell-derived Factor (SDF)-1, may protect the infarcted heart by activating pro-survival signaling in cardiomyocytes, while exerting angiogenic actions through chemotaxis of endothelial progenitors. Several members of the chemokine family may be promising therapeutic targets to attenuate adverse remodeling in patients with myocardial infarction.
1. INTRODUCTION
1.1. The inflammatory response in cardiac repair
Complete cessation of blood flow due to occlusion of a coronary vessel results in irreversible cardiomyocyte injury within 20–40 minutes of sustained severe ischemia [1]. The subendocardial myocardium is more susceptible to ischemic injury; as a result, severe myocardial ischemia leads to a “wavefront” of necrosis that extends from the subendocardial region to the subepicardium [2]. Because the adult mammalian heart has negligible regenerative capacity, sudden necrosis of a large number of cardiomyocytes in myocardial infarction activates a reparative response that ultimately leads to replacement of dead cardiomyocytes with scar tissue [3]. From a descriptive perspective, repair of the infarcted myocardium can be divided into three distinct but overlapping phases: the inflammatory phase, the proliferative phase and the maturation phase [4]. During the inflammatory phase, danger signals released from dying cardiomyocytes and damaged matrix activate the innate immune response, leading to induction of pro-inflammatory signals and infiltration of the infarct with neutrophils and pro-inflammatory monocytes. As the wound is cleared from dead cells and matrix debris, neutrophils become apoptotic and are ingested by macrophages, activating pathways that inhibit inflammation and induce resolution of the leukocytic infiltrate. Suppression of the inflammatory response marks the transition to the proliferative phase, as differentiation of reparative macrophages in the infarct activates angiogenic and fibrogenic pathways, promoting transdifferentiation and growth of myofibroblasts and proliferation of endothelial cells. Activated myofibroblasts mediate wound contraction and secrete structural matrix proteins contributing to formation of a scar. Maturation of the infarct follows, as the collagenous matrix is cross-linked, and granulation tissue cells undergo apoptosis. Infarct healing is closely intertwined with geometric remodeling of the ventricle that becomes more spherical and dilates, while infarcted segments become thinner and the non-infarcted area undergoes hypertrophy. The extent of adverse remodeling is dependent not only on the size of the infarct, but also on the qualitative characteristics of the wound; thus, the molecular signals involved in the reparative process are also crucial regulators of adverse remodeling.
Repair of the infarcted heart is dependent on sequential mobilization of immune cell subpopulations that serve diverse roles in the reparative process. Recruitment of leukocyte subsets in the infarcted heart is orchestrated by the chemokines, a family of chemotactic cytokines that interact with corresponding chemokine receptors on leukocytes mediating their activation and extravasation into the infarct. Over the last fifteen years extensive experimental evidence demonstrated a crucial role for several members of the chemokine family in the inflammatory and reparative response following myocardial infarction [5]. Our current review manuscript discusses the involvement of the chemokines in repair and remodeling of the infarcted heart. Moreover, we attempt to identify promising therapeutic approaches targeting the chemokine system in order to optimize repair of the infarcted heart and to prevent adverse cardiac remodeling.
1.2. The chemokine family
Chemokines comprise a group of chemotactic cytokines that regulate trafficking of immune cells, both in lymphoid organs and in sites of injury. Although cells of the immune system are the primary targets of chemokines; many non-immune cell types (including fibroblasts, endothelial cells, smooth muscle cells, cardiomyocytes and neurons) express chemokine receptors and respond to chemokine activation by altering their phenotype and functional properties. Thus, a growing body of evidence suggests that chemokine-mediated actions are not limited to the regulation of leukocyte trafficking. Several members of the chemokine family are known to modulate complex biological processes, such as cell proliferation, apoptosis, granule exocytosis and gene transcription. From a structural perspective, chemokines can be subdivided into CC, CXC, CX3C, and XC subfamilies based on the number of amino acids between their first two cysteines. CC chemokines are the most numerous and diverse subfamily, including at least 25 ligands in humans; most CC chemokines are potent mononuclear cell chemoattractants. CXC chemokines, on the other hand, are further classified according to the presence of the tripeptide motif glutamic acid-leucine-arginine (ELR) in the NH2 terminal region. ELR+ CXC chemokines are critically involved in chemotactic recruitment of neutrophils.
From a functional standpoint, chemokines can be divided into two categories: homeostatic chemokines are constitutively expressed in certain tissues, are responsible for basal leukocyte trafficking and are implicated in formation of lymphoid organs, and inducible chemokines which are markedly upregulated following tissue injury and participate in inflammatory reactions by mediating leukocyte activation and tissue infiltration [6, 7]. Although this generalization is somewhat oversimplified and has been challenged by evidence demonstrating that certain “homeostatic” chemokines (such as Stromal Cell Derived Factor/SDF-1) can be inducible upon immune activation [8], the concept is valuable and provides insight into the role of members of the chemokine family in pathological states [9], [7, 10]. In sites of injury, inducible chemokines are bound to glycosaminoglycans on the endothelial surface and on the matrix and are presented to circulating leukocytes, thus interacting with the corresponding chemokine receptors on the leukocyte surface.
2. Initiation of the chemokine response in myocardial infarction
In the infarcted myocardium, cardiomyocyte necrosis and injury of the extracellular matrix result in rapid release of danger signals that initiate the chemokine response. Experimental evidence suggests that selected members of the chemokine family (such as monocyte chemoattractant protein-1(MCP-1)/CCL2) may also be induced in the absence of irreversible injury, in response to ischemia-activated generation of reactive oxygen species (ROS) [11], [12]. In both canine and mouse models of brief myocardial ischemia/reperfusion chemokine upregulation is noted in the absence of infarction and is mediated by ROS generation [12], [11]. However, in the infarcted myocardium, release of danger-associated molecular patterns (DAMPs) by dying cells triggers additional potent chemokine-inducing pathways (such as the complement cascade and Toll-like receptor signaling); thus, the relative contribution of free radical generation in chemokine induction in the infarcted heart remains unclear.
2.1. The complement cascade
Activation of the complement cascade is a key component of the innate immune response following myocardial infarction. Hill and Ward [13] were the first to demonstrate that leukotactic activity in rat myocardial infarcts was in part due to C3 cleavage products. Complement activation may exert leukocyte chemotactic actions, at least in part, by inducing chemokine synthesis in ischemic tissues. C6 deficient rabbits exhibited attenuated CXCL8/Interleukin (IL)-8 expression accompanied by decreased neutrophil infiltration in the infarct [14], suggesting that the cytolytic membrane attack complex plays an important role in regulating chemokine expression.
