Preface
Natural killer cells survey host tissues for signs of infection, transformation, or stress, and true to their name, kill target cells that have become useless or are detrimental to the host. For decades, NK cells have been classified as a component of innate immunity. However, accumulating evidence in mouse and human suggests that, like T and B cells of adaptive immunity, NK cells are educated during development, possess antigen-specific receptors, undergo clonal expansion during infection, and generate long-lived memory cells. In this review, we will highlight the many stages that an NK cell progresses through during its remarkable lifetime, discussing similarities and differences with its close relative, the cytotoxic CD8+ T cell.
Introduction
A productive immune response against pathogen invasion consists of a concerted effort from many effector cell types of hematopoietic origin. Both innate and adaptive immune cells contribute to the recognition and removal of foreign pathogen materials as well as infected host cells. The best-known cell types responsible for the direct killing of infected cells are natural killer (NK) cells and cytotoxic CD8+ T cells (or CTLs). These professional killer cells are defined based on their cytolytic machinery, where killing of their targets is mediated predominantly via perforin and granzymes. NK cells and CD8+ T cells both originate from a common lymphoid progenitor and require cytokine signals through the common receptor gamma-chain (γc, also known as IL-2Rγ) family members for their survival and homeostasis. During infection, both NK cells and CD8+ T cells become activated through antigen-specific receptors and by inflammatory cytokines such as interleukin-12 (IL-12) and type I-interferons (IFNs), and produce large amounts of IFNγ1. Although they have been classified as innate immune cells, there is accumulating evidence in both mouse and human that NK cells share many attributes of T cells and B cells of adaptive immunity. For example, NK cells are educated and selected during their development, with their receptors exhibiting antigen specificity, undergo clonal expansion during infection and generate long-lived memory cells2, 3. Here, we discuss the many stages that an NK cell progresses through during its remarkable lifetime and compare it with its close relative, the cytotoxic CD8+ T cell.
Development of NK cells
NK cells have traditionally been classified as innate immune cells because of their ability to rapidly respond against target cells in the absence of prior sensitization, as well as because of the previous belief that they are cells with a short lifespan. In contrast, B and T cells are designated cells of the adaptive immune system because they generate long-lived progeny following activation of a naïve precursor and can “remember” previous encounter with antigen. Although considered as innate immune cells, NK cells comprise the third major lineage of lymphocytes, along with B and T cells4, 5. Unlike B and T cells, individual NK cells lack a unique antigen recognition receptor and do not use recombination-activating gene (RAG) enzymes for rearrangement of their receptor genes, even though transient expression of RAG and even incomplete V(D)J recombination have been observed in a low frequency of NK cells during their development6–10. NK cells are present in normal numbers in mice deficient in RAG1 or RAG211, 12.
Early studies suggested that NK cells, like B cells and myeloid-lineage cells, develop primarily in the bone marrow. Ablation or disruption of an intact bone marrow microenvironment abrogated the development and function of NK cells13,14. Unlike T cells, NK cells do not require the thymus for their development and, thus, exist at normal numbers in athymic nude mice15–17. However, a small population of CD127-expressing NK cells have been recently described to derive from the thymus through a GATA-binding protein 3 (GATA-3)-dependent pathway and arise independently from T cell precursors18. In humans, a population of CD34+ hematopoietic precursor cells were reported to develop into CD56hi NK cells in lymph nodes19. A recent examination of NK cell ontogeny suggested that NK cells can also develop in the liver20, perhaps explaining why phenotypically immature NK cells exist in the liver of adult mice21. It is not entirely clear whether these thymic-, lymph node- and liver-derived populations represent distinct NK cell lineages or merely consist of predominantly less mature peripheral cells that originated from the bone marrow. The bone marrow is certainly the site where NK cell development has been most well-characterized, and many of the cues that NK cells receive from bone marrow stromal and hematopoietic cells during their full functional maturation are discussed in this review.
In vitro studies conducted with mouse and human cells demonstrated that NK cells can be derived from early hematopoietic cells cultured with stromal elements, such as IL-7, IL-15, stem cell factor and FLT3 ligand (reviewed in Ref 4). Like with thymocytes, studies using the OP-9 cell culture system confirmed that functional NK cells could develop in vitro on stromal cells expressing specific Notch ligands, such as Delta- and Jagged-family members22–24. Interestingly, large percentages (as many as 50%) of NK1.1+ cells were recovered from early stage thymocytes that had been cultured with OP9-Jagged122–24. The cytokine IL-15 was shown to be crucial for the development of NK cells25, as mice deficient in either IL-15 or the IL-15 receptor alpha chain (IL-15Rα) have a dramatic reduction in NK cell numbers26, 27.
In vivo reconstitution studies have identified the common lymphoid progenitor (CLP) in mouse and human bone marrow as the pluripotent cell type that specifically gives rise to mature NK, B, and T cells, but not myeloid-lineage cells28. However, unlike the well-established stages of thymocyte development — CD4−CD8− double-negative (DN) stages1–4, CD4+ CD8+ double-positive (DP), and CD4+ or CD8+ single-positive (SP) stages — NK cell development and differentiation has been assigned several arbitrary stages based upon sequential acquisition of NK cell-specific markers and functional competence. The earliest NK cell progenitor is defined as a non-stromal bone marrow cell expressing CD122 (the shared β receptor chain for binding IL-2 and IL-15) alone4, 5, without any lineage-specific markers. In C57BL/6 mice, the earliest NK cell-defining markers are NK1.1 and NKG2D, followed by the inhibitory and activating Ly49 receptors21. The functional capabilities of NK cells, such as cytotoxicity and secretion of cytokines (IFNγ and tumour necrosis factor (TNF)), are acquired last in the current developmental schemes21. Although this model provides a framework for studying NK cell development, the precise rate-limiting cues that maturing NK cells receive at each stage to ultimately achieve functional competence, along with the genetic and epigenetic programming that occurs, requires further investigation.
Analogous processes in NK and T cell development
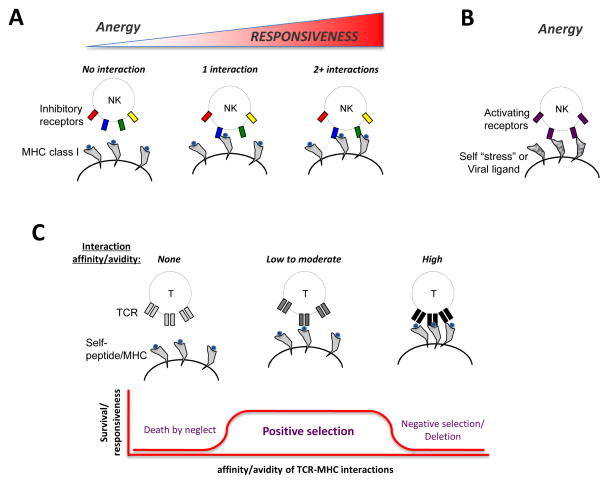
Like developing CD8+ T cells, which undergo processes such as positive and negative selection through interactions between their T cell receptor (TCR) and MHC class I molecules expressed on thymic epithelial cells and dendritic cells (DCs), developing NK cells are also “educated” or “selected” through engagement of their receptors with various MHC class I ligands (Figure 1). Immature human and mouse NK cells express inhibitory killer cell immunoglobulin-like receptors (KIR) and Ly49 receptors, respectively, during their development, and this is essential for establishing efficient “missing-self” recognition29, 30. Engagement of these receptors with cognate MHC class I results in the generation of functional NK cells in the periphery31–35, a process akin to positive selection of developing T cells (Figure 1). In addition, NK cells can vary in their responsiveness, or “tune” their capability to react, depending on the number of inhibitory receptors for autologous MHC class I expressed, as both mouse and human NK cell responsiveness increased quantitatively with each additional self MHC-specific inhibitory receptor36–38 (Figure 1). Inhibitory NK cell receptors contain immunoreceptor tyrosine-based inhibitory motifs (ITIM) in their cytoplasmic tail, which can recruit SH2 domain-containing protein tyrosine phosphatase 1 (SHP1) and SH2 domain-containing inositol-5-phosphatase (SHIP)39. Failure to engage inhibitory receptors during development, either because of lack in inhibitory receptor expression or inability to interact with MHC class I, results in peripheral NK cells that are hyporesponsive31–35.
Figure 1. Education of developing NK cells and thymocytes.
