Abstract
The foodborne mycotoxin deoxynivalenol (DON) induces a ribotoxic stress response in mononuclear phagocytes that mediate aberrant multi-organ upregulation of TNF-α, interleukins and chemokines in experimental animals. While other DON congeners also exist as food contaminants or pharmacologically-active derivatives, it is not known how these compounds affect expression of these cytokine genes in vivo. To address this gap, we compared in mice the acute effects of oral DON exposure to that of seven relevant congeners on splenic expression of representative cytokine mRNAs after 2 and 6 h. Congeners included the 8-ketotrichothecenes 3-acetyldeoxynivalenol (3-ADON), 15-acetyldeoxynivalenol (15-ADON), fusarenon X (FX), nivalenol (NIV), the plant metabolite DON-3-glucoside (D3G) and two synthetic DON derivatives with novel satiety-inducing properties (EN139528 and EN139544). DON markedly induced transient upregulation of TNF-α IL-1β, IL-6, CXCL-2, CCL-2 and CCL-7 mRNA expression. The two ADONs also evoked mRNA expression of these genes but to a relatively lesser extent. FX induced more persistent responses than the other DON congeners and, compared to DON, was: 1) more potent in inducing IL-1β mRNA, 2) approximately equipotent in the induction of TNF-α and CCL-2 mRNAs, and 3) less potent at upregulating IL-6, CXCL-2, and CCL-2 mRNAs. EN139528’s effects were similar to NIV, the least potent 8-ketotrichothecene, while D3G and EN139544 were largely incapable of eliciting cytokine or chemokine mRNA responses. Taken together, the results presented herein provide important new insights into the potential of naturally-occurring and synthetic DON congeners to elicit aberrant mRNA upregulation of cytokines associated with acute and chronic trichothecene toxicity.
Keywords: trichothecene, 8-ketotrichothecenes, cytokine, chemokine, deoxynivalenol, 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, fusarenon X, nivalenol, deoxynivalenol-3-glucoside, EN139528, EN139544
Introduction
Cereal grains are highly susceptible to Fusarium head blight (FHB) and concurrent contamination by trichothecene mycotoxins (Pestka, 2010a). Since these toxins have long been associated with human and animal toxicoses, their occurrence in food is of considerable public health concern (Pestka, 2010c). Deoxynivalenol (DON), a potent ribotoxic stressor and translational inhibitor, is the most commonly occurring trichothecene associated with FHB. Adverse effects of DON that have been reported in animal studies include anorexia, emesis, growth retardation, autoimmune-like pathophysiology, shock-like responses and, at high doses, death. One potential underlying mechanism for these toxic manifestations is the activation of the innate immune system resulting in aberrant upregulation of cytokine gene expression (Pestka, 2010b).
Exposure of mouse macrophage cell lines, human monocyte cell lines and human peripheral blood monocytes to DON evokes robust proinflammatory cytokine mRNA expression and protein secretion (Wong et al., 1998; Sugita-Konishi and Pestka, 2001; Chung et al., 2003b; He et al., 2013). These results suggest that mononuclear phagocytes in blood and tissues are critical immune targets of DON. Our laboratory has determined that cytokine gene expression results from DON-mediated pertubation of ribosomal RNA (rRNA) and sensing of damage-associated molecular patterns (DAMPs) by ribosome-associated stress kinases (Bae and Pestka, 2008; Bae et al., 2010; Pestka, 2010b). The latter activate members of the mitogen-activated protein kinase (MAPK) family including extracellular signal regulated protein kinase 1 and 2 (ERK1 and 2), p54 and p46 c-Jun N-terminal kinase 1 and 2 (JNK 1/2) and p38 (Zhou et al., 2003b; Zhou et al., 2005; Bae et al., 2010). These MAPKs mediate both transcription factor activation and mRNA stabilization resulting in increased expression of proinflammatory gene mRNAs and ultimately, proteins (Pestka, 2010b). DON’s capacity to alter translation expression of inflammation-associated genes is predominantly driven by selective transcription and mRNA stabilization, rather than regulation at the translational level (He et al., 2013). Thus, DON-induced upregulation of cytokine mRNAs correlates closely with downstream protein expression.
DON’s in vitro effects in mononuclear phagocytes can be reproduced in the mouse, a commonly used model of the human immune system. Following oral administration in the mouse, DON is rapidly absorbed and widely distributed to tissues throughout the body (Azcona-Olivera et al., 1995; Pestka and Amuzie, 2008). Consistent with our findings in mononuclear phagocytes, acute oral exposure of the mouse to DON sequentially induces in the spleen: 1) phosphorylation of ERK, JNK and p38, 2) activation of transcription factors such as NF-κB, AP-1 and CREB and 3) mRNA expression of IL-1β, IL-6 and TNF-α (Zhou et al., 2003a). Widespread tissue distribution of orally administered DON is further reflected by its capacity to induce proinflammatory cytokine mRNAs not only in the spleen, but also in the liver, lung, kidney, small intestine and brain (Azcona-Olivera et al., 1995; Zhou et al., 1997; Zhou et al., 2003a; Amuzie et al., 2008; Pestka and Amuzie, 2008). DON-induced upregulation of these genes in spleen correlates with increased serum cytokine levels of IL-1β, IL-6 and TNF-α after 2 h (Zhou et al., 1999; Islam and Pestka, 2006) suggesting that splenic cytokine mRNAs are useful as predictors of cytokine proteins in the periphery. IL-1β, IL-6 and TNF-α cause sickness and behavioral changes in experimental animals with principal pathophysiologic effects including anorexia and body weight loss (Plata-Salamán et al., 1996; Sonti et al., 1996; Plata-Salaman, 1998; Kelley et al., 2003; Wallenius et al., 2002; García et al., 2006). Therefore, it is plausible to suspect that DON-induced anorexia reflects, in part, a classic sickness response from the induction of proinflammatory cytokines.
In addition to proinflammatory cytokine upregulation, we have observed in both cultured macrophages and mice that DON induces elevated mRNA expression for several chemokines. These include C-X-C ligand 2 (CXCL-2), chemokine C-C ligand 2 (CCL-2), and C-C ligand 7 (CCL-7) (Chung et al., 2003a; Kinser et al., 2004; Pestka and Amuzie, 2008; He et al., 2013). CXCL-2 has polymorphonuclear leukocyte (PMNs) chemoattractant properties, while CCL-2 and CCL-7 mediate monocyte recruitment during inflammation (Wolpe et al., 1989; Mantovani et al., 1992; Zlotnik and Yoshie, 2000). Accordingly, some of DON’s adverse pathophysiologic effects might also be mediated by leukocyte chemotaxis evoked by aberrant chemokine expression.