2.2. Activation of TLR-mediated pathways
TLR signaling may also play an important role in chemokine upregulation in the infarcted heart. DAMPs released by dying cardiomyocytes stimulate TLR signaling activating the Nuclear Factor (NF)-κB system in resident myocardial cells and in hematopoietic cells. TLR4 appears to play an important role in mediating the post-infarction inflammatory reaction; TLR4 deficient mice exhibit suppressed inflammation following myocardial infarction [15]. Moreover, TLR2 signaling in leukocytes is also implicated in transduction of inflammatory signaling in the healing infarct [16]. In addition to its effects on leukocytes, activation of TLR signaling may also trigger inflammatory pathways in cardiomyocytes, inducing expression of CXC chemokines [17]. DAMPs are not only generated by cardiomyocyte necrosis: the rapid breakdown of extracellular matrix in ischemic tissues results in accumulation of hyaluronan fragments, which are capable of inducing chemokine synthesis in macrophages [18] and endothelial cells [19] through TLR4-dependent pathways [20].
2.3. Cytokine-induced chemokine upregulation in the infarct
Induction and release of pro-inflammatory cytokines, such as Tumor Necrosis Factor (TNF)-α and IL-1β may play an important role in mediating chemokine upregulation in the infarcted heart. TNF-α deficient mice exhibited attenuated chemokine expression in the infarcted heart, suggesting an important role for TNF-α signaling in mediating the post-infarction chemokine response [21]. Moreover, mice with complete loss of IL-1 signaling exhibited a marked reduction in chemokine expression [22], highlighting the essential role of IL-1 in the post-infarction inflammatory reaction. Release of active IL-1 in the infarct is dependent on formation of the inflammasome platform in cardiomyocytes, fibroblasts and hematopoietic cells in the infarcted region [23], [24]. In addition to cytokine-mediated actions, preformed mast cell-derived inflammatory mediators, such as histamine and tryptase, are released in the infarct and may contribute to chemokine upregulation in ischemic tissues [25], [26].
2.4. The NF-κB system
ROS generation, TLR signaling, activation of the complement cascade, and pro-inflammatory cytokines mediate chemokine upregulation through a molecular cascade that involves the NF-κB system [27]. Clearly, the genes regulated by NF-κB are diverse and do not only involve those implicated in the inflammatory response (such as chemokines, adhesion molecules and cytokines), but also genes with a prominent role in growth control and cell survival [28]. Activation of the NF-κB signaling cascade in the infarct involves all cell types implicated in cardiac injury and repair, further complicating understanding of its role in ischemic tissues.
3. Role of ELR+ CXC chemokines in the infarcted heart
CXC chemokines that contain the ELR motif are potent neutrophil chemoatractants. The prototypic ELR+ CXC chemokine CXCL8/IL-8 is a key regulator of neutrophil influx and activation in a wide range of inflammatory processes [29]. Induction of myocardial IL-8 synthesis has been documented in canine [30] and rabbit [31] models of experimental myocardial infarction. In canine infarcts, IL-8 synthesis was accentuated by reperfusion and was localized in inflammatory leukocytes and venular endothelial cells of the infarct border zone [30]. Recombinant canine IL-8 markedly increased neutrophil adhesion to isolated canine cardiac myocytes [30], suggesting a potential role in neutrophil-mediated myocardial injury. In addition to its potential role in leukocyte recruitment, IL-8 also exerts angiogenic actions; the significance of these effects in myocardial infarction remains unknown. The lack of an IL-8 homolog in mice has hampered efforts to study its role in myocardial infarction using loss-of-function approaches.
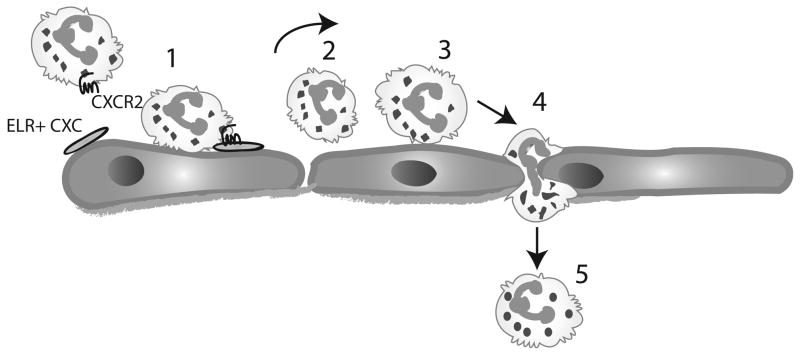
Even less information is available on the role of other ELR-containing CXC chemokines in myocardial infarction. CXCL1/Growth Related Oncogene (GRO)-α/KC, a potent neutrophil chemoattractant, is induced in a rat model of experimental myocardial infarction [32], however its role in regulating the post-infarction inflammatory response has not been established. Deficiency of CXCR2, the main receptor for the ELR-containing CXC chemokines, resulted in significantly decreased inflammatory leukocyte recruitment in murine infarcts, highlighting the crucial role of this subfamily of chemokines in neutrophil recruitment [33]. ELR+ CXC chemokines may promote leukocyte activation by binding to glycosaminoglycans on the endothelial surface. Captured and rolling leukocytes “sense” chemokines bound to the endothelium; subsequently interactions between immobilized chemokines and the corresponding leukocyte chemokine receptors activate integrins resulting in firm adhesion of the leukocyte to the endothelium (Figure 1).
Figure 1.
ELR-positive CXC chemokines mediate neutrophil recruitment in the infarcted heart. Activation of danger signals result in induction of ELR+ CXC chemokines (such as IL-8); these chemokines are bound to glycosaminoglycans on the endothelial surface. Neutrophils are captured (1) by activated endothelial cells and “sense” the immobilized chemokines and other chemoattractants (such as C5a). Rolling of neutrophils (2) on the endothelium is mediated by the selectin family of adhesion molecules. Subsequently chemokines interact with their corresponding receptor (CXCR2) and activate integrins on the neutrophil surface resulting in firm adhesion of the leukocyte to the endothelial layer (3). Transmigration of the neutrophils through the endothelial layer follows (4) and leads to infiltration of the infarct with abundant leukocytes (5).
4. ELR-negative CXC chemokines: the role of Interferon-γ-inducible Protein (IP)-10/CXCL10
CXC chemokines that lack the ELR motif do not induce neutrophil chemotaxis, but are involved in recruitment and activation of lymphocytes. Moreover, in contrast to the angiogenic actions of ELR+ CXC chemokines, members of the ELR-negative subfamily exert potent angiostatic effects in the presence of ELR-CXC chemokines, or basic fibroblast growth factor (bFGF) [34, 35]. Our experiments have demonstrated that the ELR-negative CXC chemokine IP-10/CXCL10 is markedly upregulated in canine and mouse models of reperfused infarction [36], [37]. In the canine model, IP-10 expession was upregulated in ischemic segments during the first 12h after reperfusion and was localized in the microvascular endothelium [36]. In vitro experiments demonstrated that TNF-α, which is released early after myocardial ischemia [25] markedly upregulates IP-10 expression in canine venous endothelial cells.