(a) In the bone marrow, developing NK cells interact with MHC class I on stromal and hematopoietic cells via inhibitory receptors, and the number of interactions determine the degree of responsiveness. (b) Developing NK cells that interact with self or viral ligands via activating receptors will become anergic or hyporesponsive. (c) In the thymus, developing T cells interact with self-peptide/MHC on epithelial and hematopoietic cells via T cell receptors, and affinity or avidity of these interactions determine survival and export to periphery.
Rather than “death by neglect”, a process thymocytes undergo if they cannot appropriately engage their TCRs with MHC class I (Figure 1), analogous NK cell populations that cannot ligate their inhibitory receptors do not die and are still exported to the periphery, albeit as anergic cells31–35 (Figure 1). Notably, NK cells that lack an inhibitory receptor for autologous MHC class I can respond normally in inflammatory settings33, 40, 41 and more robustly against viral infection and leukemias than their counterparts that express self-specific inhibitory receptors for MHC class I42–44. Recently, mature NK cells have been shown to undergo a “re-education” process whereby functional competence is reset following adoptive transfer into a different MHC class I environment45, 46. In these studies, anergic NK cells placed in a MHC class I-sufficient setting acquired the ability to produce effector cytokines, and functional NK cells lost their effector capabilities in an environment devoid of MHC class I45, 46, together suggesting that continuous engagement of inhibitory receptors with MHC class I is required to dictate NK cell responsiveness. Interestingly, in both mice and humans, activated and memory CD8+ T cells also express inhibitory receptors for MHC class I47, perhaps because of a need to tightly regulate these responsive T cell populations. A recent study, where MHC class I ligation of inhibitory KIR could be instantly induced by a photochemical approach, demonstrated that clustering and triggering of activating NK cell receptors immediately ceases upon inhibitory receptor ligation, and the NK cells collapse their actin cytoskeleton to strongly “retract” from the from the source of stimulation48.
Developing NK cells can also be influenced by signals received through activating receptors, which pair with immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor molecules that recruit kinases Syk and ζ-chain-associated protein kinase of 70 kDa (ZAP-70) to mediate signaling39. Analogous to negative selection of developing thymocytes, activating receptor ligation with cognate viral or self-ligands leads to anergy and a partial repertoire deletion in developing NK cells49–52 (Figure 1). Altogether, these tolerance mechanisms exist to ensure that mature NK cells do not attack healthy self-tissues.
Lineage specification in NK cells
Over the past decades, lineage-specifying transcriptional factors have been identified in B cells and T cells (including αβ T cells, γδ T cells, and NKT cells), providing important information about the genesis of these immune cell subsets. B cell development is driven by transcription factors, such as paired box gene/protein 5 (PAX5), E2A, and early B cell factor (EBF)53, whereas specific runt-related transcription factor (RUNX) and Notch family members promote T cell development54, 55 (Figure 2). Within the T cell lineage, CD4/CD8 lineage commitment in DP thymocytes occurs under the guidance of factors such as GATA-3 and ThPOK for CD4+ T cell development and runt-related transcription factor 1 (RUNX1) and RUNX3 for CD8+ T cell development54, 56. In addition, transcription factors that dictate the differentiation of mature naïve T cells into effector cells have also been characterized including T-bet for T helper 1 (TH1) cells, GATA-3 for TH2 cells, B cell lymphoma 6 (BCL6) for T follicular helper cells, forkhead box P3 (FOXP3) for regulatory T (TReg) cells, retinoic acid receptor-related orphan receptor-γt (RORγt) for TH17 cells, and T-bet and eomesodermin (EOMES) for effector CD8+ T cells57, 58.
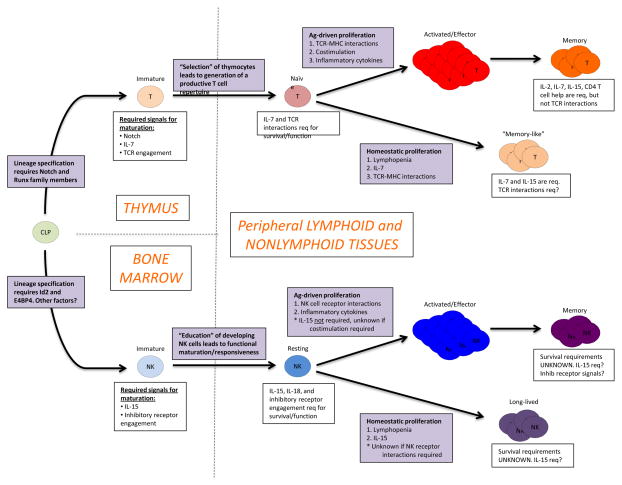
Figure 2. Factors that influence the development, homeostasis, and survival of NK cells and T cells.
A comparison of lineage specifying and external signals required for the maturation of NK cells and T cells from the common lymphoid progenitor (CLP) are shown. The CLP uses factors such as Notch and RUNX to become immature T cells, whereas ID2 and E4BP4 specify NK cell lineage precursors. Notch, IL-7, and TCR signals dictate “selection” of developing T cells in the thymus, while IL-15 and inhibitory receptor signals promote proliferation and “education” of developing NK cells in the bone marrow. Factors that influence the survival and function of T and NK cells during antigen-driven versus homeostatic proliferation are shown, including cytokine signals, cell surface receptor signals, and various other unknown signals.
Transcription factors PU.1, Ikaros, ETS1 and inhibitor of DNA binding 2 (ID2) have independently been shown to be important for NK cell lineage derivation from the CLP (Figure 2), as mice deficient in these factors have substantially reduced number of peripheral NK cells59, 60. It should be noted that these factors also influence development of other lymphocyte lineages in addition to NK cells59, 60. Similarly, NK cell development was impaired in mice deficient in TOX61, a shared DNA-binding factor also required for TReg, NKT, and CD4 T cells. A recent report using ID2–GFP reporter mice showed that the earliest known NK cell precursor highly expressed ID2 and the IL-7 receptor, prior to expression of common NK cell markers62. Other factors such as T-bet, EOMES, interferon-regulatory factor 1 (IRF1), and GATA-3 have been shown in gene-deficient mice, bone marrow chimeras, or conditional deletion mutants to be important for acquisition of full NK cell function63–66. An indirect role for IRF1 in NK cell development was discovered when IL-15 expression was observed to be defective in mice lacking this transcription factor67. Interestingly, genetic deletion of the transcription factor BCL11b in conditional knockout mice resulted in the reversion of maturing DN2 and DN3 thymocytes to killer cells that were described as NK cell-like68–71. Thus, perhaps in the absence of T cell-determining factors, the NK cell lineage becomes the default pathway of lymphocyte programming.
NK cell development requires E4BP4
Recently, the basic leucine zipper transcription factor E4BP4 (encoded by the Nfil3 gene) was proposed as a NK cell lineage-specifying factor (Figure 2). E4BP4-deficient mice showed a severe loss in mature NK cell numbers, whereas B and T cells were found at normal numbers72–74. Implicated in a wide range of biological roles including repression of viral promoter sequences and regulation of mammalian circadian clock75, E4BP4 was shown to function downstream of the IL-15 receptor in immature NK cells, as ex vivo addition of IL-15 did not rescue NK cell development in E4BP4-deficient progenitor cells72. Experimental evidence suggests that E4BP4 controls expression of GATA-3 and ID272, and ID2 is known to inhibit E protein transcription factors such as E2A, the effects of which are to inhibit B cell development while promoting the maturation of NK cells76. It should be noted that similar to the promiscuous role of T-bet in both CD4+ TH1 and CD8+ T cell differentiation, E4BP4 has been shown to influence the development of certain DC subsets, the regulation of TH2 responses, and class switching in B cells77–79. Because survival of memory CD8+ T cells is also dependent on IL-15 signals, further work is needed to determine whether E4BP4 has a role in the generation of T cell memory subsets. Additionally, the conditional deletion of E4BP4 in mature and activated NK cells will determine whether this factor continues to have a role in NK cell survival and function beyond their development. Nonetheless, there exists a clear role for transcription factors, such as E4BP4 and ID2, in the development of NK cells.