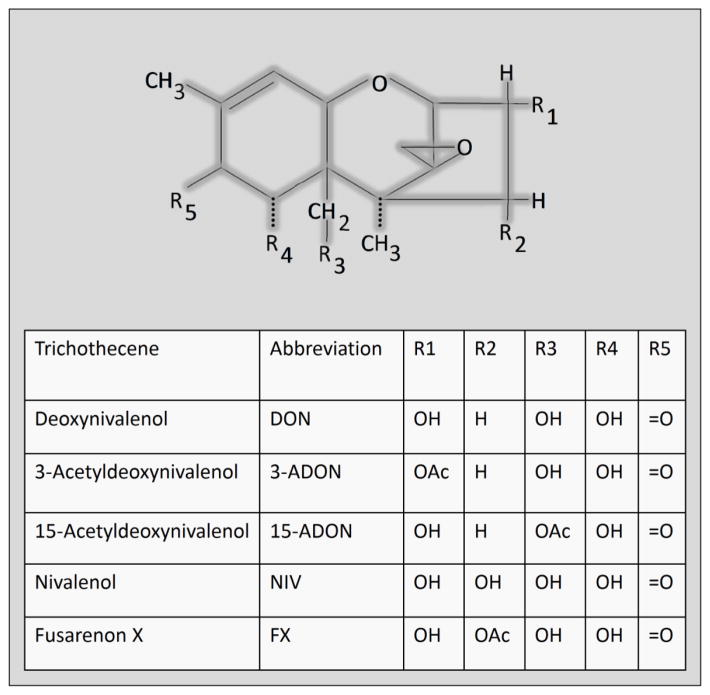
Several other 8-ketotrichothecenes besides DON are associated with FHB and can be found as contaminants in cereal-based foods (Pestka, 2010a). This family shares a common keto group at carbon 8 of the parent epoxytrichothecene nucleus and includes four congeners that are closely related to DON: 1) 3-acetyldeoxynivalenol (3-ADON), 2) 15-acetyldeoxynivalenol (15-ADON), 3) fusarenon X (FX; 4-acetylnivalenol), and 4) nivalenol (NIV) (Fig 1). DON and ADON-producing Fusarium occur in North America and Europe while NIV- producing strains predominate in Asian countries where it and FX are toxicologically highly relevant. These DON congeners have demonstrated potential to induce cytokine expression in mononuclear phagocytes (Sugita-Konishi and Pestka, 2001). Despite their presence in food and evidence for immunotoxicity in vitro, virtually nothing is known about whether these congeners affect innate immune gene expression in experimental animals.
Fig. 1.
Structures of 8-ketotrichothecene DON congeners.
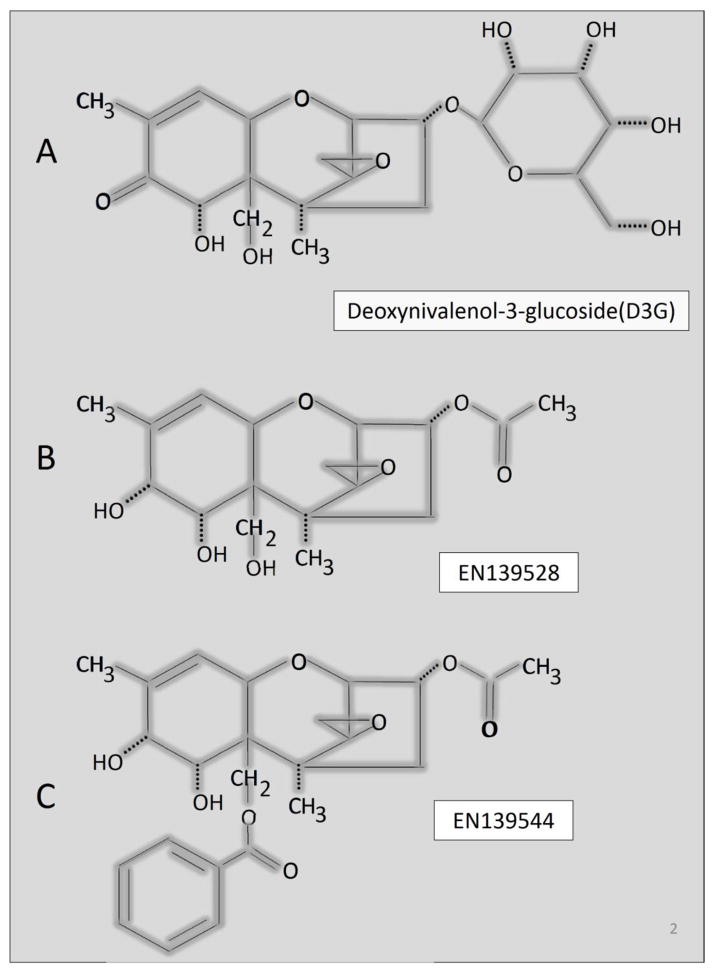
Of further concern, plants have the capacity to convert DON to the water soluble DON-3-glucoside (D3G) shown in Fig. 2A as a detoxification product (Poppenberger et al., 2003; Lemmens et al., 2005; Berthiller et al., 2011). D3G has been referred to as a masked mycotoxin because it can co-occur in cereals contaminated by DON but it is not detected by routine analytical methods (Berthiller et al., 2013). Importantly, D3G has the potential to be retroconverted to DON via enzymatic hydrolysis by gastrointestinal microbes (Berthiller et al., 2011; Nagl et al., 2012; Gratz et al., 2013). Lemmens et al. (2005) have found that the major Fusarium resistance QTL heavily utilized by wheat breeders colocalizes with the ability to efficiently convert DON to D3G. The increasing use of such cultivars could potentially increase the ratio the ratio of D3G/DON in Fusarium-infected wheat; however, there has been limited toxicological research to date on D3G’s effects on animals, particularly in regard to induction of cytokines in vivo.
Fig. 2.
Structures of natural DON plant metabolite D3G (A) and synthetic DON congeners. (EN139528 (B) and EN139544 (C).
In addition to natural congeners, DON has been derivatized to exploit unique pharmacological properties (Krantis and Durst, 2004; Durst and Krantis, 2006). Both EN139528 and EN139544 are efficacious in reducing gut motility and food intake as well as promoting weight loss but do not cause emesis. The structures of these satiety-inducing compounds are shown in Figure 2B and 2C, respectively. Specifically, in EN139528 (3α-acetoxy-12, 13-epoxy-9-methyltrichothecen-9-en-7α, 8α, 15-triol), a hydroxyl group replaces the 8-keto group and an acetyl group replaces the C3-hydroxyl group in DON, whereas in EN139544 (3α-acetoxy-15-benzoyloxy-7α, 8α-dihydroxy-12, 13-epoxytrichothec-9-ene), a benzoyloxy group replaces the C15-hydroxyl. Accordingly, these two synthetic DON congeners might have unique and promising pharmacological properties of DON without its toxic effects. It is not yet known, however, whether like DON, these synthetic congeners are capable of aberrantly inducing innate immune genes in vivo.