In order to study the role of IP-10 in cardiac injury and repair following myocardial infarction, we studied mice with global loss of IP-10. IP-10 loss did not affect the size of the acute infarct; however, IP-10 absence resulted in a hypercellular early reparative response, delayed contraction of the scar and accentuated dilative remodeling followed by rapid wall thinning and increased systolic dysfunction [37]. IP-10 null infarcts had intense infiltration with myofibroblasts suggesting that the chemokine may exert anti-fibrotic actions in the infarcted myocardium. In vitro, IP-10 had no effects on cardiac fibroblast proliferation and apoptosis, but significantly attenuated bFGF-mediated fibroblast migration. Moreover, IP-10 enhanced growth factor-induced contraction of fibroblast-populated collagen lattices [37]. The findings highlighted the potent and important effects of IP-10 on non-hematopoietic cells.
5. SDF-1/CXCL12
SDF-1 and its receptor CXCR4 have unique functions in myocardial biology. Mice with targeted deletion of SDF-1 or CXCR4 exhibited embryonic lethality associated with impaired vascular formation and with development of ventricular aortopulmonary septum defects, highlighting the role of constitutive SDF-1/CXCR4 signaling in cardiac development [38]. Studies in experimental models of myocardial infarction demonstrated that the SDF-1/CXCR4 axis is rapidly activated in the healing infarct [39], [40], [41]. In vivo experiments have produced somewhat contradictory results highlighting the complex, cell-type specific and context-dependent actions of SDF-1 signaling in the healing infarct. The bulk of the evidence suggests protective effects of SDF-1 in the infarcted myocardium. In several published studies, local infusion, or overexpression of SDF-1 in the infarcted myocardium attenuated systolic dysfunction and reduced adverse post-infarction remodeling. Three potential mechanisms of benefit have been suggested:
Activation of the SDF-1/CXCR4 axis may accentuate infarct angiogenesis through recruitment of endothelial progenitor cells (EPC), or via direct stimulation of angiogenic pathways [42], [43], [44], [45].
SDF-1/CXCR4 signaling may exert pro-survival actions on ischemic cardiomyocytes through effects involving Erk and Akt activation [46].
SDF-1 may promote myocardial regeneration through recruitment of CXCR4+/c-kit+ progenitor cells [44].
However, other studies have suggested detrimental actions of SDF-1 on the infarcted heart. Experiments using adenoviral-mediated gene therapy showed that CXCR4 overexpression in the infarcted heart accentuates inflammatory injury and increases activation of pro-apoptotic pathways worsening cardiac dysfunction [47].
Pharmacologic inhibition of SDF-1/CXCR4 using a selective small molecule CXCR4 antagonist (AMD3100) has also produced conflicting results. Chronic continuous inhibition of SDF-1/CXCR4 in mice undergoing non-reperfused infarction protocols exacerbated systolic dysfunction and accentuated adverse post-infarction cardiac remodeling [48]. However, other studies demonstrated beneficial effects of CXCR4 inhibition. CXCR4 antagonism with AMD3100 reduced infarct size and improved systolic function [49] in a rat model; however, the basis for these effects was not investigated. In another study, a single-dose AMD3100 injection administered after the onset of myocardial infarction preserved cardiac function, whereas continuous infusion worsened outcome [50]. The beneficial actions of early CXCR4 inhibition were associated with increased mobilization of bone marrow EPCs, whereas, the detrimental effects of chronic antagonism was attributed to impaired incorporation of EPCs in the infarct border zone [50]. Finally, a recent investigation using a loss-of-function approach demonstrated that CXCR4 +/- mice had reduced infarct size when compared with WT animals [51].
These conflicting observations reflect the multifunctional, pleiotropic and context-dependent actions of SDF-1/CXCR4 signaling and the complexity of the reparative process. Beacuse CXCR4 signaling regulates phenotype and function of all cell types involved in infarct healing; the outcome of strategies targeting the SDF-1/CXCR4 axis is dependent on timing and spatial localization of the intervention and on the cellular targets affected. Dose-dependent actions of SDF-1 on various cell types may affect the balance between angiogenic, pro-survival and pro-inflammatory actions of CXCR4 activation. Experiments using of conditionally-targeted mice to study cell-specific actions of SDF-1 are needed in order to understand the role of the SDF-1/CXCR4 axis in myocardial infarction and to optimally design therapeutic strategies [52], [40].
6. The role of the CC chemokines
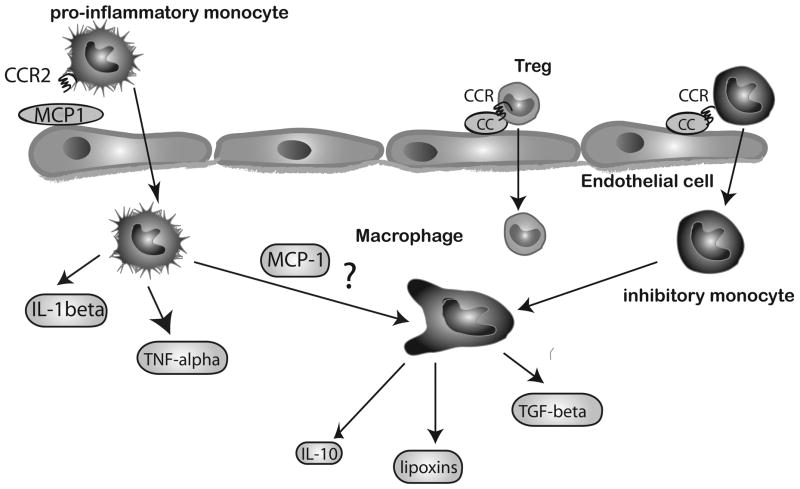
Induction of CC chemokines is a hallmark of the inflammatory and reparative response following myocardial infarction (Figure 2). Perhaps the best characterized CC chemokine, CCL2/MCP-1, is markedly upregulated in canine [53], rat [54, 55] and mouse models [56] of experimental myocardial infarction. In the canine model, induction of myocardial MCP-1 mRNA in infracted segments was accentuated by reperfusion and peaked 3h after the acute event [53]. MCP-1 in the infarct was predominantly expressed by infiltrating leukocytes and venular endothelial cells. In the mouse model, MCP-1 was also markedly, but transiently induced, peaking after 3–6h of reperfusion [57].
Figure 2.
