Abstract
Background
The impact of patient age on the risks of death or rehospitalization after primary prevention implantable cardioverter-defibrillator (ICD) placement is uncertain.
Methods and Results
Data from 5 major ICD trials were merged: MADIT-I, MUSTT, MADIT-II, DEFINITE, and SCD-HeFT . Median age at enrollment was 62 (interquartile range 53-70) years. Compared with their younger counterparts, older patients had a greater burden of comorbid illness. In unadjusted exploratory analyses, ICD recipients were less likely to die than non-recipients in all age groups: hazard ratio (HR) 0.48, 95% posterior credible interval (PCI) 0.33-0.69 among patients <55 years; HR 0.69, 95%PCI 0.53-0.90 among patients 55-64 years; HR 0.67, 95%PCI 0.53-0.85 among patients 65-74 years; and HR 0.54, 95%PCI 0.37-0.78 among patients > 75 years. Sample sizes were limited among patients > 75 years. In adjusted Bayesian Weibull modeling, point estimates indicate ICD efficacy persists but is attenuated with increasing age. There was evidence of an interaction between age and ICD treatment on survival (two-sided posterior tail probability of no interaction < 0.01). Using an adjusted Bayesian logistic regression model, there was no evidence of an interaction between age and ICD treatment on rehospitalization (two-sided posterior tail probability of no interaction 0.44).
Conclusions
In this analysis, the survival benefit of the ICD exists but is attenuated with increasing age. The latter finding may be due to the higher burden of comorbid illness, competing causes of death, or limited sample size of older patients. There was no evidence that age modifies the association between ICD treatment and rehospitalization.
Keywords: implantable cardioverter-defibrillator, aging, meta-analysis
Introduction
Clinical trials have demonstrated the efficacy of the implantable cardioverter-defibrillator (ICD) in reducing the risk of sudden cardiac death.1-5 Consequently, ICD placement has become widespread, particularly among older patients. Greater than 40% of new ICDs are placed among patients > 70 years of age and greater than 10% among patients > 80 years of age.6 As the population ages,7 the number of ICDs placed in older patients is expected to grow. Uncertainty regarding ICD efficacy among older patients exists,8 however, as they were underrepresented in individual clinical trials. This dearth of data is problematic. On the one hand, undergoing a potentially hazardous and expensive procedure without realizing a clinical benefit is objectionable. On the other hand, withholding an effective therapy is also undesirable. The impact of age on the likelihood of rehospitalization after ICD placement is similarly incompletely understood.
Pooling of clinical trial data permits more efficient appraisal of treatment effects among subgroups by increasing sample sizes. Given the small number of patients in subgroups of interest in individual trials, a consortium consisting of the investigators of 9 major ICD trials was created. Restricted to patients enrolled in primary prevention ICD trials, the current analysis examined whether age modifies the relationship between ICD treatment and mortality or rehospitalizations.
Methods
Data sources and study selection
Patient-level data previously collected during 9 primary and secondary prevention trials were merged. The de-identified data set was granted exempt status by the Duke Institutional Review Board. Primary prevention clinical trials randomizing patients to an ICD or usual care were included in this analysis: the Multicenter Automatic Defibrillator Implantation Trial I (MADIT-I),1 the Multicenter UnSustained Tachycardia Trial (MUSTT),2 MADIT-II,3 the Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation trial,4 and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT)5 (Table 1). Although patients were not randomized to ICD or usual care in MUSTT, the trial was nevertheless included because the observed treatment effect of electrophysiologically-guided antiarrhythmic therapy was due to better outcomes among ICD recipients rather than antiarrhythmic drug recipients.9 For the purposes of the present analysis, MUSTT ICD recipients received active intervention and nonrecipients usual care. The Antiarrhythmics Verus Implantable Defibrillators trial10 the Cardiac Arrest Study Hamburg trial11 were excluded given their focus on secondary prevention, and the amiodarone arms of SCD-HeFT5 or MUSTT were excluded on the basis of amiodarone's potentially confounding effect. The Coronary Artery Bypass Graft trial12 and Defibrillator in Acute Myocardial Infarction Trial13 were also excluded based on the potential benefit of surgical revascularization on ventricular arrhythmia and the exclusion of patients with a recent myocardial infarction (MI) in current professional guidelines,14 respectively. Within the included trials, individual patient inclusion criteria were heart failure (New York Heart Association Class I-III), a left ventricular ejection fraction (LVEF) of < 35%, and the availability of important covariates. Accordingly, patients without heart failure symptoms or with New York Heart Association Class IV symptoms (53 from MADIT-II), a LVEF of > 35% (2 from MADIT-I, 77 from MUSTT, 3 from SCD-HeFT) or with time from MI to randomization less than 40 days (16 from MADIT-I, 12 from MADIT-II, 89 from MUSTT, and 2 from SCD-HeFT) as well as those missing values for variables that define the inclusion criteria (87 from MADIT II, 248 from MUSTT and 49 from SCD-HeFT) were excluded from this study.
Table 1.
