Transforming Growth Factor (TGF)-β is a central regulator of cellular function in health and disease. Extensive evidence suggests that activation of TGF-β signaling cascades regulates cell survival, proliferation, migration and differentiation, and is critically implicated in tissue inflammation, repair, remodeling and fibrosis [1], [2], [3]. Most tissues contain large stores of latent TGF-β [4]; generation and release of active TGF-β following injury can modulate phenotype and function of all cell types involved in reparative, inflammatory and fibrotic responses. TGF-β signals by binding and sequentially transphosphorylating type II and type I receptors on the cell surface [5]. Seven type I receptors, also known as activin receptor like kinases (ALKs), and five type II receptors have been described. Most cellular effects of TGF-β are mediated through binding to ALK5 and subsequent activation of cascades that involve the intracellular effectors Smad2 and Smad3. In certain cell types (such as endothelial cells), TGF-β may also signal by activating ALK1, thus transducing Smad1 and Smad5 cascades [6]. Differential cell-specific activation of distinct type I receptors may explain the functional complexity of the pathophysiologic actions of the members of the TGF-β superfamily.
Like most tissues, the heart contains a significant amount of latent TGF-β. Following cardiac injury, release of proteases, oxidative stress and induction of matricellular proteins cooperate to activate preformed stores of TGF-β, [7], while de novo synthesis of TGF-β isoforms contributes to accentuation of the response [8]. In the pressure-overloaded myocardium, release of bioactive TGF-β in the cardiac interstitium elicits responses in both cardiomyocytes and interstitial cells, critically regulating geometry and function of the remodeling ventricle [9]. TGF-β signaling cascades have been implicated in the pathogenesis of cardiac hypertrophy and interstitial fibrosis [9], [10], [11], suggesting that targeting the TGF-β system may hold promise in the treatment of heart failure [12], [13]. However, the pleiotropic, multifunctional and context-dependent actions of TGF-β signaling raise significant concerns regarding the outcome of interventions targeting TGF-β in patients with heart disease.
The study by Engebretsen and co-workers [14] provides a highly informative illustration of the potential benefits and perils associated with manipulation of the TGF-β cascade in a clinically relevant rodent model of heart failure. The authors studied the effects of ALK5 inhibition in the pressure overloaded myocardium by treating mice undergoing transverse aortic constriction protocols with SM16, an orally active ALK5 inhibitor. ALK5 inhibition attenuated diastolic dysfunction, reducing fibrosis and decreasing deposition of cross-linked collagen in the cardiac interstitium. However, these beneficial effects came at a heavy cost: mice treated with SM16 had increased mortality (due to rupture of the ascending aorta), exhibited accentuated chamber dilation and developed inflammatory valve lesions. The findings highlight the risks of pharmacologic interventions targeting the TGF-β cascade in cardiac remodeling. Although blockade of TGF-β-induced fibrosis is an attractive therapeutic target for patients with heart failure, broad non-specific inhibition of TGF-β signaling in the remodeling myocardium may have catastrophic consequences. In order to understand the basis for these adverse events and to optimally design treatment strategies targeting TGF-β in patients with heart disease, we need to dissect the cell-specific effects of TGF-β cascades in the remodeling heart.