Members of the CC chemokine family mediate recruitment of mononuclear cells into the infarcted myocardium. MCP-1, the best-studied CC chemokine, is markedly upregulated in the infarct and is responsible for recruitment of pro-inflammatory monocytes that express CCR2. These cells are capable of producing pro-inflammatory cytokines (such as IL-1β and TNF-α) and have potent phagocytotic properties. Inhibitory monocytes exhibit a late time course of recruitment in the infarcted myocardium; the CC chemokine/Chemokine receptor (CCR) pairs involved in their trafficking remain poorly understood. CCR5 signaling may be involved in recruitment of regulatory T cells (Tregs). In addition to its actions on recruitment of mononuclear cells, MCP-1 may also regulate macrophage differentiation and may have effects on fibroblasts, cardiomyocytes and endothelial cells (not shown).
Our laboratory investigated the role of MCP-1 in infarct healing and cardiac remodeling by examining the effects of MCP-1 gene disruption and antibody neutralization in a mouse model of reperfused myocardial infarction [58]. Although MCP-1 loss had no effects on scar size following myocardial infarction, MCP-1 −/− mice exhibited attenuated dilative remodelling, associated with decreased and delayed macrophage infiltration in the healing infarct, and with markedly reduced pro-inflammatory gene expression. Although MCP-1 loss protected from adverse remodelling, this came at a cost: MCP-1 null animals exhibited delayed replacement of injured cardiomyocytes with granulation tissue and defective phagocytotic removal of dead cardiomyocytes. On the other hand, MCP-1 antibody inhibition did not affect recruitment of macrophages in the infarct, but resulted in defects comparable with the pathological findings noted in infarcted MCP-1 −/− animals [58]. Two additional studies provide further support to the role of the MCP-1/CCR2 axis in mediating dilative post-infarction remodeling. Mice with genetic loss of CCR2 were protected from chamber dilation following myocardial infarction [59]. Moreover, anti-MCP-1 gene therapy reduced adverse remodeling in a murine model of experimental infarction [60].
What are the mechanisms responsible for the effects of MCP-1 absence in the infarcted heart? Impaired recruitment of pro-inflammatory monocytes (known to express the MCP-1 receptor, CCR2) [61] may be the most important consequence of MCP-1 loss in the infarcted heart. These cells exhibit high phagocytotic capacity and a pro-inflammatory profile (Figure 2); their absence from the infarcted heart may explain the alterations observed in MCP-1 null animals. However, the effects of MCP-1 may extend beyond its monocyte chemoattractant properties: MCP-1 may promote macrophage differentiation and cytokine expression and may have direct effects on myofibroblast phenotype [58]. Suppression of macrophage-derived synthesis of pro-inflammatory cytokines, decreased macrophage activation, and impaired myofibroblast differentiation and function may be important additional mechanisms responsible for attenuated left ventricular remodeling in MCP-1 null mice. It should be noted, however, that our in vitro experiments failed to demonstrate consistent effects of MCP-1 on fibroblast gene expression [62].
Although our experiments did not demonstrate a role for MCP-1 in extending cardiomyocyte injury, several lines of evidence suggest actions of MCP-1 (either direct or indirect) on cardiomyocyte survival. In a rat model of experimental myocardial infarction, administration of a neutralizing antibody to MCP-1 significantly reduced infarct size, presumably through attenuation of inflammatory activity [54]. More recently, administration of a competitive MCP-1 inhibitor reduced infarct size in a mouse model of myocardial ischemia/reperfusion [63]. In vitro experiments suggested that MCP-1 may promote ICAM-1-dependent adhesive interactions between neutrophils and cardiomyocytes [64]. Moreover, MCP-1 binding to its receptor, CCR2, induces expression of a transcription factor, MCP-induced protein (MCPIP), that is expressed in cardiomyocytes and monocytes and causes cell death [65], [66].
Several other members of the CC chemokine family are also upregulated in healing myocardial infarcts and may play a role in regulation of the reparative response. CCL7/MCP-3 is upregulated in the infarcted myocardium and may stimulate recruitment of mesenchymal progenitor cells and of circulating angiogenic cells in the site of injury [67], [68]. Although pro-angiogenic and reparative effects of MCP-3 may be beneficial for infarct healing, the pro-inflammatory properties of the chemokine may also induce injurious effects [69]. CCL3/Macrophage Inflammatory Protein (MIP)-1α and CCL4/MIP-1β are also mononuclear cell chemoattractants, although less efficient than MCP-1 [70]. Distinct chemokine/chemokine receptor pairs may be responsible for recruitment of monocyte subpopulations with unique phenotypic properties. Robust induction of MIP-1α and -β is noted in reperfused murine infarcts [57]; however, the importance of these chemokines in myocardial injury and repair has not been systematically investigated. Increased levels of CCL5/RANTES were found in the serum from patients with acute myocardial infarction [71]; myocardial RANTES expression was also upregulated in a mouse model of coronary occlusion [72]. RANTES neutralization significantly reduced infarct size and attenuated systolic dysfunction in a mouse model of non-reperfused infarction [72]. However, the basis for these protective effects remain poorly understood.
7. Fractalkine/CX3CL1
Fractalkine (CX3CL1) is a unique chemokine that exists in both soluble and membrane-anchored forms. As a pro-inflammatory mediator, fractalkine may play a role in the pathogenesis of the vulnerable atherosclerotic plaque [73]. Increased myocardial fractalkine expression has been reported in infarcted and remodeling hearts [74], [75]; mononuclear cells expressing the fractalkine receptor are recruited in the infarct during the proliferative phase of healing and exhibit a reparative phenotype [61]. Stimulation with soluble fractalkine increased expression of pro-inflammatory and matrix-degrading genes by fibroblasts and cardiomyocytes. Fractalkine neutralization delayed the progression of chamber enlargement following myocardial infarction suggesting that fractalkine signaling may be implicated in the pathogenesis of dilative remodeling [74].
8. Inhibition of the chemokine response following myocardial infarction
Tissue repair is dependent on activation of endogenous inhibitory signals that restrain the inflammatory reaction preventing uncontrolled or extended inflammatory injury. These STOP signals are critical for cardiac repair: because in the myocardium normal function is dependent on optimal preservation of structure, prolongation or extension of the inflammatory reaction following cardiac injury may have catastrophic consequences, leading to extensive matrix degradation, accentuated cardiomyocyte apoptosis, increased dilative remodeling and progressive systolic dysfunction [4].