Accordingly, while DONs toxic effects have been previously linked to altered expression of cytokine genes, surprisingly little is known about the capacity of naturally-occurring and synthetic DON congeners to elicit similar responses. This gap in understanding precludes our ability to predict in vivo toxic potencies of these compounds. We believe that relative tissue levels of cytokine mRNAs in the well-characterized spleen can serve as biomarkers of innate immune system dysregulation by DON congeners. The aim of this study was to compare the effects of DON and its congeners on splenic TNF-α, interleukin and chemokine mRNA expression in the mouse. The results provide important new insights into the comparative abilities of these naturally-occurring and synthetic DON congeners to mediate toxicity by evoking a cytokine storm.
Materials and methods
Toxins
DON, 3-ADON, 15-ADON, FX and NIV were isolated, and purity (>98%) was verified by elemental analysis and LC-MS as previously described (Flannery et al., 2011; Wu et al., 2012). EN139528 and EN139544 were available at a purity of (> 98%) based on High Field proton HNMR as previously described (Durst and Krantis, 2006). D3G was either purified from DON-treated wheat as previously described (Berthiller et al., 2011), or generated by in vitro enzymatic synthesis and preparative HPLC purification (manuscript in preparation).
Laboratory animals
Animal treatment followed National Institutes of Health guidelines and was approved by the Michigan State University Institutional Animal Care and Use Committee (MSU-IACUC). Female B6C3F1 (C57BL/6 x C3HeN) mice (10–12 weeks old) were obtained from Charles River Breeding (Portage, MI) and housed individually in polycarbonate cages in a room maintained at 21–24°C and 40–55% relative humidity under a 12 h light (6:00–18:00 h)/dark (18:00–6:00 h) cycle. This mouse strain was employed because it is used for immunotoxicology studies by the National Toxicology Program and has been utilized extensively by our laboratory to study the effects of DON (Azcona-Olivera et al., 1995; Zhou et al. 1997; Kinser et al., 2004). The female gender was chosen because its docile characteristics enabled ease of handling.
Experimental design
The general experimental design is depicted in Fig. 3. Briefly, groups of mice (n=6) were fasted for 8 h and then orally gavaged at the initiation of the dark cycle with 2.5 mg/kg bw DON, D3G, 3-ADON, 15-ADON, FX, NIV, EN139528 or EN139544 in 100 μl phosphate-buffered saline (PBS), respectively, and cohorts sacrificed at 2 h and 6 h after exposure. PBS (100 μl) was used as the vehicle control. At experiment termination, mice were anesthetized by IP injection with 100 μl 56 mg/ml sodium pentobarbital. Spleens were removed and immediately immersed in RNAlater (Ambion Inc., Austin, TX) for 24 h, and then frozen at −80°C for real-time PCR. Fasting was utilized to ensure uniform uptake of DON. The 2.5 mg/kg BW comparator dose and 2 and 6 h time points employed here were chosen based on our previous mouse studies with DON showing that 1) this amount reliably induced splenic cytokine and mRNAs and 2) these effects were maximal at 2 to 4 h (Azcona-Olivera et al., 1995; Chung et al., 2003b; Kinser et al., 2004; Islam and Pestka, 2006; Amuzie et al., 2008; Amuzie et al., 2009). The responses to this dose were less than those which could be maximally achieved with DON at 10 to 25 mg/kg BW. Therefore, the use of the comparator dose and the 2 and 6 h time points enabled discernment between both increased and decreased mRNA expression by the congeners relative to DON.
Fig. 3.
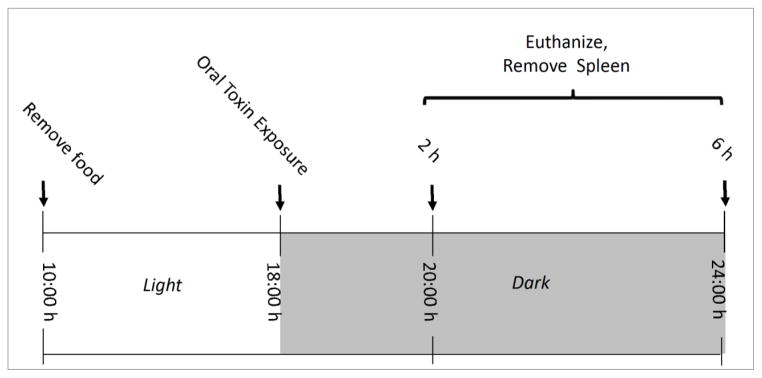
Experimental design.
Quantitative real-time PCR
RNA was isolated using Tri Reagent (Molecular Research Center, Inc., Cincinnati, OH). An ABI PRISM 7900HT Sequence Detection system using Taqman One-Step Real-time PCR Master Mix and Assay-on-Demand primer/probes (40 cycles, see supplementary materials) (Applied Biosystems, Foster City, NY) were utilized to measure IL-1β, IL-6, TNF-α, CXCL-2, CCL-2 and CCL-7 mRNA in mouse spleens. Fold changes of cytokines were determined using β2-microglobulin RNA control and 2(−ΔΔCt) method (Livak and Schmittgen, 2001).
Statistics
Data were plotted and statistically analyzed using SigmaPlot 11 for Windows (Jandel Scientific, San Rafael, CA). Means were considered significantly different at p ≤ 0.05. A one-way ANOVA using Holm-Sidak method was used to analyze significant differences between treatments and the control. If normality failed, Kruskal-Wallis ANOVA on Ranks was executed with the Student-Newman-Keuls test or Dunnett’s Method.
Results
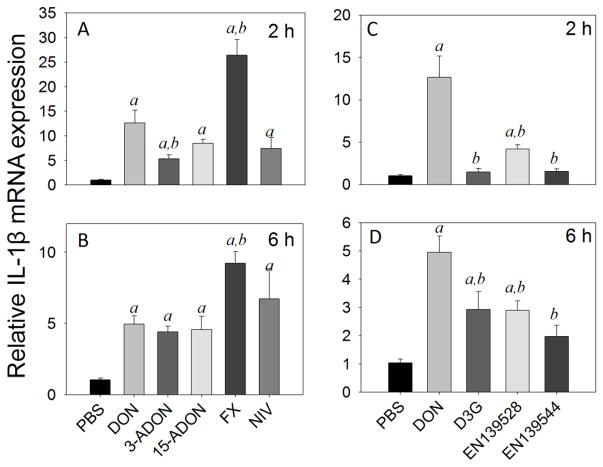
DON, 3-ADON, 15-ADON, FX, and NIV upregulated IL-1β mRNA by 13-, 5-, 8-, 26-, and 7-fold at 2 h, respectively (Fig 4A), and by 5-, 4.4-, 4.5-, 9-, and 7-fold over control at 6 h, respectively (Fig 4B). Following D3G, EN139528 and EN139544 exposure, IL-1β mRNA expression was 1.4-, 4- and 1.5-fold over control at 2 h, respectively (Fig 4C), and 3-, 3- and 2-fold over control at 6 h, respectively (Fig 4D). The IL-1β mRNA responses to all three of the latter congeners were significantly lower than that for DON at both time points (<0.05).