The cellular effects of TGF-β in the remodeling myocardium
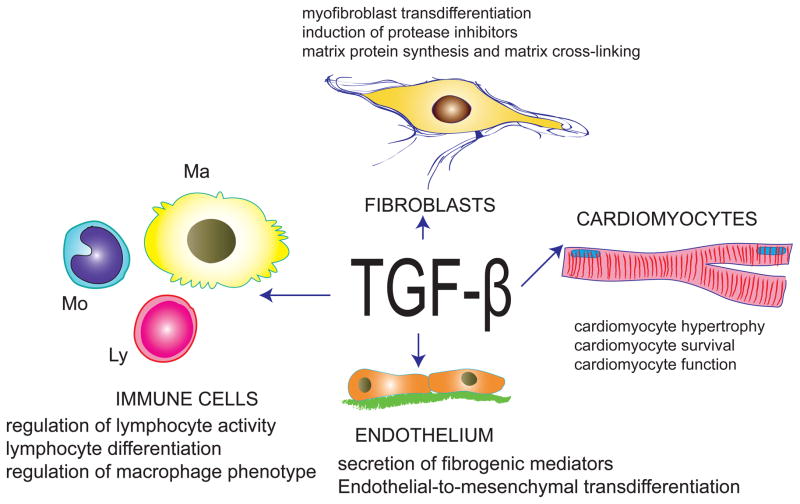
In the pressure-overloaded myocardium, TGF-β modulates phenotype and function of both cardiomyocytes and interstitial cells (Figure). Extensive evidence has documented important effects of TGF-β on the response of cardiomyocytes to stress. In vitro, TGF-β has been shown to mediate the hypertrophic and pro-apoptotic effects of angiotensin II [15], [16]. In vivo, studies using genetic strategies for cell-specific suppression of the type II receptor suggested that cardiomyocyte-specific TGF-β signaling promotes maladaptive cardiac remodeling, hypertrophy and dysfunction in the pressure overloaded heart. In the infarcted myocardium, cardiomyocyte TGF-β signaling is also implicated in adverse remodeling by suppressing synthesis of protective, anti-inflammatory mediators [17]. In vitro effects of TGF-β on cardiac fibroblasts are also well-documented. TGF-β critically regulates cardiac fibroblast phenotype, promoting myofibroblast transdifferentiation, enhancing matrix protein synthesis, and inducing a matrix preserving phenotype characterized by increased synthesis of protease inhibitors [2],[18]. Unfortunately, challenges in development of tools for fibroblast-specific gene targeting [19] have hampered our understanding of the in vivo significance of TGF-β signaling in fibroblasts. Although the effects of TGF-β on vascular endothelial cells in the pressure-overloaded myocardium have not been systematically investigated, TGF-β-mediated endothelial to mesenchymal transdifferentiation has been demonstrated in the remodeling myocardium [20] and may contribute to the pathogenesis of cardiac fibrosis. Immune cells (including macrophages and lymphocytes) are also critically modulated by TGF-β [21]. Considering the growing body of evidence suggesting involvement of lymphocyte and monocyte subpopulations in the pathogenesis of heart failure [22], effects of TGF-β on immune cells may be important in cardiac remodeling; however, experiments directly testing this hypothesis have not been performed. Understanding the cell-specific actions of TGF-β signaling in the remodeling heart is crucial in order to design therapeutic strategies that target maladaptive responses without disrupting protective processes.
Figure.
TGF-β exerts a wide range of effects on cardiomyocytes, fibroblasts, endothelial cells and immune cells (macrophages, Ma; lymphocytes, Ly; monocytes, Mo) that may play an important role in cardiac remodeling.
The perils of ALK5 inhibition
Considering the wide range of TGF-β-mediated actions in all cell types involved in cardiac remodeling, how can we explain the effects of ALK5 inhibition in the pressure overloaded myocardium? The beneficial effects of ALK5 inhibition on diastolic function may reflect attenuated fibroblast activation and reduced deposition of cross-linked collagen in the cardiac interstitium, or effects on cardiomyocyte hypertrophy. However, global inhibition of the matrix-preserving actions of TGF-β may have catastrophic consequences, resulting in an overactive matrix metalloproteinase system, protease-mediated matrix degradation and subsequent perturbation of the matrix balance in the remodeling heart. Loss of matrix support may cause chamber dilation; disruption of matrix-cardiomyocyte interactions may promote apoptosis of cardiomyocytes, further accentuating systolic dysfunction. Perturbation of matrix metabolism through inhibition of TGF-β signaling may also cause vascular events. In the current study, the authors observed a high incidence of fatal rupture of the ascending aorta upon administration of the ALK5 inhibitor in mice undergoing transverse aortic constriction protocols. ALK5 inhibition may impair deposition of matrix in the ascending aorta; in the presence of a pressure load, increased wall tension may result in rupture. This observation serves as a warning regarding potentially catastrophic effects of TGF-β inhibition in patients at high risk for aortic aneurysmal disease.
Activation of Smad-dependent and Smad-independent cascades: an opportunity for specific therapeutic interventions?
Because of its critical involvement in cardiac remodeling and fibrosis, the TGF-β cascade is an attractive therapeutic target. However, identification of safe and effective therapeutic strategies will require dissection of the downstream cascades transducing TGF-β signals. Understanding the distinct effects of Smad-dependent and Smad-independent TGF-β signaling may identify specific therapeutic targets, while avoiding disruption of protective effects. For example, in the pressure overloaded myocardium, the deleterious effects of TGF-β signaling in cardiac remodeling were suggested to be mediated through Smad-independent cardiomyocyte-specific signaling cascades [9].