Timely and effective suppression of chemokine signaling may be a key event in protection of the infarcted heart from adverse remodeling. Multiple inhibitory signals and cellular effectors may co-operate to limit, contain and suppress the chemokine response. Defects in suppression of chemokine signaling may be responsible for progressive dilation of the infarcted ventricle and may extend cardiac injury. Although the molecular pathways implicated in suppression of chemokine expression and activation following myocardial infarction remain poorly understood, several recent investigations have identified important inhibitory pathways:
8.1. The role of IL-10 and Transforming Growth Factor (TGF)-β
Soluble inhibitory mediators, such as IL-10 and TGF-β1, may be released in the infarct suppressing chemokine synthesis by endothelial cells and macrophages. IL-10 is markedly induced in the infarcted myocardium, showing a late and prolonged time course; IL-10 expression is localized in T lymphocytes and in a subset of macrophages [76]. Associative in vivo studies and in vitro experiments suggested that IL-10 may suppress pro-inflammatory cytokine and chemokine synthesis and may contribute to stabilization of the matrix by inducing expression of protease inhibitors. However, experiments examining the role of IL-10 in post-infarction inflammation using IL-10 −/− mice produced contradictory results: Yang and co-workers suggested that IL-10 −/− mice had markedly increased mortality and exhibited enhanced myocardial inflammation following reperfused myocardial infarction [77]. In contrast, our laboratory found no effects of IL-10 disruption on survival of mice undergoing reperfused infarction protocols, but showed that. Although IL-10 −/− mice had higher peak myocardial TNF-α and MCP-1 mRNA levels following infarction than wildtype animals [78], these relatively subtle alterations had no effects on chamber dilation following myocardial infarction.
TGF-β, a highly pleiotropic and multifunctional mediator with context-dependent actions, has also been implicated in suppression of chemokine-driven inflammation following myocardial infarction, orchestrating the transition from inflammatory activation to scar formation. TGF-β suppresses chemokine synthesis by macrophages and endothelial cells, attenuates adhesion molecule expression and inhibits the immune response by promoting differentiation of regulatory T cells (Tregs) [79]. On the other hand, TGF-β induces myofibroblast transdifferentiation, stimulates synthesis of extracellular matrix proteins [80], and inhibits matrix degradation by upregulating expression of protease inhibitors [81]. In healing infarcts, TGF-β may serve as a “master switch” that links repression of chemokine synthesis with activation of a fibrogenic program [82].
8.2. Inhibition of chemokine-mediated leukocyte-endothelial interactions
A growing body of evidence suggests that suppression of inflammation is associated with induction of inhibitory signals that suppress chemokine-mediated leukocyte activation. Growth Differentiation Factor (GDF)-15, a member of the TGF-β superfamily, inhibits chemokine-triggered conformational integrin activation in neutrophils, thus limiting activation of inflammatory leukocytes and preventing adhesion to the endothelium [83]. Loss of the suppressive effects of GDF-15 in post-infarction inflammation results in an increased incidence of cardiac rupture [83].
8.3. Inhibition of innate immune responses
Our laboratory has recently identified Interleukin Receptor-Associated Kinase (IRAK)-M as an essential negative regulatory mechanism that prevents uncontrolled TLR/IL-1-driven inflammation following infarction. Unlike IRAK-1 and IRAK-4, IRAK-M does not transduce pro-inflammatory signals, but is predominantly expressed in macrophages and limits TLR/IRAK-1-dependent NF-κB signaling. IRAK-M is upregulated in both macrophages and fibroblasts in the infarcted heart; IRAK-M absence is associated with accentuated post-infarction remodeling, increased inflammation and enhanced MMP activation in the infarct [84].
8.4. The role of the extracellular matrix in negative regulation of chemokine-driven inflammation
Following myocardial infarction, the extracellular matrix undergoes dynamic changes that play a key role in regulating inflammatory activity [85], [86]. Because matrix fragments serve as important “danger signals” that activate inflammation following infarction, removal of matrix fragments generated in the injured heart is an essential step to restrain and limit the post-infarction inflammatory response [85]. Studies from our laboratory suggested that clearance of low molecular weight hyaluronan fragments generates important inhibitory signals in the infarcted heart, suppressing cytokine and chemokine synthesis [87].
During the proliferative phase of infarct healing induction of matricellular proteins may also modulate chemokine synthesis. Thrombospondin (TSP) -1, an anti-inflammatory matricellular protein with potent angiostatic actions and a crucial role in TGF-β activation is selectively upregulated in the infarct border zone and suppresses the inflammatory reaction, serving as a barrier that prevents extension of inflammatory activity into the viable non-infarcted myocardium [88]. Other matricellular proteins, such as osteonectin, osteopontin, tenascin-C and periostin are also induced in the infarcted heart and may regulate chemokine expression by modulating cytokine and growth factor signaling. [89], [90].
8.5. Expression of decoy receptors
Negative regulation of chemokine signaling may involve binding to receptors that do not transduce signal, but serve as a molecular trap that removes the chemokine from the circulation (decoy receptors). A recently published study demonstrated that the decoy chemokine receptor D6 is expressed in healing infarcts and protects the heart from excessive inflammation and adverse remodeling [91].
8.6. Are chemokines involved in negative regulation of the post-infarction inflammatory response?
Myocardial infarcts are sequentially infiltrated with monocyte subsets that exhibit distinct properties. Early recruitment of pro-inflammatory CCR2+ cells is followed by infiltration of the infarct with reparative monocyte subpopulations [61]. Activation of specific chemokine/chemokine receptor pairs may be responsible for recruitment of inhibitory mononuclear cell subsets in the infarcted myocardium. Recent studies from our laboratory demonstrated that loss of the chemokine receptor CCR5 is associated with accentuated inflammation and worse adverse remodeling following myocardial infarction [92]. CCR5 absence was associated with impaired recruitment of Tregs in the infarcted heart.
9. Chemokines as therapeutic targets in myocardial infarction
Interfering with the inflammatory response in order to reduce extension of cardiac injury following myocardial infarction is not a new concept. The intense inflammatory reaction observed in the infarct border zone and the potential of infiltrating leukocytes to induce cytotoxic cardiomyocyte injury supported the notion that post-infarction inflammation may extend ischemic damage. This attractive concept was tested by examining the effectiveness of targeted anti-inflammatory approaches in large animal models of reperfused myocardial infarction. Extensive experimental evidence demonstrated that neutralization of adhesion molecules, chemokines and cytokines protected the ischemic and reperfused myocardium, leading to impressive reduction of the size of the infarct by 40–50% [93]. Unfortunately, clinical trials failed to translate the concept into a successful therapeutic approach. For example, although anti-integrin approaches induced impressive infarct size reduction in experimental animal models, clinical trials showed no effects of early CD11b/CD18 integrin inhibition in human patients with ST elevation myocardial infarction [94], [95]. Moreover, in a large clinical trial, treatment with pexelizumab to inhibit the complement cascade, an essential upstream pathway of the innate immune response, did not improve outcome in patients undergoing a percutaneous coronary intervention for acute myocardial infarction [96]. Enthusiasm regarding the use of strategies targeting inflammatory pathways has, understandably, waned. Considering the failures of the past, is there any reason for optimism regarding the potential usefulness of strategies targeting the chemokine system?