Peripheral NK cell localization and homeostasis
Trafficking of mature NK cells
Once they leave the bone marrow, the NK cells that have gained functional competence have the capability to respond robustly against infection and provide immunosurveillance against tumours. During basal homeostasis, mature peripheral NK cells reside in the blood, spleen, liver, lung, and various other organs80 (Figure 2). NK cells are found at low frequency in the lymph nodes, even though the majority of resting NK cells (>90%) in mouse spleen and blood express L-selectin (CD62L), which mediates naïve T cell recruitment into lymph nodes from circulation. In mice deficient for L-selectin or L-selectin ligands, NK cells were not properly recruited to lymph nodes and, thus, could not control tumour metastasis to secondary lymphoid organs in a mouse melanoma model81. The general homing of NK cells between circulation, lymphoid organs, and non-lymphoid organs has not been completely characterized, even though many chemokines (CC-chemokine ligand 3 (CCL3, also known as MIP1α), CCL4, CCL5, CCL19, CXC-chemokine ligand 12 (CXCL12), CXCL16 and CX3C-chemokine ligand 1 (CX3CL1)) have been implicated in NK cell localization, and expression of integrins (such as α2 integrin and α4 integrin), adhesion molecules (such as macrophage receptor 1 (MAC1) and DNAX accessory molecule 1 (DNAM1)) and chemokine receptors (such as CC-chemokine receptor 1 (CCR1), CCR5, CCR7, CX3C-chemokine receptor 1 (CXCR3), CXCR4, CXCR6, and CX3C-chemokine receptor 1 (CX3CR1)) are regulated during NK cell development and homeostasis in mice and humans82. Recently, the bioreactive lipid sphingosine-1-phosphate (S1P) has been implicated in NK cell retention and egress from sites such as the bone marrow and lymph nodes, with expression of the dedicated S1P receptor (S1P5) on NK cells observed to be regulated by T-bet83, 84.
At steady-state, NK cells are generally localized to the red pulp of the spleen and the sinusoidal regions of the liver85, 86; however, during viral infection they infiltrate the splenic white pulp and liver parenchyma near infected foci87–89. Thus, NK cell homeostasis leads to their presence in both lymphoid and non-lymphoid tissues, where NK cells are poised to rapidly respond against pathogen invasion. The precise mechanisms by which NK cells traffic between and within organs during homeostasis and following infection remain to be revealed.
Continuous maturation of peripheral NK cells
Recent evidence suggests that both NK cells and T cells continue to mature in the periphery following egress from the bone marrow and thymus, respectively. Using reporter mice to identify recent thymic emigrants, maturation of T cells was shown to continue post-thymically, with progressive acquisition of phenotypic changes and immune functions90. Similarly, continuous NK cell maturation in the periphery has been characterized by the expression of surface markers such as CD11b and killer cell lectin-like receptor subfamily G, member 1 (KLRG1), and loss of CD27 and TNF-related apoptosis-inducing ligand (TRAIL)91–94.
IL-18 has recently been described to “prime” NK cells either during their development or in the periphery (Figure 2), as resting splenic NK cells unable to receive IL-18 signals were found to be defective in cytotoxicity and cytokine secretion following ex vivo stimulation95–97. IL-18 has also been implicated in the basal homeostasis of γδ T cells, suggesting that IL-18 commonly influences other innate-like lymphocytes prior to activation98. During herpes simplex virus-1 (HSV-1) infection, mice deficient in IL-18 were susceptible (showing higher viral burden and decreased survival compared with control mice), having profound defects in the ability of NK cells to produce IFNγ and mediate cytotoxicity99.
Interestingly, because mature NK cell responsiveness can be continuously modulated based on constant interactions between inhibitory receptors with MHC class I in their environment45, 46 (Figure 2), the NK cell population exhibits a novel versatility less observed in T cells, where an individual NK cell can adapt to new environments to either gain or lose functional competence, thereby maintaining tolerance to self while responding against pathogens and tumours. Additional signals that mature peripheral NK cells require at steady-state for survival, basal turnover, and maintenance of function require further investigation.
IL-15 maintains NK cell survival
Cytokines of the common cytokine receptor γ chain (γC) family are critical for the development of lymphoid lineage cells (Figure 2). Mice deficient in the γC have defective maturation in the B, T, and NK cell compartments. IL-2 and IL-15, along with other inflammatory cytokines, promote clonal expansion of T cells (Figure 2), with elevated or prolonged levels of IL-2 leading to apoptosis of effector T cells25. IL-7 and IL-15 then protect memory precursor cells from cell death during the contraction phase of the T cell response100 (Figure 2). IL-15 also has an important role during NK cell development and continues to be crucial in the homeostasis and survival of peripheral NK cells25 (Figure 2). In adoptive transfer experiments, the half-life of mature NK cells was measured to be roughly one week; however, in the absence of IL-15, nearly all transferred NK cells rapidly disappear within 48 hours101–104. Memory CD8+ T cells, which have been likened to NK cells, also depend on IL-15 for their homeostasis and survival25. DCs and macrophages mediate trans-presentation of IL-15105–107, as Cre-induced specific deletion of the IL-15Rα in these professional antigen-presenting cells resulted in the loss of both NK cells and memory CD8+ T cells, although T cells were more affected than NK cells108. Transgenic expression of IL-15R specifically in DCs or treatment of mice with soluble IL-15 and IL-15Rα complexes also promoted expansion of NK cells, resulting in elevated numbers and function109, 110; however, transgenic overexpression of IL-15 led to fatal leukemia111. IL-15 signalling in NK cells acts by increasing the expression and activity of BCL2 and its family members104, 112, 113, and at the same time suppressing the transcription factor forkhead box O3A (FOXO3A) and pro-apoptotic factor BCL-2-interacting mediator of cell death (BIM)113.
Given the known influence of IL-15 on immune cells, this cytokine has become an immunotherapeutic target. For example, administration or overexpression of IL-15 has been shown to protect mice against a variety of infections where cytotoxic NK and T cells were boosted in effector function114, 115. Conversely, blockade of IL-15 using mutant IL-15-Fc fusion proteins or a soluble IL-15α receptor chain has shown efficacy in treatment of arthritis and allograft transplant survival in mice116–118.
NK cells “fill the space” during lymphopenia
Immunocompetence is defined as having a sufficient number of functional immune cells ready to respond against pathogen invasion. Thus, cells of the immune system will undergo homeostatic, or space-driven, proliferation during times of lymphopenia to restore overall cell numbers (Figure 2). Like B and T cells, NK cells can rapidly expand in number when placed into a lymphopenic environment. Adoptive transfer of mature splenic NK cells in RAG and γC doubly-deficient mice (which lack B, T cells, and NK cells) or in mice treated with sublethal doses of radiation, results in rapid division, as measured by CFSE-labelling or BrdU-incorporation101, 103, 104, 119. In these studies, the kinetics of NK cell homeostatic proliferation mimicked CD8+ T cells and was more rapid than that of CD4+ T cell and B cell expansion119. Like CD8+ T cells, NK cells become phenotypically activated during homeostatic proliferation, upregulating markers, such as CD11a, CD44, CD122, and Ly6C120–122. In addition, both homeostatically expanding NK cells and CD8+ T cells can rapidly degranulate and secrete IFNγ during antigen receptor triggering because of increased transcript expression, as demonstrated in lymphocytes from Yeti mice, where expression of yellow fluorescent protein (YFP) is driven by the IFNγ promoter119. This heightened ability of lymphocytes to respond may represent an evolved mechanism that ensures the host can effectively defend itself during lymphopenia, when it is most susceptible to pathogens.
Homeostatic proliferation generates long-lived NK cells
Mature peripheral NK cells were previously considered terminally differentiated effector cells incapable of self-renewal. However, prior studies evaluating adoptive transfer of NK cells into lymphopenic hosts did not investigate the long-term consequences of homeostatic proliferation. In a recent study, homeostatic proliferation of NK cells was demonstrated to result in an unexpected longevity of the transferred mature NK cells119, tracked using a congenic marker (Figure 2). The homeostasis-driven NK cells were found to reside in both lymphoid and non-lymphoid organs for greater than 6 months and were able to self-renew and slowly turn over at steady-state similar to memory T cells119. Furthermore, the long-lived NK cells retained their functionality many months after initial transfer and responded robustly to viral infection119. However, unlike long-lived CD8+ and CD4+ T cells that have undergone homeostatic proliferation and retain a “memory-like” phenotype and function (Figure 2), the expanded NK cells reverted to a quiescent phenotype and function equivalent to resting NK cells, and responded with comparable kinetics against viral challenge119. Homeostatically driven CD8+ T cells were shown to require CD4+ T cell help for protective capability123 and deferred to “true” antigen-experienced memory CD8+ T cells when mice were challenged with pathogens124. Similar studies remain to be done to investigate the requirements for robust homeostatic proliferation of NK cells, and to compare the function of space- versus antigen-driven NK cells. Nonetheless, the ability of mature NK cells to self-renew and possibly persist in the host for months or years will be of clinical importance for reconstitution of immune compartments during viral infections such as HIV, or during NK cell adoptive immunotherapy for the treatment of certain cancers (Box 1).