Smad-dependent signaling may also regulate phenotype and function of cardiomyocytes and interstitial cells in cardiac remodeling. Whether Smad2 and Smad3 cascades play distinct roles in the pathogenesis of cardiac dysfunction, hypertrophy and fibrosis, remains unknown. In vitro experiments have suggested distinct (and sometimes opposing) effects of Smad2 and Smad3 signaling in regulation of cellular functions [23]. Smad3 mediates extracellular matrix protein synthesis and induction of protease inhibitors in TGF-β-stimulated cardiac fibroblasts [18]. These actions may promote interstitial fibrosis and diastolic dysfunction in the remodeling pressure-overloaded myocardium; however, the matrix-preserving effects of Smad3 may also protect the chamber from dilation in the presence of increased intracardiac pressures. In vivo experiments using fibroblast-specific gene targeting strategies are needed to dissect the functional role of Smad-dependent fibroblast activation in remodeling and dysfunction of the pressure overloaded heart. The availability of Smad3 inhibitors [24] may provide an interesting therapeutic strategy for patients with cardiac fibrosis and diastolic heart failure; what is needed is careful dissection of the relative role of Smad-dependent and Smad-independent pathways in the remodeling myocardium.
In addition to its potential role in the pathogenesis of heart failure, TGF-β is a promising therapeutic target in many non-cardiac conditions, including cancer [25], scleroderma [26] and several chronic fibrotic diseases. Because of the high prevalence of subclinical cardiovascular disease in the general population, complete and non-selective TGF-β inhibition in vulnerable patients may cause serious cardiac or vascular side effects. Thus, understanding the effects of interventions targeting the TGF-β cascade on the myocardium has broad clinical implications.
Acknowledgments
Dr Frangogiannis’ laboratory is supported by NIH grants R01 HL76246 and R01 HL85440.
Footnotes
DISCLOSURES: None.
References
- 1.Lan HY. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–67. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–74. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 6.Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc Res. 2005;65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–88. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol. 1999;31:667–78. doi: 10.1006/jmcc.1998.0902. [DOI] [PubMed] [Google Scholar]
- 9.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, et al. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–12. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, et al. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–38. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 11.Tan SM, Zhang Y, Connelly KA, Gilbert RE, Kelly DJ. Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol. 2010;298:H1415–25. doi: 10.1152/ajpheart.01048.2009. [DOI] [PubMed] [Google Scholar]
- 12.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–6. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–80. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 14.Engebretsen KV, Skardal K, Bjornstad S, Marstein HS, Skrbic B, Sjaastad I, et al. Attenuated development of cardiac fibrosis in left ventricular pressure overload by SM16, an orally active inhibitor of ALK5. J Mol Cell Cardiol. 2014 doi: 10.1016/j.yjmcc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Schroder D, Heger J, Piper HM, Euler G. Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat. J Mol Med (Berl) 2006;84:975–83. doi: 10.1007/s00109-006-0090-0. [DOI] [PubMed] [Google Scholar]
- 16.Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, et al. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002;109:787–96. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rainer PP, Hao S, Vanhoutte D, Lee DI, Koitabashi N, Molkentin JD, et al. Cardiomyocyte-specific transforming growth factor beta suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ Res. 2014;114:1246–57. doi: 10.1161/CIRCRESAHA.114.302653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–28. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2013;305:H1363–72. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 21.Travis MA, Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–24. doi: 10.1161/CIRCULATIONAHA.113.007101. [DOI] [PubMed] [Google Scholar]
- 23.Kretschmer A, Moepert K, Dames S, Sternberger M, Kaufmann J, Klippel A. Differential regulation of TGF-beta signaling through Smad2, Smad3 and Smad4. Oncogene. 2003;22:6748–63. doi: 10.1038/sj.onc.1206791. [DOI] [PubMed] [Google Scholar]
- 24.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 25.Drabsch Y, ten Dijke P. TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012;31:553–68. doi: 10.1007/s10555-012-9375-7. [DOI] [PubMed] [Google Scholar]
- 26.Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5:200–6. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]