Considering their marked induction in the infarcted myocardium, and their well-documented role in cardiac repair and in remodeling of the infarcted heart, certain members of the chemokine family may be promising targets for patients with myocardial infarction. However, successful implementation of chemokine-based strategies will require realistic approaches and a revision of the traditional paradigm on the role of inflammation in post-ischemic cardiac injury. Over the last decade, studies using genetically targeted mice have provided evidence against the concept of inflammatory cardiomyocyte injury. Mice with disruption of key pro-inflammatory pathways, such as the P-selectin/ICAM-1 double knockout mice [97], the IL-1 type 1 receptor null mice [22] and the MCP-1 −/− animals [58] had no reduction in infarct size, despite a marked attenuation of inflammatory activity. Thus, published antibody neutralization studies may have overstated the significance of inflammation-related extension of ischemic cardiomyocyte death. Chemokine-driven inflammatory injury may not accentuate ischemic cardiomyocyte death; however, a growing body of evidence suggests that inflammatory chemokines may play an important role in the pathogenesis of dilative remodeling following myocardial infarction. The evidence for a role of the MCP-1/CCR2 axis is particularly strong; in mouse models of myocardial infarction, genetic disruption of MCP-1 or CCR2, and MCP-1 antagonism had beneficial effects significantly attenuating chamber dilation [58], [59], [63]. Thus, MCP-1 inhibition may hold promise as a strategy for attenuation of adverse remodeling; this approach may be particularly effective in patients with evidence of defective suppression of inflammatory signaling. These patients may be identified using biomarkers (such as serum MCP-1 levels) that may reflect accentuated or prolonged chemokine responses [98], [99].
Through its unique effects in activation of progenitor cells, angiogenesis and pro-survival signaling, SDF-1 may also hold promise as therapy for patients with myocardial infarction. Because SDF-1 is rapidly cleaved by MMP-2 generating a neurotoxic substance, use of protease-resistant forms of SDF-1 may be needed for effective treatment [44]. However, a word of caution should be raised regarding the pro-inflammatory properties of SDF-1 that may accentuate matrix degradation and increase fibrosis following infarction.
10. Conclusions
Over the last 15 years, extensive in vivo experimentation has contributed to an explosion in our knowledge on the role of the chemokines in repair and remodeling of infarcted hearts. In addition to the expected role of several inducible members of the family in recruitment of inflammatory cells in the infarct, we have identified new and interesting actions of chemokines on phenotype and function of non-hematopoietic cells, such as fibroblasts and cardiomyocytes. Current and future research in the field needs to focus on several distinct directions. First, the dissection of the cellular targets of chemokines in the infarcted heart is crucial for understanding their actions. Second, study of mechanisms involved in termination of chemokine signals in the infarct will contribute to understanding the consequences of a dysregulated inflammatory cascade and its impact on cardiac remodeling. Third, identification of specific chemokine/chemokine receptor interactions responsible for recruitment of mononuclear cell subsets with distinct properties will contribute essential knowledge for understanding the cellular basis of the reparative response. Mechanistic studies in the field need to be accompanied by translationally-oriented work to identify patient subpopulations most likely to benefit from specific approaches targeting the chemokine system.
Acknowledgments
FUNDING: Dr Frangogiannis’ laboratory is funded by NIH R01 HL-76246 and R01 HL-85440 and the Wilf Family Cardiovascular Research Institute.
References
- 1.Jennings RB, Murry CE, Steenbergen C, Jr, Reimer KA. Development of cell injury in sustained acute ischemia. Circulation. 1990;82:II2–12. [PubMed] [Google Scholar]
- 2.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–94. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 3.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–39. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 4.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–73. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738–47. [PubMed] [Google Scholar]
- 6.Clark-Lewis I, Kim KS, Rajarathnam K, Gong JH, Dewald B, Moser B, Baggiolini M, Sykes BD. Structure-activity relationships of chemokines. J Leukoc Biol. 1995;57:703–11. doi: 10.1002/jlb.57.5.703. [DOI] [PubMed] [Google Scholar]
- 7.Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- 8.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 9.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–15. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 10.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 11.Lakshminarayanan V, Lewallen M, Frangogiannis NG, Evans AJ, Wedin KE, Michael LH, Entman ML. Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am J Pathol. 2001;159:1301–11. doi: 10.1016/S0002-9440(10)62517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nossuli TO, Frangogiannis NG, Knuefermann P, Lakshminarayanan V, Dewald O, Evans AJ, Peschon J, Mann DL, Michael LH, Entman ML. Brief murine myocardial I/R induces chemokines in a TNF-alpha-independent manner: role of oxygen radicals. Am J Physiol Heart Circ Physiol. 2001;281:H2549–58. doi: 10.1152/ajpheart.2001.281.6.H2549. [DOI] [PubMed] [Google Scholar]
- 13.Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971;133:885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilgore KS, Park JL, Tanhehco EJ, Booth EA, Marks RM, Lucchesi BR. Attenuation of interleukin-8 expression in C6-deficient rabbits after myocardial ischemia/reperfusion. J Mol Cell Cardiol. 1998;30:75–85. doi: 10.1006/jmcc.1997.0573. [DOI] [PubMed] [Google Scholar]
- 15.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–9. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 16.Arslan F, Smeets MB, O’Neill LA, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 17.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res. 2006;72:384–93. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 18.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–13. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate dermal endothelial recognition of injury through TLR4. J Biol Chem. 2004 doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 20.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–84. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 21.Maekawa N, Wada H, Kanda T, Niwa T, Yamada Y, Saito K, Fujiwara H, Sekikawa K, Seishima M. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-alpha. J Am Coll Cardiol. 2002;39:1229–35. doi: 10.1016/s0735-1097(02)01738-2. [DOI] [PubMed] [Google Scholar]