BOX 1. NK cells in the clinic: promising therapeutic potential and outstanding questions.
Reconstitution of the host immune system with hematopoietic stem cell transplants after radiation or chemotherapy of cancer patients, or in humans with natural immunodeficiencies (such as SCID patients), can result in homeostatic proliferation of NK cells and other hematopoietic cell types. In the past decade, the adoptive transfer of in vitro-cultured and activated human NK cells has been applied towards the treatment of certain cancers174. Although adoptive NK cell therapy shows promise in the clinic, many questions arise in light of the novel findings on the longevity of NK cells following homeostatic proliferation. For example, are there detrimental side effects, such as graft-versus-host disease, or other unforeseen dangers to healthy host tissues associated with having a long-lived population of self-renewing NK cells poised to kill cellular targets? Conversely, are there unexpected benefits associated with adoptive NK cell immunotherapy beyond the destruction of cancers, such as leukemias? Thought to be a potent but transient therapy in humans, adoptive transfer of NK cells may allow these cells to persist for months to years in the patient, mediating continuous surveillance against recurrence of cancer. Furthermore, in hematopoietic stem cell transplant patients, co-transfer of long-lived NK cells might protect against opportunistic pathogens (such as human cytomegalovirus reactivation) over the several months it takes for B and T cells to fully reconstitute the immune system. Understanding the nature of NK cells undergoing homeostatic proliferation using mouse models will provide insights crucial to the implementation of NK cell-based treatments in the clinic.
Dynamic response of NK cells during viral infection
During viral infection, homeostasis is perturbed, and mature NK cells become activated, proliferate robustly, and contribute to both innate and adaptive immunity125. NK cells, together with CTLs, directly target infected cells during viral invasion, and deficiency of NK cells in mice and humans results in susceptibility to many viral infections and severe clinical outcomes1, 126. Like CD8+ T cell responses against various pathogens, a clonal-like expansion has been described in the Ly49H-bearing subset of NK cells during mouse cytomegalovirus (MCMV) infection127 (Figure 3). In certain mouse strains, such as C57BL/6, Ly49H+ NK cells can undergo 3 to 10-fold expansion in absolute numbers (measured in spleen and liver) over the course of 6–7 days after viral infection127, 128. Although this expansion is not as prolific as the several thousand-fold expansion measured in antigen-specific T cell responses129, adoptive transfer of relatively small numbers of wild-type NK cells into Ly49H-deficient hosts demonstrated that during infection, Ly49H+ NK cells are fully capable of 100- and 1000-fold expansion in the spleen and liver, respectively128.
Figure 3. Specific and nonspecific response of NK cells and CD8+ T cells during infection.
(a) During viral infection, NK cells and CD8+ T cells mount “specific” responses upon antigen receptor triggering. TCR-MHC interactions, costimulation, and pro-inflammatory cytokines such as interleukin-12 (IL-12) and type I interferons (IFNs) are the three signals thought to promote the activation and clonal expansion of naïve CD8+ T cells. Similarly, resting NK cells receive signals via activating receptors and inflammatory cytokine receptors; it is unclear whether costimulatory molecules play an important role in NK cell activation and expansion. The short and long term functional outcomes are shown. (b) IL-12 and type I IFNs can directly act on memory CD8+ T cells and resting NK cells in the absence of antigen receptor triggering, leading to “nonspecific” or “bystander” responses. The short and long term functional outcomes are shown.
Interestingly, a similar clonal-like expansion has been observed in human NK cell responses against viral infection. The natural killer group 2, member C (NKG2C)-bearing NK cell subset is highly enriched within the total NK cell population in humans that are seropositive for human cytomegalovirus (HCMV)130, 131. In these studies, HCMV-seronegative donors typically contained less than 1% NKG2C+ NK cells in peripheral blood, whereas seropositive donors ranged from less than 5% to as many as 50–60% NKG2C+ NK cells within the total NK cell population. A challenge arises in the analysis and interpretation of these data sets because of the unknown time point of HCMV infection or reactivation, explaining the large spectrum of NKG2C+ NK cell percentages in different individuals. One recent study documented a B and T cell-deficient newborn that presented with complications stemming from HCMV infection, and greater than 80% of this child’s NK cell population expressed NKG2C at a time point where viral loads were increasing, suggesting a prolific expansion of this subset132. Longitudinal studies have demonstrated a clonal-like expansion of human NK cell subsets during HCMV infection in immunosuppressed solid-organ transplant recipients who have reactivated or become acutely infected with HCMV133. In these patients, NKG2C+ NK cells undergo a substantial expansion within the first week after virus detection and the elevated frequency of these NKG2C+ NK cells persists after control of the virus133.
Interestingly, a hantavirus outbreak in northern Sweden in 2007 allowed clinicians to prospectively investigate the immune response to infection in a setting where initial viral infection was well-documented134. This study demonstrated that human NKG2C+ NK cells rapidly proliferated following hantavirus infection134, with a kinetics and magnitude that strikingly mimics the clonal-like expansion of mouse Ly49H+ NK cells against MCMV infection128. Of note, this expansion of NKG2C+ NK cells in the hantavirus-infected individuals only occurred in HCMV-seropositive but not HCMV-seronegative patients, suggesting that these NKG2C+ NK cells may have been primed by prior recognition of HCMV. Together, these mouse and human studies demonstrate that NK cell clones have a proliferative capacities not appreciated previously.
Signals for NK and T cell activation
Some of the cues that promote NK cell activation and proliferation during viral infection have been characterized, whereas others remain to be investigated. Triggering of TCR on T cells by viral peptides bound to MHC molecules constitutes “Signal 1” in T cell activation135 and similarly, in the case of MCMV infection recognition of cognate antigen through the Ly49H activating NK cell receptor promotes NK cell activation and clonal proliferation during viral infection (Figure 3a). During MCMV infection in C57BL/6 mice, only virus-specific Ly49H+ NK cells kill infected cells bearing the viral m157 glycoprotein and expand significantly in overall numbers to provide protection against virus127, 128, 136. The NK cell subsets that don’t express Ly49H can respond in a nonspecific “bystander” fashion, but do not undergo a clonal-like expansion or afford any protection against MCMV127, 128, 136 (Figure 3b).
T cells require co-stimulation through CD28 as “Signal 2” for activation of effector function135, but it is not clear whether NK cells require this signal for full effector function in vivo during virus infection (Figure 3a). Early in vitro studies suggested that although resting NK cells expressed CD28 at much lower levels than naïve T cells, CD28 triggering on NK cells was required for optimal cytokine secretion and proliferation, but not killing137. These findings can now be revisited in vivo by measuring virus-specific responses in NK cells from mice lacking CD28.
In certain mouse models of infection, T cells have been shown to require “Signal 3” in the form of pro-inflammatory cytokines IL-12 and type I-IFNs for optimal activation and proliferation129. The early nonspecific NK cell response against MCMV, characterized by the secretion IFNγ and TNF, but not IL-2, is also dependent primarily on IL-12 and type I IFNs produced by the conventional DC (cDC) 138–140 (Figure 3b). In addition to cDCs, plasmacytoid DC (pDC) also contribute to NK cell-mediated protection via secretion of large amounts of IFNα and IFNβ following triggering of Toll-like receptors (TLRs) and intracellular sensors of viral nucleic acids (Figure 3), as specific pDC ablation led to decreased NK cell activity and increased MCMV titers141. Interestingly, the crucial IL-15 signals required for NK cell development, basal homeostasis, and survival were not essential for NK cell activation, proliferation, or function during acute MCMV infection, as the anti-viral NK cell response was primarily driven by IL-12 even in mice lacking the γC142. Conversely, IL-12 and type I IFNs are not thought to play a role during NK cell development, as both cytokine and cytokine receptor gene-deficient mice have normal NK cell numbers and phenotypes143–145.