Fig. 4.
Comparative effects of DON congeners on IL-1β mRNA expression. Mice were orally gavaged with 8-ketotrichothecenes (A,B) and D3G, EN139528, EN139544 (C,D) at 2.5 mg/kg bw and then spleens removed at 2 h (A,C) or 6 h (B,D) post administration IL-1β mRNA expression was determined by real-time PCR. Data are mean ± SEM (n=6/gp) of mRNA fold change relative to vehicle control and were analyzed as described in Fig. 4 legend
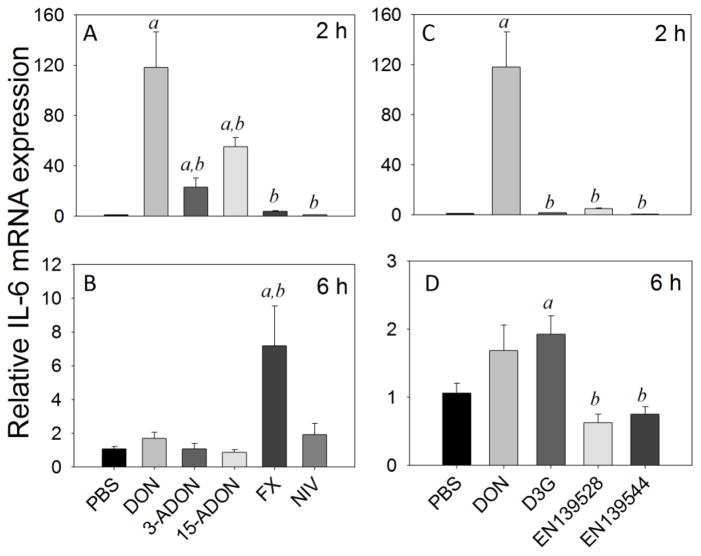
IL-6 mRNA expression was elevated by DON (118-fold), 3-ADON (23-fold), and 15-ADON (55-fold) at 2 h but not by FX and NIV (Fig 5A). IL-6 mRNA expression was unaffected by D3G, EN139528 or EN139544 at 2 h (Fig. 5C). Responses to all congeners at 2 h were significantly lower than that for DON (<0.05). By 6 h, splenic IL-6 mRNAs were unaffected by the congeners with the exceptions of FX and D3G which were modestly increased by 7-fold (Fig 5B) and 2-fold (Fig. 5D), respectively.
Fig. 5.
Comparative effects of DON congeners on IL-6 mRNA expression. Mice were orally gavaged with 8-ketotrichothecenes (A,B) and D3G, EN139528, EN139544 (C,D) at 2.5 mg/kg bw and then spleens removed at 2 h (A,C) or 6 h (B,D) post administration IL-6 mRNA expression was determined by real-time PCR. Data are mean ± SEM (n=6/gp) of mRNA fold change relative to vehicle control and were analyzed as described in Fig. 4 legend.
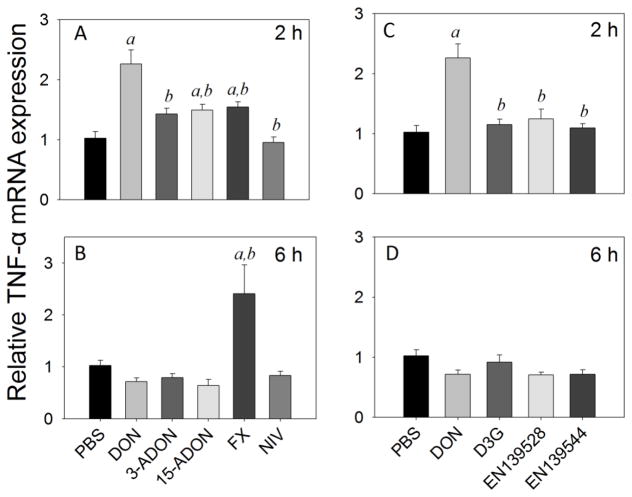
TNF-α mRNA levels were modestly elevated by DON (2-fold), 15-ADON (1.5-fold) and FX (1.5-fold) at 2 h (Fig. 6A). Only FX (2.4-fold) caused TNF-α mRNA elevation at 6 h (Fig 4B). TNF-α mRNA expression was not affected at either time point by NIV (Fig. 6A, B) or by D3G, EN139528 or EN139544 (Fig. 6C, D).
Fig. 6.
Comparative effects of DON congeners on TNF-α mRNA expression. Mice were orally gavaged with 8-ketotrichothecenes (A,B) and D3G, EN139528, EN139544 (C,D) at 2.5 mg/kg bw and then spleens removed at 2 h (A,C) or 6 h (B,D) post administration TNF-α mRNA expression was determined by real-time PCR. The letter a indicates significant differences between responses to DON or DON congener and vehicle control (p ≤ 0.05). The letter b indicates significant differences between responses to congener and DON (p ≤ 0.05).
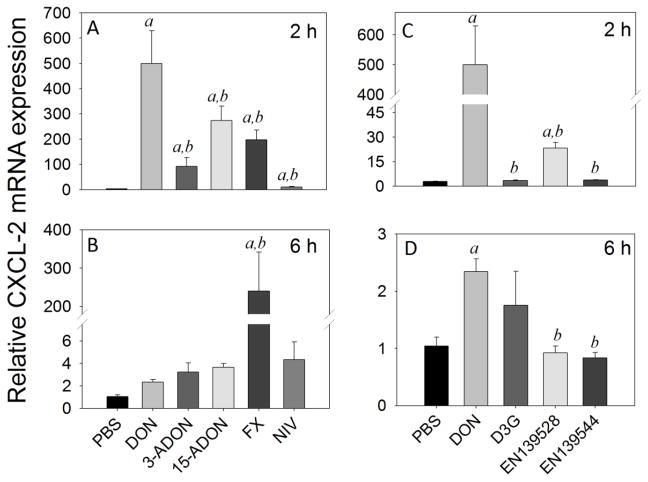
Splenic CXCL-2 mRNA expression levels were markedly increased by DON (500-fold), 3-ADON (92-fold), 15-ADON (273-fold), FX (198-fold), and NIV (10-fold) at 2 h (Fig 7A). EN139528 moderately elevated CXCL-2 mRNA expression by 12-fold at 2 h post-exposure while D3G and EN139544 had no effect (Fig. 7C). Responses to all congeners at 2 h were significantly lower than that for DON (<0.05). While expression at 6 h remained robust in FX-treated mice (240-fold), it was markedly less in those animals treated with DON (2-fold), 3-ADON (3-fold), 15-ADON (4-fold), and NIV (4-fold) (Fig. 7B). D3G, EN139528 and EN139544 did not affect CXCL-2 mRNA expression at 6 h (Fig. 7D).