- 22.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 24.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–30. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 26.Somasundaram P, Ren G, Nagar H, Kraemer D, Mendoza L, Michael LH, Caughey GH, Entman ML, Frangogiannis NG. Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. J Pathol. 2005;205:102–11. doi: 10.1002/path.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res. 2011;108:1122–32. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 28.Stancovski I, Baltimore D. NF-kappaB activation: the I kappaB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 29.Zeilhofer HU, Schorr W. Role of interleukin-8 in neutrophil signaling. Curr Opin Hematol. 2000;7:178–82. doi: 10.1097/00062752-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Kukielka GL, Smith CW, LaRosa GJ, Manning AM, Mendoza LH, Daly TJ, Hughes BJ, Youker KA, Hawkins HK, Michael LH, et al. Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J Clin Invest. 1995;95:89–103. doi: 10.1172/JCI117680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivey CL, Williams FM, Collins PD, Jose PJ, Williams TJ. Neutrophil chemoattractants generated in two phases during reperfusion of ischemic myocardium in the rabbit. Evidence for a role for C5a and interleukin-8. J Clin Invest. 1995;95:2720–8. doi: 10.1172/JCI117974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation. 2001;103:2296–302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- 33.Tarzami ST, Miao W, Mani K, Lopez L, Factor SM, Berman JW, Kitsis RN. Opposing effects mediated by the chemokine receptor CXCR2 on myocardial ischemia-reperfusion injury: recruitment of potentially damaging neutrophils and direct myocardial protection. Circulation. 2003;108:2387–92. doi: 10.1161/01.CIR.0000093192.72099.9A. [DOI] [PubMed] [Google Scholar]
- 34.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, Chan SY, Rocziak S, Shanafelt AB. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–57. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 35.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van Damme J, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol. 1995;57:752–62. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- 36.Frangogiannis NG, Mendoza LH, Lewallen M, Michael LH, Smith CW, Entman ML. Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J. 2001;15:1428–30. doi: 10.1096/fj.00-0745fje. [DOI] [PubMed] [Google Scholar]
- 37.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–83. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaruba MM, Franz WM. Role of the SDF-1-CXCR4 axis in stem cell-based therapies for ischemic cardiomyopathy. Expert Opin Biol Ther. 2010;10:321–35. doi: 10.1517/14712590903460286. [DOI] [PubMed] [Google Scholar]
- 39.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 40.Frangogiannis NG. The stromal cell-derived factor-1/CXCR4 axis in cardiac injury and repair. J Am Coll Cardiol. 2011;58:2424–6. doi: 10.1016/j.jacc.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 41.Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25:293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- 42.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, DiMaio JM, Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–31. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. Faseb J. 2007;21:3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 44.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–92. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki T, Fukazawa R, Ogawa S, Kanno S, Nitta T, Ochi M, Shimizu K. Stromal cell-derived factor-1alpha improves infarcted heart function through angiogenesis in mice. Pediatr Int. 2007;49:966–71. doi: 10.1111/j.1442-200X.2007.02491.x. [DOI] [PubMed] [Google Scholar]
- 46.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–63. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Chemaly E, Liang L, Kho C, Lee A, Park J, Altman P, Schecter AD, Hajjar RJ, Tarzami ST. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol. 2010;176:1705–15. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai S, Yuan F, Mu J, Li C, Chen N, Guo S, Kingery J, Prabhu SD, Bolli R, Rokosh G. Chronic AMD3100 antagonism of SDF-1alpha-CXCR4 exacerbates cardiac dysfunction and remodeling after myocardial infarction. J Mol Cell Cardiol. 2010;49:587–97. doi: 10.1016/j.yjmcc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Proulx C, El-Helou V, Gosselin H, Clement R, Gillis MA, Villeneuve L, Calderone A. Antagonism of stromal cell-derived factor-1alpha reduces infarct size and improves ventricular function after myocardial infarction. Pflugers Arch. 2007;455:241–50. doi: 10.1007/s00424-007-0284-5. [DOI] [PubMed] [Google Scholar]
- 50.Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, Ito A, Misener S, Tanaka T, Klyachko E, Kobayashi K, Tongers J, Roncalli J, Tsurumi Y, Hagiwara N, Losordo DW. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010;107:11008–13. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liehn EA, Tuchscheerer N, Kanzler I, Drechsler M, Fraemohs L, Schuh A, Koenen RR, Zander S, Soehnlein O, Hristov M, Grigorescu G, Urs AO, Leabu M, Bucur I, Merx MW, Zernecke A, Ehling J, Gremse F, Lammers T, Kiessling F, Bernhagen J, Schober A, Weber C. Double-edged role of the CXCL12/CXCR4 axis in experimental myocardial infarction. J Am Coll Cardiol. 2011;58:2415–23. doi: 10.1016/j.jacc.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 52.Agarwal U, Ghalayini W, Dong F, Weber K, Zou YR, Rabbany SY, Rafii S, Penn MS. Role of cardiac myocyte CXCR4 expression in development and left ventricular remodeling after acute myocardial infarction. Circ Res. 2010;107:667–76. doi: 10.1161/CIRCRESAHA.110.223289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar AG, Ballantyne CM, Michael LH, Kukielka GL, Youker KA, Lindsey ML, Hawkins HK, Birdsall HH, MacKay CR, LaRosa GJ, Rossen RD, Smith CW, Entman ML. Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation. 1997;95:693–700. doi: 10.1161/01.cir.95.3.693. [DOI] [PubMed] [Google Scholar]
- 54.Ono K, Matsumori A, Furukawa Y, Igata H, Shioi T, Matsushima K, Sasayama S. Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest. 1999;79:195–203. [PubMed] [Google Scholar]
- 55.Kakio T, Matsumori A, Ono K, Ito H, Matsushima K, Sasayama S. Roles and relationship of macrophages and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the ischemic and reperfused rat heart. Lab Invest. 2000;80:1127–36. doi: 10.1038/labinvest.3780119. [DOI] [PubMed] [Google Scholar]
- 56.Tarzami ST, Cheng R, Miao W, Kitsis RN, Berman JW. Chemokine expression in myocardial ischemia: MIP-2 dependent MCP-1 expression protects cardiomyocytes from cell death. J Mol Cell Cardiol. 2002;34:209–21. doi: 10.1006/jmcc.2001.1503. [DOI] [PubMed] [Google Scholar]
- 57.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–77. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–9. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 59.Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165:439–47. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayashidani S, Tsutsui H, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2003;108:2134–40. doi: 10.1161/01.CIR.0000092890.29552.22. [DOI] [PubMed] [Google Scholar]
- 61.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–92. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 63.Liehn EA, Piccinini AM, Koenen RR, Soehnlein O, Adage T, Fatu R, Curaj A, Popescu A, Zernecke A, Kungl AJ, Weber C. A new monocyte chemotactic protein-1/chemokine CC motif ligand-2 competitor limiting neointima formation and myocardial ischemia/reperfusion injury in mice. J Am Coll Cardiol. 2010;56:1847–57. doi: 10.1016/j.jacc.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 64.Ban K, Ikeda U, Takahashi M, Kanbe T, Kasahara T, Shimada K. Expression of intercellular adhesion molecule-1 on rat cardiac myocytes by monocyte chemoattractant protein-1. Cardiovasc Res. 1994;28:1258–62. doi: 10.1093/cvr/28.8.1258. [DOI] [PubMed] [Google Scholar]