In addition to directing the nonspecific NK cell response during MCMV infection (Figure 3b), there is evidence that pro-inflammatory cytokines are crucial for the proliferation and effector function of antigen-specific Ly49H+ NK cells, similar to “Signal 3” for T cells139 (Sun and Lanier, unpublished observations). Interestingly, like resting NK cells, memory CD8+ T cells have been described to possess the “innate-like” ability to produce IFNγ and proliferate following exposure to proinflammatory cytokines in the absence of TCR signals and costimulation146, 147 (Figure 3b). Altogether, these data suggest that parallel mechanisms of signal integration through activating receptors, costimulatory receptors, and cytokine receptors facilitate a productive effector response in both NK cells and T cells.
NK cell memory
In addition to clonal expansion, much evidence in recent years suggests that NK cells also possess features of adaptive immunity such as longevity and immune memory, manifested as the ability to mount recall responses (BOX 2). Following the prolific expansion phase of Ly49H+ NK cells during MCMV infection, the effector NK cells undergo apoptosis 1–2 weeks later127, 128, 148 resembling the contraction phase observed when effector T cells undergo activation-induced cell death149. Interestingly, the contraction phase of the NK cell response occurs in a manner resembling the contraction phase of CD4+ T cell response, during which a gradual but continuous decline in cell numbers is observed150.
BOX 2. The evidence for NK cell memory.
Immunological memory is a classical hallmark that distinguishes adaptive immune B and T cells from all other cells of the hematopoietic lineage. However, evidence for immune memory exists in invertebrates, including crustaceans, flies, beetles, and mosquitoes and more primitive species, such as tunicates, sea urchins, and sea sponges, which lack lymphocytes possessing rearranged antigen receptors175. Early transplantation studies involving the NK cell-mediated rejection of parental bone marrow cells transferred into F1 progeny recipients (“hybrid resistance”) first suggested that immune memory could reside also in NK cells. Interestingly, when the F1 offspring had been previously primed with parental bone marrow cells, they rejected a second graft from the same parent more efficiently176, implicating NK cell-mediated “recall” responses in graft rejection. Previous studies had also suggested that NK cell memory may be responsible for enhanced cytokine secretion following exposure to inflammatory cytokines, or more potent tumour-specific responses following stimulation177–179.
More recent studies demonstrated that NK cells mediate contact hypersensitivity responses to chemical haptens in RAG-deficient mice (lacking B and T cells), but not in mice deficient for both RAG and γC (lacking B, T, and NK cells)180. Secondary responses were only elicited by the hapten to which mice had been originally exposed and not by a different hapten180. The contact hypersensitivity observed in the RAG-deficient mice was mediated by a CXC-chemokine receptor 6 (CXCR6)-expressing NK cell population residing in the liver181. During mouse cytomegalovirus infection, adoptively transferred NK cells bearing the Ly49H receptor proliferate 100–1000-fold in lymphoid and non-lymphoid tissues, resulting in a long-lived pool of memory NK cells capable of more potent effector function, self-renewal, recall response, and greater protection against viral challenge128, 161. In a subsequent study, exposure to the pro-inflammatory cytokines interleukin-12 (IL-12) and IL-18 in vitro also led to generation of memory NK cells capable of more robust interferon γ production following activating receptor stimulation compared with resting NK cells182. In humans, acute infection with human cytomegalovirus (HCMV) results in the preferential expansion of NK cells expressing the activating NKG2C receptor133. Similarly, acute hantavirus infection led to the prolific expansion and long-term persistence of NKG2C-expressing NK cells134. In this study, greater numbers of NKG2C+ NK cells were observed at the peak of expansion in HCMV-seropositive patients (but not HCMV-seronegative patients), suggesting that a more robust response was measured in this HCMV-responsive NK cell subset134.
IL-7 and IL-15 dictate the homeostasis of effector T cells during the contraction phase, but the cytokines that influence the resolution of the effector NK cell response are not well understood, although it is likely that they also involve IL-15 (Figure 2). The survival of effector T cells during the contraction phase and transition to memory cells is dictated by pro- and anti-apoptotic factors of the BCL2 family, such as BCL2 and BIM151–155, respectively, and evidence suggests that these factors regulate the contraction phase of the NK cell response against MCMV infection as well (Bezman, Sun, and Lanier, unpublished data). Although early IL-21 signalling has been described to be important for functional maturation and activation of NK cells156–158, a regulatory role for IL-21 may exist in NK cells during proliferation, restricting the NK cell response while promoting T cell responses159. This dichotomous effect of IL-21 on NK cells is not unprecedented, as another γC family member, IL-2, can act to promote T cell proliferation, as well as induce apoptosis depending on the phase and context of the T cell response 25, 160.
Following the contraction of effector NK cells, long-lived memory NK cells can be detected for several months in adoptive transfer studies128. Memory NK cells were found to reside in both lymphoid and non-lymphoid organs and could mediate rapid and potent effector functions when stimulated ex vivo128. The mechanisms behind the trafficking of effector NK cells to peripheral organs and the precise signals required to maintain long-lived NK cells at those sites are currently unknown. Memory Ly49H+ NK cells were shown to be self-renewing and could undergo secondary and tertiary expansion following each round of expansion and contraction, expressing higher levels of Ly49H receptor (measured by mean fluorescence intensity) after each contraction phase161. Interestingly, following acute HCMV and hantavirus infection, NKG2C+ NK cells become NKG2Chi during clonal proliferation and express the CD57 marker on long-lived cells after the contraction phase, providing a possible memory marker for human NK cells133, 134. The higher expression of the virus-specific Ly49H and CD94-NKG2C receptors in mouse and human NK cells, respectively, following infection suggests a form of “avidity maturation” in the NK cell receptors that have previously engaged their cognate ligands, similar to what is observed in CD8+ T cells during infection162.
Reactivity of naïve, effector, and memory NK and CD8+ T cells
The spontaneity of killing and speed of effector function mediated by NK cells led Eva Klein to first coin them “natural killers” in 1975163, and frequent comparisons are drawn to activated CTL2, 125, but not resting CD8+ T cells. However, recent studies using the MCMV model showed that NK cells can also exist as both resting and activated/effector cells, distinguished by their ability to degranulate and secrete effector cytokines, such as IFNγ127, 128, 136. CD8+ T cells can exist in at least 3 states of reactivity depending on their ability to kill and secrete effector cytokines during ex vivo TCR triggering: low for naïve CD8+ T cells, high for activated/effector CD8+ T cells, and intermediate for memory CD8+ T cells (Figure 4). We believe a similar model can be applied to NK cell responses against viral infection; however, a naïve NK cell is in a more responsive state than a naive CD8+ T cell (Figure 4). Naïve resting NK cells have the capability to respond to activating receptor triggering and their IFNγ transcript levels are similar to those of memory CD8+ T cells, as detected using IFNγ-YFP reporter mice164. The equivalent IFNγ transcript levels between resting NK cells and memory CD8+ T cells translate to functional similarities between these two cell types, as both can secrete IFNγ following stimulation with pro-inflammatory cytokines alone127, 146, 147. Memory NK cells have a greater responsiveness than resting NK cells, and ligation of activating receptors leads to greater cytokine production and degranulation (measured by lysosome-associated membrane protein 1 (LAMP-1) expression) on a per cell basis128. Thus, perhaps resting NK cells possess a comparable state of reactivity to memory CD8+ T cells, and likewise memory NK cell reactivity is similar to that of activated/effector CD8+ T cells (Figure 4).
Figure 4. Comparing the reactivity of NK cells and CD8+ T cells during differentiation.
The state of reactivity of resting NK cells and naïve CD8+ T cells, activated/effector and memory NK and CD8+ T cells is marked by the expression of activation and differentiation markers (KLRG1, CD62L, and Ly6C) and their relative ability to mediate cytotoxicity and secrete effector cytokines is indicated. (TCM = Central memory T cell, TEM = Effector memory T cell, lo = low, int = intermediate, hi = high)
The overall memory CD8+ T cell compartment has been described to contain at least two subsets, central and effector memory T cell populations165, 166, whereas memory Ly49H+ NK cells appear to be a homogeneous population closely resembling the effector memory CD8+ T cell subset both in phenotype (high KLRG1 and low CD62L expression) and function (high IFNγ production upon stimulation)128 (Figure 4). In this comparison between responding NK and CD8+ T cells, the lymphocyte with the greatest state of reactivity is the activated/effector NK cell (Figure 4), which secretes cytokines spontaneously127, 128, 136 and does not require ex vivo ligation of activating receptors for its effector function. Although NK and T cells differ fundamentally in the generation of antigen recognition receptors, their similar nature and response suggests an evolutionary ancestry between these two cytotoxic cell lineages that have been distinctly categorized as innate and adaptive167.