Fig. 7.
Comparative effects of DON congeners on CXCL-2 mRNA expression. Mice were orally gavaged with 8-ketotrichothecenes (A,B) and D3G, EN139528, EN139544 (C,D) at 2.5 mg/kg bw and then spleens removed at 2 h (A,C) or 6 h (B,D) post administration CXCL-2 mRNA expression was determined by real-time PCR. Data are mean ± SEM (n=6/gp) of mRNA fold change relative to vehicle control and were analyzed as described in Fig. 4 legend.
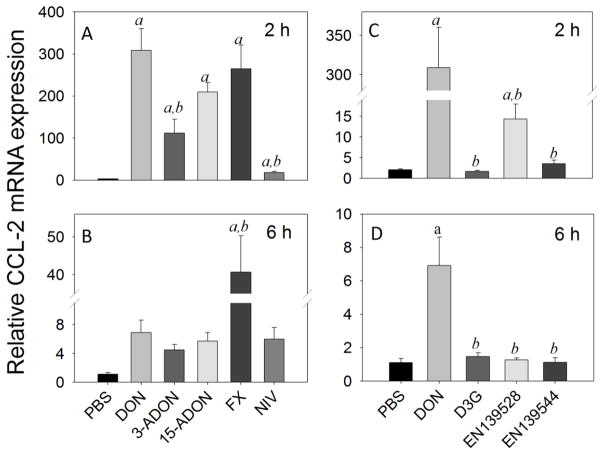
As determined for CXCL-2, robust increases in CCL-2 mRNA expression were observed after 2 h in mice exposed orally to DON (309-fold), 3-ADON (112-fold), 15-ADON (210-fold), and FX (265-fold), with moderate elevation by NIV (10-fold) (Fig 8A). By 6 h, CCL-2 mRNA levels declined to 7-, 4-, 6-, 41-, and 6-fold over control in DON-, 3-ADON-, 15-ADON, FX- and NIV- treated animals, respectively (Fig. 8B). CCL-2 mRNA expression at 2 h was moderately upregulated by EN139528 (14-fold) (Fig. 8C) and returned to basal level by 6 h (Fig. 8D). Contrastingly, D3G and EN139544 were without effect at either time point (Fig. 8C, D). Responses to all three latter congeners were significantly lower than that for DON at both time points (<0.05).
Fig. 8.
Comparative effects of DON congeners on CCL-2 mRNA expression. Mice were orally gavaged with 8-ketotrichothecenes (A,B) and D3G, EN139528, EN139544 (C,D) at 2.5 mg/kg bw and then spleens removed at 2 h (A,C) or 6 h (B,D) post administration CCL-2 mRNA expression was determined by real-time PCR. Data are mean ± SEM (n=6/gp) of mRNA fold change relative to vehicle control and were analyzed as described in Fig. 4 legend.
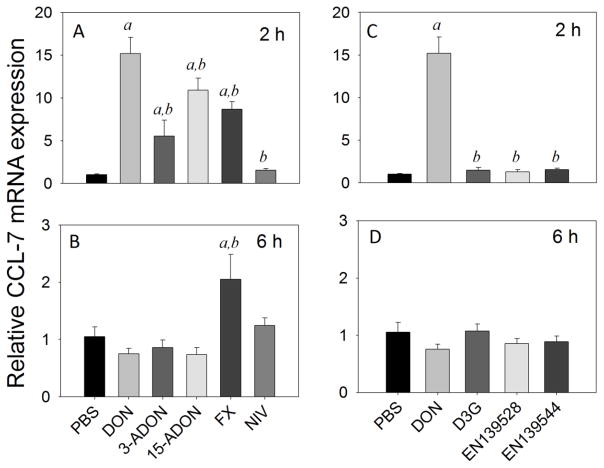
Upregulation of splenic CCL-7 mRNAs was apparent at 2 h in mice treated with DON (15-fold), 3-ADON (6-fold), 15-ADON (11-fold) and FX (9-fold), while NIV was without effect (Fig 9A). Responses to all of these congeners at 2 h were significantly lower than that for DON (<0.05). By 6 h, CCL-7 mRNA expression returned to basal levels in mice treated with DON and all its congeners, except for FX (2-fold) (Fig. 9B). Exposure to D3G, EN139528 and EN139544 did not alter CCL-7 mRNA expression in mouse spleen at 2 h (Fig. 9C) or 6 h (Fig. 9D).
Fig. 9.
Comparative effects of DON congeners on CCL-7 mRNA expression. Mice were orally gavaged with 8-ketotrichothecenes (A,B) and D3G, EN139528, EN139544 (C,D) at 2.5 mg/kg bw and then spleens removed at 2 h (A,C) or 6 h (B,D) post administration CCL-7 mRNA expression was determined by real-time PCR. Data are mean ± SEM (n=6/gp) of mRNA fold change relative to vehicle control and were analyzed as described in Fig. 4 legend.
Discussion
DON evokes a wide spectrum of pathophysiological effects in experimental animals that are attributable, in part, to a ribotoxic stress-mediated cytokine storm (Pestka, 2010b). Measurement of in vivo cytokine protein levels is complicated by several factors (Svetic et al., 1991). Cytokine assays are not sufficiently sensitive for the detection of minute concentrations secreted in tissue. Also, adsorption and uptake of cytokines by cells bearing specific receptors impairs accurate quantitation of secreted cytokine. Because of these issues, here we utilized cytokine mRNAs as biomarkers to compare the potential of DON to its natural and synthetic DON congeners to aberrantly induce innate immune activation in the mouse spleen. As expected from our previous findings (Azcona-Olivera et al., 1995; Zhou et al., 1997; Chung et al., 2003b; Kinser et al., 2004), oral exposure of mice to 2.5 mg/kg bw DON dramatically increased TNF-α, IL-1β, IL-6, CXCL-2, CCL-2 and CCL-7 mRNA expression in spleen at 2 h post-exposure. When these responses were used as reference points for comparing the relative effects of the other congeners and their response durations, several key findings were evident (summarized in Table 1). First, as observed for DON, the 8-ketotrichothecenes 3-ADON and 15-ADON evoked transient cytokine and chemokine mRNA expression but to a lesser extent than the parent compound. Second, relative to DON, FX was 1) more potent in inducing IL-1β mRNA at 2 h, 2) approximately equipotent in the induction of TNF-α and CCL-2 mRNA expression at 2 h, and 3) less potent at upregulating IL-6, CXCL-2, and CCL-7 mRNAs at 2 h. Third, cytokine and chemokine responses to FX generally persisted longer (i.e. were still detectable at 6 h) than DON or the other congeners. Fourth, of the 8-ketotrichothecenes tested, NIV was the least potent. Finally, regarding the effects of other DON congeners, EN139528’s mirrored those of NIV, whereas, except for slight elevations in IL-1β mRNA expression, D3G and EN139544 were largely incapable of evoking cytokine or chemokine responses in the mouse.