- 65.Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, Younce C, Binkley PF, Kolattukudy PE. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. 2006;98:1177–85. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bidzhekov K, Zernecke A, Weber C. MCP-1 induces a novel transcription factor with proapoptotic activity. Circ Res. 2006;98:1107–9. doi: 10.1161/01.RES.0000223483.12225.80. [DOI] [PubMed] [Google Scholar]
- 67.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, McCarthy PM, Penn MS. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–51. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 68.Bousquenaud M, Schwartz C, Leonard F, Rolland-Turner M, Wagner D, Devaux Y. Monocyte chemotactic protein 3 is a homing factor for circulating angiogenic cells. Cardiovasc Res. 2012;94:519–25. doi: 10.1093/cvr/cvs140. [DOI] [PubMed] [Google Scholar]
- 69.Westermann D, Savvatis K, Lindner D, Zietsch C, Becher PM, Hammer E, Heimesaat MM, Bereswill S, Volker U, Escher F, Riad A, Plendl J, Klingel K, Poller W, Schultheiss HP, Tschope C. Reduced degradation of the chemokine MCP-3 by matrix metalloproteinase-2 exacerbates myocardial inflammation in experimental viral cardiomyopathy. Circulation. 2011;124:2082–93. doi: 10.1161/CIRCULATIONAHA.111.035964. [DOI] [PubMed] [Google Scholar]
- 70.Uguccioni M, D’Apuzzo M, Loetscher M, Dewald B, Baggiolini M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1 alpha and MIP-1 beta on human monocytes. Eur J Immunol. 1995;25:64–8. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- 71.Parissis JT, Adamopoulos S, Venetsanou KF, Mentzikof DG, Karas SM, Kremastinos DT. Serum profiles of C-C chemokines in acute myocardial infarction: possible implication in postinfarction left ventricular remodeling. J Interferon Cytokine Res. 2002;22:223–9. doi: 10.1089/107999002753536194. [DOI] [PubMed] [Google Scholar]
- 72.Montecucco F, Braunersreuther V, Lenglet S, Delattre BM, Pelli G, Buatois V, Guilhot F, Galan K, Vuilleumier N, Ferlin W, Fischer N, Vallee JP, Kosco-Vilbois M, Mach F. CC chemokine CCL5 plays a central role impacting infarct size and post-infarction heart failure in mice. Eur Heart J. 2012;33:1964–74. doi: 10.1093/eurheartj/ehr127. [DOI] [PubMed] [Google Scholar]
- 73.Flierl U, Schafer A. Fractalkine--a local inflammatory marker aggravating platelet activation at the vulnerable plaque. Thromb Haemost. 2012;108:457–63. doi: 10.1160/TH12-04-0271. [DOI] [PubMed] [Google Scholar]
- 74.Xuan W, Liao Y, Chen B, Huang Q, Xu D, Liu Y, Bin J, Kitakaze M. Detrimental effect of fractalkine on myocardial ischaemia and heart failure. Cardiovasc Res. 2011;92:385–93. doi: 10.1093/cvr/cvr221. [DOI] [PubMed] [Google Scholar]
- 75.Husberg C, Nygard S, Finsen AV, Damas JK, Frigessi A, Oie E, Waehre A, Gullestad L, Aukrust P, Yndestad A, Christensen G. Cytokine expression profiling of the myocardium reveals a role for CX3CL1 (fractalkine) in heart failure. J Mol Cell Cardiol. 2008;45:261–9. doi: 10.1016/j.yjmcc.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 76.Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165:2798–808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 77.Yang Z, Zingarelli B, Szabo C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation. 2000;101:1019–26. doi: 10.1161/01.cir.101.9.1019. [DOI] [PubMed] [Google Scholar]
- 78.Zymek P, Nah DY, Bujak M, Ren G, Koerting A, Leucker T, Huebener P, Taffet G, Entman M, Frangogiannis NG. Interleukin-10 is not a critical regulator of infarct healing and left ventricular remodeling. Cardiovasc Res. 2007;74:313–22. doi: 10.1016/j.cardiores.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–95. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bassols A, Massague J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988;263:3039–45. [PubMed] [Google Scholar]
- 81.Laiho M, Saksela O, Andreasen PA, Keski-Oja J. Enhanced production and extracellular deposition of the endothelial-type plasminogen activator inhibitor in cultured human lung fibroblasts by transforming growth factor-beta. J Cell Biol. 1986;103:2403–10. doi: 10.1083/jcb.103.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–6. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–8. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, Tian Q, Dobaczewski M, Frangogiannis NG. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol. 2012;32:2598–608. doi: 10.1161/ATVBAHA.112.300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dobaczewski M, de Haan JJ, Frangogiannis NG. The extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium. J Cardiovasc Transl Res. 2012;5:837–47. doi: 10.1007/s12265-012-9406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, Frangogiannis NG. CD44 Is Critically Involved in Infarct Healing by Regulating the Inflammatory and Fibrotic Response. J Immunol. 2008;180:2625–33. doi: 10.4049/jimmunol.180.4.2625. [DOI] [PubMed] [Google Scholar]
- 88.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 89.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d’Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206:113–23. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–88. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cochain C, Auvynet C, Poupel L, Vilar J, Dumeau E, Richart A, Recalde A, Zouggari Y, Yin KY, Bruneval P, Renault G, Marchiol C, Bonnin P, Levy B, Bonecchi R, Locati M, Combadiere C, Silvestre JS. The chemokine decoy receptor D6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2012;32:2206–13. doi: 10.1161/ATVBAHA.112.254409. [DOI] [PubMed] [Google Scholar]
- 92.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177–87. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simpson PJ, Todd RF, 3rd, Fantone JC, Mickelson JK, Griffin JD, Lucchesi BR. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988;81:624–9. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baran KW, Nguyen M, McKendall GR, Lambrew CT, Dykstra G, Palmeri ST, Gibbons RJ, Borzak S, Sobel BE, Gourlay SG, Rundle AC, Gibson CM, Barron HV. Double-blind, randomized trial of an anti-CD18 antibody in conjunction with recombinant tissue plasminogen activator for acute myocardial infarction: limitation of myocardial infarction following thrombolysis in acute myocardial infarction (LIMIT AMI) study. Circulation. 2001;104:2778–83. doi: 10.1161/hc4801.100236. [DOI] [PubMed] [Google Scholar]
- 95.Faxon DP, Gibbons RJ, Chronos NA, Gurbel PA, Sheehan F. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J Am Coll Cardiol. 2002;40:1199–204. doi: 10.1016/s0735-1097(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 96.Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D, Jr, O’Neill WW, Todaro TG, Vahanian A, Van de Werf F. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. Jama. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 97.Briaud SA, Ding ZM, Michael LH, Entman ML, Daniel S, Ballantyne CM. Leukocyte trafficking and myocardial reperfusion injury in ICAM-1/P-selectin-knockout mice. Am J Physiol Heart Circ Physiol. 2001;280:H60–7. doi: 10.1152/ajpheart.2001.280.1.H60. [DOI] [PubMed] [Google Scholar]
- 98.Gonzalez-Quesada C, Frangogiannis NG. Monocyte chemoattractant protein-1/CCL2 as a biomarker in acute coronary syndromes. Current Atherosclerosis Reports. 2009;11:131–138. doi: 10.1007/s11883-009-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frangogiannis NG. Biomarkers: hopes and challenges in the path from discovery to clinical practice. Transl Res. 2012;159:197–204. doi: 10.1016/j.trsl.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]