Concluding remarks
The precise mechanisms of NK cell development and homeostasis are actively being investigated. The discovery of NK cell memory generates many new questions, some of which also remain to be answered in the study of immune memory in T cells. Moreover, questions that T cell biologists have raised (and answered, in many circumstances) in the previous decades can now be applied towards understanding how NK cells can also become long-lived cells capable of recall responses. For instance, what molecular and cellular interactions are important for the optimal generation of NK cell homeostasis and memory? Crucial cues from DCs and CD4+ T cells are required for proper generation and maintenance of memory CD8+ T cells129, 168. Does the viral glycoprotein m157 have to be present on DCs in order to prime Ly49H+ NK cells, or are other cell types sufficient to drive NK cell activation, proliferation and generation of memory? Do helper CD4+ T cells play a direct or indirect role in memory NK cell generation and maintenance? What environment signals are responsible for proper NK homing to sites of infection or inflammation? CD8+ T cells are known to require only a brief period of stimulation (2–24 hours) to commit to at least 7–10 cell divisions, which can occur in the absence of further TCR ligation169–171. How does the duration of antigen encounter influence the programmed expansion and memory generation in NK cells? CD8+ T cell activation, differentiation, and memory generation have been shown to be dependent on factors such as T-bet, EOMES, BCL6, ID2, and B lymphocyte-induced maturation protein 1 (BLIMP1), among others129, 165. Do similar transcription factors control NK cell differentiation? Finally, can we track NK cell differentiation and fate of memory cells using tracing methods described for T cells (e.g. barcoding172, 173)? As these and other questions are clarified, additional complexity will undoubtedly be exposed in the regulation of NK cell development, maturation, homeostasis, activation, function, and memory. The goal of immunization is to prevent infectious disease, and we present NK cells as a novel immune compartment to be targeted in vaccine strategies.
Online summary.
NK cell development in the bone marrow consists of an “education” process involving inhibitory receptor engagement of MHC class I, in similar manner to thymic “selection” dictated by TCR–MHC interactions.
NK cell lineage specification depends upon intrinsic programming by transcription factors such as ID2 and E4BP4.
After exiting the bone marrow, NK cells undergo a progressive phenotypic and functional maturation. Peripheral NK cell homeostasis and survival require IL-15.
In a lymphopenic environment, NK cells rapidly undergo homeostatic proliferation in order to fill the NK cell ‘niche’. This expansion results in the generation of long-lived NK cells capable of robust effector responses.
During certain viral infections in mice and humans, NK cells are activated, undergo prolific clonal expansion, and become long-lived memory cells capable of increased responsiveness; this response closely resembles the CD8+ T cell response against pathogens.
Acknowledgments
The authors thank Steve Jameson, Jeroen van Heijst, and Georg Gasteiger for helpful discussions and critical reading of the manuscript. J.C.S. is supported by the Searle Scholars Program and NIH grant AI085034. L.L.L. is an American Cancer Society Professor and supported by NIH grants AI068129, AI066897, and CA095137.
GLOSSARY
- Perforin and Granzymes
these proteins comprise the cytolytic molecules secreted by NK cells and cytotoxic CD8+ T cells. Perforin subunits assemble into a pore-forming structure in the membrane of the target cell, allowing granzymes (a family of proteolytic enzymes) to activate caspases and induce apoptosis in the target cell
- Clonal expansion
The process whereby individual B and T cells expressing a unique antigen-specific receptor will encounter cognate ligand and rapidly proliferate to give rise to a large number of progeny that express the same antigen-specific receptor (i.e. clones)
- NK1.1
Also known as NKRP1C, this activating receptor associates with the adaptor molecule FCεRIγ and is expressed on all NK and NKT cells in C57BL/6 mice. Although cross-linking of the receptor using the anti-NK1.1 mAb (clone PK136) induces NK cell-mediated cytotoxicity and effector cytokine secretion, the in vivo function of NK1.1 and nature of its ligand(s) remain unknown
- NKG2D
An activating receptor constitutively expressed on NK cells and activated CD8+ T cells that recognizes a family of induced host ligands on the surface of “stressed” cells. DNA instability and damage arising from radiation, chemicals, infection, or transformation can lead to cellular “stress” and the upregulation of NKG2D ligands (H60, MULT1, and RAE-1 family in mice, and MIC and ULBP families in humans)
- Ly49 receptors
A family of NK cell receptors in mice that contain inhibitory members that bind to MHC class I and inhibit NK cell responses and activating members that predominantly associates with the adaptor molecule DAP12 to initiate effector functions upon encounter of target cells expressing cognate ligands. The ligands for the majority of activating Ly49 receptors are currently unknown; however, some are likely virally encoded components
- “Missing-self”
A hypothesis proposed by Klas Karre suggesting that NK cells preferentially recognize and kill host cells that have lost expression of self-MHC class I molecules, or in other words, cells that are “missing self”. Uninhibited by ITIM-containing receptors for MHC class I, NK cells can target virally infected or transformed cells that downregulate MHC class I to evade detection by CD8+ T cells
- Positive selection
a process during T cell development whereby CD4+ CD8+ (double-positive) thymocytes are selected to survive and mature into single-positive CD4+ or CD8+ T cells based on an appropriate degree (low to intermediate) of stimulation through the TCR
Biographies
Joseph C. SunNIHMS681815-supplement.pdf
Joseph C. Sun received his Ph.D. in Immunology in the laboratory of Michael Bevan at the University of Washington, Seattle, WA, USA. He then trained as a postdoctoral fellow at the University of California at San Francisco, San Francisco, CA, USA, under the guidance of Lewis Lanier. He is currently a Searle Scholar and Assistant Professor in the Immunology Program at Memorial Sloan-Kettering Cancer Center, New York, NY, USA, and his research group focuses on the general biology of Natural Killer cells.
Lewis L. LanierNIHMS681815-supplement.pdf
Lewis L. Lanier received his Ph.D. in Immunology in the laboratory of Geoffrey Haughton at the University of North Carolina, Chapel Hill, NC, USA. He is currently an American Cancer Society Professor and Chairman of the Department of Microbiology and Immunology at the University of California San Francisco, San Francisco, CA, USA. His research group focuses on Natural Killer cell receptors and their roles in immunity to tumours and pathogens and their potential participation in autoimmune disorders.