Table 1.
Summary of fold changes in splenic cytokine mRNA expression induced by oral exposure to DON and its congeners
| Compound | IL-1β | IL-6 | TNF-α | CXCL-2 | CCL-2 | CCL7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h | 6 h | 2 h | 6 h | 2 h | 6 h | 2 h | 6 h | 2 h | 6 h | 2 h | 6 h | |
| DON | 13a | 5a | 118a | 1 | 2a | 1 | 500 | 2 | 310a | 7 | 15a | 1 |
| 3-ADON | 5a b | 4a | 23a b | 1 | 1b | 1 | 92a b | 3 | 110ab | 4 | 6a b | 1 |
| 15 -ADON | 8a | 5a | 55a b | 1 | 1.5a b | 1 | 270a b | 4 | 210a | 6 | 11a b | 1 |
| FX | 26a b | 9a b | 1b | 7a b | 1.5a b | 2.4a b | 200a b | 240a b | 270a | 41ab | 9b | 2a b |
| NIV | 7a | 7a | 1b | 1 | 1b | 1 | 10a b | 4 | 10ab | 6 | 1b | 1 |
| D3G | 1b | 3a b | 1b | 2a | 1b | 1 | 1b | 1 | 1b | 1b | 1b | 1 |
| EN139528 | 4a b | 3a b | 1b | 1b | 1b | 1 | 12a b | 1 | 14ab | 1b | 1b | 1 |
| EN139544 | 2b | 2b | 1b | 1b | 1b | 1 | 1b | 1 | 1b | 1b | 1 | 1 |
Significantly different from vehicle-treated animals at given time point.
Significantly different from DON-treated animals at given time point.
The kinetics of cytokine mRNA responses to DON indicated that upregulation of these genes was maximal at 2 h but were reduced or returned to basal levels at 6 h. In vitro studies suggest that the likely reason for the decreased response at 6 h is mRNA degradation caused by reduced MAPK activation (Wong et al., 2001; Chung et al. 2000c; Gray and Pestka, 2007). These kinetic findings are consistent with our previous observations that splenic IL-1β, IL-6 and TNF-α mRNA responses to DON in B6C3F1 mice peaked at 2 h (Islam and Pestka, 2006). It is further important to note that in this prior study, plasma IL-1β, IL-6 and TNF-α were also induced by DON at 2 h but not at 1, 4, 8 or 24 h. Thus, splenic mRNA expression is indeed predictive of systemic cytokine concentrations in DON-treated animals.
This is the first report to demonstrate that 8-ketotrichothecenes 3-ADON, 15-ADON, FX and NIV differentially induce proinflammatory cytokine and chemokine expression in vivo. Given the pleiotropic effects of these inflammatory mediators and their capacity to cause sickness responses and shock-related effects, aberrant upregulation of these genes by DON congeners is toxicologically relevant. While 3-ADON and 15-ADON were generally less potent than DON, chronic exposure to these congeners nevertheless could result in a sustained proinflammatory state. In support of this contention, we previously found that chronic feeding of 15-ADON to female B6C3F1 mice reduced food consumption and body weight gain but it was slightly less potent than DON in causing these effects (Pestka et al., 1986).
Interestingly, compared to other 8-ketotrichothecenes, NIV had only very slight effects on cytokine and chemokine mRNA expression, whereas FX, an acetylated form of NIV (Fig. 1) induced robust and persistent expression of these genes. Of further importance, FX was differentially more or less potent than DON depending on the cytokine being measured. The discordant findings with NIV and FX might be explained by inherent differences in absorption, distribution, metabolism and excretion of these congeners relative to DON. When the mouse is orally dosed with NIV, a large proportion of the toxin passes through the gastrointestinal lumen without being absorbed (Poapolathep et al., 2003). Inefficient absorption of NIV following oral exposure probably diminishes its impact on splenic cytokine and chemokine expression. Unlike NIV, absorption of FX from the gastrointestinal tract following oral exposure is more efficient (5- to 10- fold higher than NIV) (Poapolathep et al., 2003). Elimination of FX follows two-compartment clearance kinetics (t1/2α = 0.878 h, t1/2β = 37.63 h). Comparatively, DON is cleared more rapidly than FX, with an initial rapid phase of elimination (t1/2α = 0.36 h) and a slower terminal elimination phase (t1/2β = 7.62 h) (Azcona-Olivera et al., 1995). The longer half-lives for FX compared to DON likely explains its more persistent effects and should be an important consideration in future safety assessments.
From a comparative risk assessment perspective, another key finding of this study was that D3G was essentially devoid of the ability to induce cytokine expression in the mouse. This lack of activity might be related to D3G’s inability to elicit a ribotoxic stress response which plays an important role in cytokine induction. Consistent with this notion, D3G has been shown to be much less inhibitory for wheat ribosome function in vitro (Poppenberger et al., 2003). D3G also differs from DON because it is water soluble and therefore likely to be absorbed to a lower extent. An important caveat to this interpretation is the potential for gastrointestinal microbiota to produce hydrolytic enzymes capable of converting D3G back to DON (Berthiller et al., 2011; Gratz et al., 2013; Nagl et al., 2012). It is possible that these enzymes do not function at a sufficient rate to impact the systemic bioavailability of the conversion endproduct, DON, because cleavage occurs late, in the colon, whereas most DON is rapidly taken up by the small intestine. Further clarification is required relative to species differences in gut microbiomes and their inherent capacity to bioactivate D3G by hydrolyzing it to DON.
The synthetic DON congeners EN139528 and EN139544 have been reported to have relatively lower toxicity scores than DON and other trichothecenes which led to their selection for preclinical testing for satiety induction (Durst and Krantis, 2004; Durst and Krantis, 2006). Our results support this contention since EN139528’s effects mirrored those of NIV, the least potent 8-ketrotrichothecene, and EN139544 was largely incapable of eliciting cytokine or chemokine responses in the mouse. The inactivity of the latter compound might relate to its bulky benzoyl-residue which might sterically hinder interaction with the ribosomal target and onset of the ribotoxic stress response. Minimally, the negligible effects of these compounds on innate immune gene expression in vivo could be considered along with other relevant preclinical data when assessing the safety of these and other putative drugs developed from DON.
The 2.5 mg/kg BW dose employed in this study approximates the total daily consumption of DON in mice consuming food containing 12.5 mg/kg of the toxin (Forsell et al., 1986). This DON concentration is relevant because this trichothecene has been detected in corn- and wheat-based foods at levels exceeding 15 mg/kg (Abouzied et al., 1991), and furthermore, the European guidance level for corn byproducts intended for feed use is 12 mg/kg. It is also important to emphasize that it was our intention to compare gravimetric equivalents rather than molar equivalents, since this is in line with conventional measurement of 8-ketotrichothecenes and D3G in cereals and associated food products as well as putative usage of EN139528 and EN139544 as pharmacotherapeutic agents. It is nevertheless recognized that small differences in potencies of the compared compounds might be, in part, attributable to inherent disparities in molecular weights.
Taken together, this comparative research employing cytokine biomarkers is important because it establishes a framework on which to base future studies of the acute and chronic effects of natural and synthetic DON congeners. The results presented herein indicate that the 8-ketotrichothecenes produced naturally by Fusarium graminearum exhibit variable potentials to dysregulate innate immune gene expression in vivo. It is highly likely that, similar to DON, ribotoxic stress-driven activation of MAPKs is involved in the cytokine responses to the DON congeners but this will require additional investigation. Relative to DON, the effects of D3G, EN139528 and EN139544 were minimal or absent. Further investigation is needed on the effects of these congeners on different animal species as well as their metabolism by enzymes from mammalian and microbial sources.
Supplementary Material
Highlights.
We compared effects of DON congeners on biomarker proinflammatory genes in mice
Oral DON induced splenic IL-1β, IL-6, TNF-α,CXCL-2, CCL-2 and CCL-7 mRNAs
8-ketotrichothecene ranking for biomarkers was FX≈DON>15ADON>3ADON>NIV
Plant metabolite DON-3-glucoside failed to induce proinflammatory biomarkers
Synthetic DON congeners EN139528 and EN139544 did not affect biomarkers
Acknowledgments
Funding
This study was supported by USDA NIFA Award (2011-0635), USDA Wheat and Barley SCAB Initiative Award 59-0206-9-058 and by Public Health Service Grant ES03553 from the National Institutes of Health. GA and FB received funding from the Vienna Science and Technology Fund (WWTF LS12-021). FB also thanks the Austrian Federal Ministry of Economy, Family and Youth, the Austrian National Foundation for Research, Technology and Development.
We would like to acknowledge the excellent technical advice of Dr. Rudi Krska as well as Mary Rosner, for assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abouzied MM, Azcona JI, Braselton WE, Pestka JJ. Immunochemical assessment of mycotoxins in 1989 grain foods: evidence for deoxynivalenol (vomitoxin) contamination. Appl Environ Microbiol. 1991;57:672–677. doi: 10.1128/aem.57.3.672-677.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuzie CJ, Harkema JR, Pestka JJ. Tissue distribution and proinflammatory cytokine induction by the trichothecene deoxynivalenol in the mouse: comparison of nasal vs. oral exposure. Toxicol. 2008;248:39–44. doi: 10.1016/j.tox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Amuzie CJ, Shinozuka J, Pestka JJ. Induction of suppressors of cytokine signaling by the trichothecene deoxynivalenol in the mouse. Toxicol Sci. 2009;111:277–287. doi: 10.1093/toxsci/kfp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcona-Olivera JI, Ouyang Y, Murtha J, Chu FS, Pestka JJ. Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): relationship to toxin distribution and protein synthesis inhibition. Toxicol. Appl Pharmacol. 1995;133:109–120. doi: 10.1006/taap.1995.1132. [DOI] [PubMed] [Google Scholar]
- Bae H, Gray JS, Li M, Vines L, Kim J, Pestka JJ. Hematopoietic cell kinase associates with the 40S ribosomal subunit and mediates the ribotoxic stress response to deoxynivalenol in mononuclear phagocytes. Toxicol Sci. 2010;115:444–452. doi: 10.1093/toxsci/kfq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae HK, Pestka JJ. Deoxynivalenol induces p38 interaction with the ribosome in monocytes and macrophages. Toxicol Sci. 2008;105:59–66. doi: 10.1093/toxsci/kfn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiller F, Crews C, Dall’Asta C, De Saeger S, Haesaert G, Karlovsky P, Oswald IP, Seefelder W, Speijers G, Stroka J. Masked mycotoxins: A review. Mol Nutr Food Res. 2013;57:165–186. doi: 10.1002/mnfr.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiller F, Krska R, Domig KJ, Kneifel W, Juge N, Schuhmacher R, Adam G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol Lett. 2011;206:264–267. doi: 10.1016/j.toxlet.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YJ, Jarvis B, Pestka J. Modulation of lipopolysaccharide-induced proinflammatory cytokine production by satratoxins and other macrocyclic trichothecenes in the murine macrophage. J Toxicol Environ Health A. 2003a;66:379–391. doi: 10.1080/15287390306363. [DOI] [PubMed] [Google Scholar]
- Chung YJ, Yang GH, Islam Z, Pestka JJ. Up-regulation of macrophage inflammatory protein-2 and complement 3A receptor by the trichothecenes deoxynivalenol and satratoxin G. Toxicol. 2003b;186:51–65. doi: 10.1016/s0300-483x(02)00605-4. [DOI] [PubMed] [Google Scholar]
- Chung YJ, Zhou HR, Pestka JJ. Transcriptional and posttranscriptional roles for p38 mitogen-activated protein kinase in upregulation of TNF-alpha expression by deoxynivalenol (vomitoxin) Toxicol Appl Pharmacol. 2003c;193:188–201. doi: 10.1016/s0041-008x(03)00299-0. [DOI] [PubMed] [Google Scholar]
- Durst T, Krantis A. A multi-ring regulator of gut motility and food intake. 2,004,009,074A2. [last accessed 01/11/2014];WO Patent. 2004 http://www.google.com/patents/WO2004009074A2?cl=en.
- Durst T, Krantis A. Multi-ring compounds for regulating gut motility, food intake and weight gain. 2,006,006,082. [last accessed 01/11/2014];WO Patent. 2006 https://www.google.com/patents/WO2006006082A2?cl=en.