References
- 1.Sun JC, Lanier LL. In: The Immune Response to Infection. Kaufmann S, Rouse B, Sacks D, editors. ASM Press; Washington, D.C: 2010. pp. 197–207. [Google Scholar]
- 2.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL. NK cells and immune “memory”. J Immunol. 2011;186:1891–7. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–86. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–29. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 6.Borghesi L, et al. B lineage-specific regulation of V(D)J recombinase activity is established in common lymphoid progenitors. J Exp Med. 2004;199:491–502. doi: 10.1084/jem.20031800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–30. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 8.Kouro T, Kumar V, Kincade PW. Relationships between early B- and NK-lineage lymphocyte precursors in bone marrow. Blood. 2002;100:3672–80. doi: 10.1182/blood-2002-02-0653. [DOI] [PubMed] [Google Scholar]
- 9.Pilbeam K, et al. The ontogeny and fate of NK cells marked by permanent DNA rearrangements. J Immunol. 2008;180:1432–41. doi: 10.4049/jimmunol.180.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokota T, et al. Unique properties of fetal lymphoid progenitors identified according to RAG1 gene expression. Immunity. 2003;19:365–75. doi: 10.1016/s1074-7613(03)00231-0. [DOI] [PubMed] [Google Scholar]
- 11.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 12.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V, Ben-Ezra J, Bennett M, Sonnenfeld G. Natural killer cells in mice treated with 89strontium: normal target-binding cell numbers but inability to kill even after interferon administration. J Immunol. 1979;123:1832–8. [PubMed] [Google Scholar]
- 14.Seaman WE, et al. beta-Estradiol reduces natural killer cells in mice. J Immunol. 1978;121:2193–8. [PubMed] [Google Scholar]
- 15.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16:230–9. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 16.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–21. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 17.Su HC, Ishikawa R, Biron CA. Transforming growth factor-beta expression and natural killer cell responses during virus infection of normal, nude, and SCID mice. J Immunol. 1993;151:4874–90. [PubMed] [Google Scholar]
- 18.Vosshenrich CA, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–24. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 19.Freud AG, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Andrews DM, Smyth MJ. A potential role for RAG-1 in NK cell development revealed by analysis of NK cells during ontogeny. Immunol Cell Biol. 2010;88:107–16. doi: 10.1038/icb.2009.94. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–8. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 22.Lehar SM, Dooley J, Farr AG, Bevan MJ. Notch ligands Delta 1 and Jagged1 transmit distinct signals to T-cell precursors. Blood. 2005;105:1440–7. doi: 10.1182/blood-2004-08-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–56. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 24.Williams NS, et al. Differentiation of NK1.1+, Ly49+ NK cells from flt3+ multipotent marrow progenitor cells. J Immunol. 1999;163:2648–56. [PubMed] [Google Scholar]
- 25.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 28.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 29.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 30.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 31.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Chalifour A, et al. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30:337–47. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–23. doi: 10.1182/blood-2004-08-3156. References 33–35 describe how inhibitory receptor engagement of MHC class I during development endows NK cells with functional competence in the periphery, a process known as NK cell “education” or “licensing”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson S, et al. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med. 2005;201:1145–55. doi: 10.1084/jem.20050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 36.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–41. doi: 10.1182/blood-2008-05-156836. References 36–38 describe how the number of inhibitory receptors for autologous MHC class I determines the degree of NK cell responsiveness in mouse and human, with the highest range of functional competence correlating with greatest number of self-MHC specific inhibitory receptors. [DOI] [PubMed] [Google Scholar]
- 37.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182:4572–80. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu J, et al. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179:5977–89. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 39.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun JC, Lanier LL. Cutting edge: viral infection breaks NK cell tolerance to “missing self”. J Immunol. 2008;181:7453–7. doi: 10.4049/jimmunol.181.11.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–54. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 42.Hsu KC, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–84. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller JS, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–61. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orr M, Murphy W, Lanier LL. “Unlicensed” Natural Killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010 doi: 10.1038/ni.1849. in press. Reference 44 demonstrates that “unlicensed” NK cells (or NK cells lacking an inhibitory receptor against autologous MHC class I) proliferate more robustly and mediate greater protection than “licensed” NK cells during viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207:2073–9. doi: 10.1084/jem.20100986. References 45 and 46 demonstrate that NK cells can re-adjust their responsiveness and functionality when adoptively transferred into a MHC class I-different mouse, suggesting that NK cells require constant interactions with MHC class I to maintain functional competence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207:2065–72. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 48.Abeyweera TP, Merino E, Huse M. Inhibitory signaling blocks activating receptor clustering and induces cytoskeletal retraction in natural killer cells. J Cell Biol. 2011;192:675–90. doi: 10.1083/jcb.201009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6:938–45. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oppenheim DE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–37. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 51.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205:1819–28. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tripathy SK, et al. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–41. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- 54.Collins A, Littman DR, Taniuchi I. RUNX proteins in transcription factor networks that regulate T-cell lineage choice. Nat Rev Immunol. 2009;9:106–15. doi: 10.1038/nri2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–74. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 56.Bosselut R. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol. 2004;4:529–40. doi: 10.1038/nri1392. [DOI] [PubMed] [Google Scholar]
- 57.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–11. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 58.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hesslein DG, Lanier LL. Transcriptional control of natural killer cell development and function. Adv Immunol. 2011;109:45–85. doi: 10.1016/B978-0-12-387664-5.00002-9. [DOI] [PubMed] [Google Scholar]
- 60.Ramirez K, Kee BL. Multiple hats for natural killers. Curr Opin Immunol. 2010;22:193–8. doi: 10.1016/j.coi.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat Immunol. 2010;11:945–52. doi: 10.1038/ni.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carotta S, Pang SH, Nutt SL, Belz GT. Identification of the earliest NK-cell precursor in the mouse BM. Blood. 2011;117:5449–52. doi: 10.1182/blood-2010-11-318956. [DOI] [PubMed] [Google Scholar]
- 63.Duncan GS, Mittrucker HW, Kagi D, Matsuyama T, Mak TW. The transcription factor interferon regulatory factor-1 is essential for natural killer cell function in vivo. J Exp Med. 1996;184:2043–8. doi: 10.1084/jem.184.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 65.Samson SI, et al. GATA-3 promotes maturation, IFN-gamma production, and liver-specific homing of NK cells. Immunity. 2003;19:701–11. doi: 10.1016/s1074-7613(03)00294-2. [DOI] [PubMed] [Google Scholar]
- 66.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 67.Ogasawara K, et al. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 1998;391:700–3. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 68.Ikawa T, et al. An essential developmental checkpoint for production of the T cell lineage. Science. 2010;329:93–6. doi: 10.1126/science.1188995. [DOI] [PubMed] [Google Scholar]
- 69.Kastner P, et al. Bcl11b represses a mature T-cell gene expression program in immature CD4(+)CD8(+) thymocytes. Eur J Immunol. 2010;40:2143–54. doi: 10.1002/eji.200940258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L, Leid M, Rothenberg EV. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science. 2010;329:89–93. doi: 10.1126/science.1188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li P, et al. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science. 2010;329:85–9. doi: 10.1126/science.1188063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gascoyne DM, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–24. doi: 10.1038/ni.1787. References 72–74 describe a specific NK cell deficiency in mice lacking the transcription factor E4BP4 (also known as Nfil3) [DOI] [PubMed] [Google Scholar]
- 73.Kamizono S, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med. 2009;206:2977–86. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kashiwada M, et al. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proc Natl Acad Sci U S A. 2010;107:821–6. doi: 10.1073/pnas.0909235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cowell IG. E4BP4/NFIL3, a PAR-related bZIP factor with many roles. Bioessays. 2002;24:1023–9. doi: 10.1002/bies.10176. [DOI] [PubMed] [Google Scholar]
- 76.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–30. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kashiwada M, Cassel SL, Colgan JD, Rothman PB. NFIL3/E4BP4 controls type 2 T helper cell cytokine expression. EMBO J. 2011;30:2071–82. doi: 10.1038/emboj.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kashiwada M, Pham NL, Pewe LL, Harty JT, Rothman PB. NFIL3/E4BP4 is a key transcription factor for CD8{alpha}+ dendritic cell development. Blood. 2011 doi: 10.1182/blood-2010-07-295873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Motomura Y, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. 2011;12:450–9. doi: 10.1038/ni.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of NK cells. Nat Rev Immunol. 2011 doi: 10.1038/nri3065. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen S, Kawashima H, Lowe JB, Lanier LL, Fukuda M. Suppression of tumor formation in lymph nodes by L-selectin-mediated natural killer cell recruitment. J Exp Med. 2005;202:1679–89. doi: 10.1084/jem.20051473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gregoire C, et al. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jenne CN, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–81. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walzer T, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–44. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 85.Andrews DM, et al. NK1.1+ cells and murine cytomegalovirus infection: what happens in situ? J Immunol. 2001;166:1796–802. doi: 10.4049/jimmunol.166.3.1796. [DOI] [PubMed] [Google Scholar]
- 86.Dokun AO, Chu DT, Yang L, Bendelac AS, Yokoyama WM. Analysis of in situ NK cell responses during viral infection. J Immunol. 2001;167:5286–93. doi: 10.4049/jimmunol.167.9.5286. [DOI] [PubMed] [Google Scholar]
- 87.Bekiaris V, et al. Ly49H+ NK cells migrate to and protect splenic white pulp stroma from murine cytomegalovirus infection. J Immunol. 2008;180:6768–76. doi: 10.4049/jimmunol.180.10.6768. [DOI] [PubMed] [Google Scholar]