- Flannery BM, Wu W, Pestka JJ. Characterization of deoxynivalenol-induced anorexia using mouse bioassay. Food and Chemical Toxicology. 2011;49:1863–1869. doi: 10.1016/j.fct.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsell JH, Witt MF, Tai JH, Jensen R, Pestka JJ. Effects of 8-week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food Chem Toxicol. 1986;24:213–219. doi: 10.1016/0278-6915(86)90231-0. [DOI] [PubMed] [Google Scholar]
- García MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahrén B, Enerback S, Ohlsson C. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- Gratz SW, Duncan G, Richardson AJ. The human fecal microbiota metabolizes deoxynivalenol and deoxynivalenol-3-glucoside and may be responsible for urinary deepoxy-deoxynivalenol. Appl Environ Microbiol. 2013;79:1821–1825. doi: 10.1128/AEM.02987-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS, Pestka JJ. Transcriptional regulation of deoxynivalenol-induced IL-8 expression in human monocytes. Toxicol Sci. 2007;99:502–511. doi: 10.1093/toxsci/kfm182. [DOI] [PubMed] [Google Scholar]
- He K, Pan X, Zhou HR, Pestka JJ. Modulation of inflammatory gene expression by the ribotoxin deoxynivalenol involves coordinate regulation of the transcriptome and translatome. Toxicol Sci. 2013;131:153–163. doi: 10.1093/toxsci/kfs266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Pestka JJ. LPS priming potentiates and prolongs proinflammatory cytokine response to the trichothecene deoxynivalenol in the mouse. Toxicol Appl Pharmacol. 2006;211:53–63. doi: 10.1016/j.taap.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17:112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kinser S, Jia Q, Li M, Laughter A, Cornwell PD, Corton JC, Pestka JJ. Gene expression profiling in spleens of deoxynivalenol-exposed mice: immediate early genes as primary targets. J Toxicol Environ Health, Part A. 2004;67:1423–1441. doi: 10.1080/15287390490483827. [DOI] [PubMed] [Google Scholar]
- Krantis A, Durst T. A multi-ring regulator of gut motility and food intake. 2,004,009,074 WO Patent. 2004
- Lemmens M, Scholz U, Berthiller F, Dall’Asta C, Koutnik A, Schuhmacher R, Adam G, Buerstmayr H, Mesterhazy A, Krska R, Ruckenbauer P. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol Plant Microbe Interact. 2005;18:1318–1324. doi: 10.1094/MPMI-18-1318. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- Nagl V, Schwartz H, Krska R, Moll WD, Knasmuller S, Ritzmann M, Adam G, Berthiller F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol Letts. 2012;213:367–373. doi: 10.1016/j.toxlet.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka J. Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J. 2010a;3:323–347. [Google Scholar]
- Pestka JJ. Deoxynivalenol-induced proinflammatory gene expression: mechanisms and pathological sequelae. Toxins. 2010b;2:1300–1317. doi: 10.3390/toxins2061300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol. 2010c;84:663–679. doi: 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Amuzie CJ. Tissue distribution and proinflammatory cytokine gene expression following acute oral exposure to deoxynivalenol: comparison of weanling and adult mice. Food Chem Toxicol. 2008;46:2826–2831. doi: 10.1016/j.fct.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ, Lin WS, Forsell JH. Decreased feed consumption and body-weight gain in the B6C3F1 mouse after dietary exposure to 15-acetyldeoxynivalenol. Food Chem Toxicol. 1986;24:1309–1313. doi: 10.1016/0278-6915(86)90063-3. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR. Cytokines and anorexia: a brief overview, Sem. Oncol. 1998;25:64–72. [PubMed] [Google Scholar]
- Plata-Salamán CR, Sonti G, Borkoski JP, Wilson CD, Ffrench-Mullen MHJ. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol Behav. 1996;60:867–875. [PubMed] [Google Scholar]
- Poapolathep A, Sugita-Konishi Y, Doi K, Kumagai S. The fates of trichothecene mycotoxins, nivalenol and fusarenon-X, in mice. Toxicon. 2003;41:1047–1054. doi: 10.1016/s0041-0101(03)00089-8. [DOI] [PubMed] [Google Scholar]
- Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R, Krska R, Kuchler K, Glossl J, Luschnig C, Adam G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J Biol Chem. 2003;278:47905–47914. doi: 10.1074/jbc.M307552200. [DOI] [PubMed] [Google Scholar]
- Sonti G, Ilyin S, Plata-Salaman C. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am J Physiol -Reg, Int Comp Physiol. 1996;270:R1394–R1402. doi: 10.1152/ajpregu.1996.270.6.R1394. [DOI] [PubMed] [Google Scholar]
- Sugita-Konishi Y, Pestka JJ. Differential upregulation of TNF-alpha, IL-6, and IL-8 production by deoxynivalenol (vomitoxin) and other 8-ketotrichothecenes in a human macrophage model. J Toxicol Environ Health A. 2001;64:619–636. doi: 10.1080/152873901753246223. [DOI] [PubMed] [Google Scholar]
- Svetic A, Finkelman FD, Jian YC, Dieffenbach CW, Scott DE, McCarthy KF, Steinberg AD, Gause WC. Cytokine goat expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J Immunol. 1991;147:2391–2397. [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc National Acad Sci. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S, Schwartz RZC, Pestka JJ. Superinduction of TNF-alpha and IL-6 in macrophages by vomitoxin (deoxynivalenol) modulated by mRNA stabilization. Toxicology. 2001;16:139–149. doi: 10.1016/s0300-483x(01)00331-6. [DOI] [PubMed] [Google Scholar]
- Wong SS, Zhou HR, Marin-Martinez M, Brooks K, Pestka J. Modulation of IL-1 beta, IL-6 and TNF-alpha secretion and mRNA expression by the trichothecene vomitoxin in the RAW 264.7 murine macrophage cell line. Food Chem Toxicol. 1998;36:409–419. doi: 10.1016/s0278-6915(97)00167-1. [DOI] [PubMed] [Google Scholar]
- Wu W, Flannery BM, Sugita-Konishi Y, Watanabe M, Zhang H, Pestka JJ. Comparison of murine anorectic responses to the 8-ketotrichothecenes 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, fusarenon X and nivalenol. Food Chem Toxicol. 2012;50:2056–2061. doi: 10.1016/j.fct.2012.03.055. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Islam Z, Pestka JJ. Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression in spleens of mice exposed to the trichothecene vomitoxin. Toxicol Sci. 2003a;72:130–142. doi: 10.1093/toxsci/kfg006. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Yan D, Pestka JJ. Differential cytokine mRNA expression in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): dose response and time course. Toxicol Appl Pharmacol. 1997;144:294–305. doi: 10.1006/taap.1997.8132. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Harkema JR, Yan D, Pestka JJ. Amplified proinflammatory cytokine expression and toxicity in mice coexposed to lipopolysaccharide and the trichothecene vomitoxin (deoxynivalenol) J Toxicol Environ Health A. 1999;57:115–136. doi: 10.1080/009841099157818. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Jia Q, Pestka JJ. Ribotoxic stress response to the trichothecene deoxynivalenol in the macrophage involves the SRC family kinase Hck. Toxicol Sci. 2005;85:916–926. doi: 10.1093/toxsci/kfi146. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Lau AS, Pestka JJ. Role of double-stranded RNA-activated protein kinase R (PKR) in deoxynivalenol-induced ribotoxic stress response. Toxicol Sci. 2003b;74:335–344. doi: 10.1093/toxsci/kfg148. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: A new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.