- 88.Salazar-Mather TP, Lewis CA, Biron CA. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1alpha delivery to the liver. J Clin Invest. 2002;110:321–30. doi: 10.1172/JCI15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–75. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fink PJ, Hendricks DW. Post-thymic maturation: young T cells assert their individuality. Nat Rev Immunol. 2011;11:544–9. doi: 10.1038/nri3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiossone L, et al. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–96. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 92.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–24. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 93.Huntington ND, et al. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178:4764–70. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 94.Takeda K, et al. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 2005;105:2082–9. doi: 10.1182/blood-2004-08-3262. [DOI] [PubMed] [Google Scholar]
- 95.Chaix J, et al. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–31. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoshino K, et al. Cutting edge: generation of IL-18 receptor-deficient mice: evidence for IL-1 receptor-related protein as an essential IL-18 binding receptor. J Immunol. 1999;162:5041–4. [PubMed] [Google Scholar]
- 97.Takeda K, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–90. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 98.Andrews DM, et al. Homeostatic defects in interleukin 18-deficient mice contribute to protection against the lethal effects of endotoxin. Immunol Cell Biol. 2011 doi: 10.1038/icb.2010.168. [DOI] [PubMed] [Google Scholar]
- 99.Reading PC, et al. IL-18, but not IL-12, regulates NK cell activity following intranasal herpes simplex virus type 1 infection. J Immunol. 2007;179:3214–21. doi: 10.4049/jimmunol.179.5.3214. [DOI] [PubMed] [Google Scholar]
- 100.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 101.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–70. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 102.Koka R, et al. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–84. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–76. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ranson T, et al. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–93. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 105.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 106.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–17. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–25. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mortier E, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–22. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 109.Castillo EF, Stonier SW, Frasca L, Schluns KS. Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation. J Immunol. 2009;183:4948–56. doi: 10.4049/jimmunol.0900719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–80. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fehniger TA, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193:219–31. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cooper MA, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–8. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 113.Huntington ND, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–63. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsunobuchi H, et al. A protective role of interleukin-15 in a mouse model for systemic infection with herpes simplex virus. Virology. 2000;275:57–66. doi: 10.1006/viro.2000.0455. [DOI] [PubMed] [Google Scholar]
- 115.Umemura M, Nishimura H, Hirose K, Matsuguchi T, Yoshikai Y. Overexpression of IL-15 in vivo enhances protection against Mycobacterium bovis bacillus Calmette-Guerin infection via augmentation of NK and T cytotoxic 1 responses. J Immunol. 2001;167:946–56. doi: 10.4049/jimmunol.167.2.946. [DOI] [PubMed] [Google Scholar]
- 116.Ferrari-Lacraz S, et al. Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J Immunol. 2004;173:5818–26. doi: 10.4049/jimmunol.173.9.5818. [DOI] [PubMed] [Google Scholar]
- 117.Ruchatz H, Leung BP, Wei XQ, McInnes IB, Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998;160:5654–60. [PubMed] [Google Scholar]
- 118.Smith XG, et al. Selective blockade of IL-15 by soluble IL-15 receptor alpha-chain enhances cardiac allograft survival. J Immunol. 2000;165:3444–50. doi: 10.4049/jimmunol.165.6.3444. [DOI] [PubMed] [Google Scholar]
- 119.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med. 2011;208:357–68. doi: 10.1084/jem.20100479. Reference 119 demonstrates that homeostatic proliferation drives NK cells to become long-lived cells with robust effector function and proliferative potential even 6 months following adoptive transfer into lymphopenic recipient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–56. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–64. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–7. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 123.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–81. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 124.Cheung KP, Yang E, Goldrath AW. Memory-like CD8+ T cells generated during homeostatic proliferation defer to antigen-experienced memory cells. J Immunol. 2009;183:3364–72. doi: 10.4049/jimmunol.0900641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 126.Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- 127.Dokun AO, et al. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–6. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 128.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61. doi: 10.1038/nature07665. Reference 128 demonstrates the ability of NK cells to undergo massive clonal expansion and generate memory cells against MCMV infection. Upon viral challenge, memory Ly49H+ NK cells undergo a secondary recall response, mediating greater protection against MCMV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–92. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 130.Guma M, et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–71. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 131.Guma M, et al. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006;194:38–41. doi: 10.1086/504719. [DOI] [PubMed] [Google Scholar]
- 132.Kuijpers TW, et al. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–5. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- 133.Lopez-Verges S, et al. Expansion of a unique CD57+NKG2Chi natural killer subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1110900108. in press References 133–134 are the first longitudinal studies in humans to demonstrate a virus-specific clonal expansion of a NK cell subset, which maintains elevated numbers for months, suggesting the existence of memory NK cells in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bjorkstrom NK, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dorner BG, et al. Coordinate expression of cytokines and chemokines by NK cells during murine cytomegalovirus infection. J Immunol. 2004;172:3119–31. doi: 10.4049/jimmunol.172.5.3119. [DOI] [PubMed] [Google Scholar]
- 137.Nandi D, Gross JA, Allison JP. CD28-mediated costimulation is necessary for optimal proliferation of murine NK cells. J Immunol. 1994;152:3361–9. [PubMed] [Google Scholar]
- 138.Andoniou CE, et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–9. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 139.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–81. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 140.Krug A, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–19. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 141.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–66. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun JC, Ma A, Lanier LL. Cutting edge: IL-15-independent NK cell response to mouse cytomegalovirus infection. J Immunol. 2009;183:2911–4. doi: 10.4049/jimmunol.0901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Magram J, et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–81. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 144.Muller U, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 145.Wu C, Ferrante J, Gately MK, Magram J. Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159:1658–65. [PubMed] [Google Scholar]
- 146.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–93. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–50. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 148.Robbins SH, Tessmer MS, Mikayama T, Brossay L. Expansion and contraction of the NK cell compartment in response to murine cytomegalovirus infection. J Immunol. 2004;173:259–66. doi: 10.4049/jimmunol.173.1.259. [DOI] [PubMed] [Google Scholar]
- 149.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–19. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 150.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–9. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 151.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–4. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 152.Hildeman DA, et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–67. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 153.Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci U S A. 2003;100:14175–80. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Prlic M, Bevan MJ. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc Natl Acad Sci U S A. 2008;105:16689–94. doi: 10.1073/pnas.0808997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wojciechowski S, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–75. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172:2048–58. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- 157.Parrish-Novak J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 158.Vosshenrich CA, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–21. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 159.Kasaian MT, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–69. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 160.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–87. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 161.Sun JC, Beilke JN, Lanier LL. Immune memory redefined: characterizing the longevity of natural killer cells. Immunol Rev. 2010;236:83–94. doi: 10.1111/j.1600-065X.2010.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–10. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–7. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 164.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 167.Sun JC, Lanier LL. Natural killer cells remember: an evolutionary bridge between innate and adaptive immunity? Eur J Immunol. 2009;39:2059–64. doi: 10.1002/eji.200939435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 169.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–22. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mercado R, et al. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–9. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 171.van Stipdonk MJ, et al. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–5. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 172.Schepers K, et al. Dissecting T cell lineage relationships by cellular barcoding. J Exp Med. 2008;205:2309–18. doi: 10.1084/jem.20072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schumacher TN, Gerlach C, van Heijst JW. Mapping the life histories of T cells. Nat Rev Immunol. 2010;10:621–31. doi: 10.1038/nri2822. [DOI] [PubMed] [Google Scholar]
- 174.Gill S, Olson JA, Negrin RS. Natural killer cells in allogeneic transplantation: effect on engraftment, graft- versus-tumor, and graft-versus-host responses. Biol Blood Marrow Transplant. 2009;15:765–76. doi: 10.1016/j.bbmt.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kurtz J, Franz K. Innate defence: evidence for memory in invertebrate immunity. Nature. 2003;425:37–8. doi: 10.1038/425037a. [DOI] [PubMed] [Google Scholar]
- 176.Cudkowicz G, Stimpfling JH. Induction of Immunity and of Unresponsiveness to Parental Marrow Grafts in Adult F-1 Hybrid Mice. Nature. 1964;204:450–3. doi: 10.1038/204450a0. [DOI] [PubMed] [Google Scholar]
- 177.Carson WE, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gidlund M, Orn A, Wigzell H, Senik A, Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978;273:759–61. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- 179.Glas R, et al. Recruitment and activation of natural killer (NK) cells in vivo determined by the target cell phenotype. An adaptive component of NK cell-mediated responses. J Exp Med. 2000;191:129–38. doi: 10.1084/jem.191.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–16. doi: 10.1038/ni1332. Together with reference 128, references 180–182 demonstrate that NK cells have the capability to generate memory responses against a wide range of antigens and pathogens. [DOI] [PubMed] [Google Scholar]
- 181.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]