Primary Prevention ICD Trial Characteristics
| Clinical Trial | Participating Countries | Year of Main Publication | Eligible age range at entry (yrs) | Eligible LVEF | Cardiomyopathy | Factorial Comparison | No. of participants | Mean follow-up (yrs) |
|---|---|---|---|---|---|---|---|---|
| MADIT-I1 | USA, Italy, Germany | 1996 | 25-80 | ≤ 35% | Ischemic | ICD v. conventional medical therapy | 95 vs 101 | 2.40 vs 2.07 |
| MUSTT2 | United States, Canada | 1999 | <80 | ≤ 40% | Ischemic | ICD or antiarrhythmic drugs v. placebo | 167 vs 537 | 3.55 vs 3.16 |
| MADIT-II3 | USA, Netherlands, Germany, Israel | 2002 | >21 | ≤ 30% | Ischemic | ICD v. conventional medical therapy | 742 vs 490 | 1.71 vs 1.64 |
| DEFINITE4 | USA | 2004 | 21-80 | <36% | Nonischemic | ICD v. conventional medical therapy | 229 vs 229 | 2.55 vs 3.40 |
| SCD-HeFT5 | USA, Canada | 2005 | >18 | ≤ 35% | Ischemic or nonischemic | ICD v. placebo v. amiodarone | 829 vs 1692 | 3.40 vs 3.33 |
Statistical Analysis
The primary endpoint was all-cause mortality. The secondary endpoint was rehospitalization for any reason. For descriptive analyses, the following age categories were chosen to balance interest with adequate sample sizes: <55, 55-64, 65-74, and >75 years. Patients > 65 years of age were considered “older,” but younger categories were included to better understand trends. Baseline characteristics were summarized as percentages for categorical variables and medians with 25th and 75th percentiles for continuous variables. The association of age group with each characteristic was assessed using the χ2 and equality of medians tests as appropriate. Given the multilevel resolution of the data, that is, with patients within trials, we also assessed the association of age group with each characteristic using generalized linear mixed models with random effects for trials. This allowed us to borrow information across trials while also accounting for possible correlated observations within the same trial. Baseline data according to age were also stratified by trial and by sex.
Time zero was the day of randomization in both study arms. The absolute number of each endpoint was stratified according to age group and sex as well as the presence or absence of an ICD. Kaplan-Meier curves showing the survival of ICD recipients relative to non-recipients were generated. Differences in the log-hazard ratios were assessed with a stratified Cox-Proportional hazards model allowing for separate baseline hazard functions for each trial. Cause-of-death data was not examined due to the degree of missingness.
Using Cox proportional hazards models, we examined factors associated with death in univariate fashion. In adjusted analyses, Bayesian Weibull survival regression modeling15, 16 was used to combine trial data and address missingness. Specifically, a model including continuously-valued age and ICD therapy as well as their interaction was fitted. Additional covariates were selected on the basis of clinical relevance among those available across trials and included sex, race, LVEF, New York Heart Association class, indicator of QRS duration greater or equal to 120, indicator of ischemic disease (prior coronary artery bypass grafting, prior myocardial infarction, or history of ischemic heart disease), and the use of angiotensin-converting enzyme inhibitors, β-blockers and history of diabetes. Within our Bayesian Weibull survival model, we performed multiple imputation to address missingness of QRS duration or history of diabetes. The imputations were performed for each trial based upon the empirical distribution of the variable of interest. To account for the possible heterogeneity in treatment effect or in the underlying risk of death from all causes between patients in the different trials, trial-specific treatment effects and parameters defining trial-specific baseline hazard functions were considered random effects. The significance of the interaction value was evaluated with the two-sided tailed posterior probability for a null interaction as a Bayesian analogue to the frequentist p-value. For additional details, see the Statistical Appendix. We also fitted similar unadjusted models, that is, that do not include additional covariates beyond age and treatment for comparison. The interaction of age with ICD treatment on the secondary endpoint of rehospitalization was assessed in an analogous fashion in a Bayesian logistic regression model17 without or with the same adjustment variables with patients from MUSTT and DEFINITE removed given non-availability of the secondary outcome. In sensitivity analyses, we fitted a quadratic model on age with an interaction with ICD treatment on the endpoints of death and rehospitalization and assessed the interaction. Additional details about our models are provided in the Supplementary Appendix. Analyses were performed with R version 3.1.0 and WinBugs version 1.4.3.18
Results
Baseline characteristics
The final study population consisted of 3,530 patients from 5 clinical trials. The median age in the overall sample was 62 (interquartile range 53-70) years. The number of patients > 75 years of age was 390 (11.0%). Some differences in baseline characteristics according to age group were present at baseline (Table 2). Compared with their younger counterparts, older patients were more commonly white, more frequently had advanced heart failure symptoms, and were more likely to have a number of comorbidities, including atrial fibrillation, hypertension, peripheral vascular disease, and pulmonary disease. They were also more likely to have been revascularized either surgically or percutaneously. They more commonly had an elevated creatinine, a left bundle-branch block , and a widened QRS. Further, they were less likely to be taking a β-blocker or an angiotensin-converting enzyme inhibitor. Differences were also observed when the baseline data were stratified by trial (Supplementary Table 1) and by sex (Supplementary Table 2).
Table 2.
Baseline Characteristics According to Age at Enrollment*
| Age at Enrollment |
|||||
|---|---|---|---|---|---|
| Characteristic | <55 (n=1010) | 55-64 (n=1055) | 65-74 (n=1075) | ≥75 (n=390) | P† |
| Randomized to ICD, % | 52.2 | 50.1 | 51.6 | 58 | 0.127 |
| Age, median (IQR) | 48 (43-52) | 60 (57-62) | 69 (67-72) | 78 (76-80) | |
| Male, % | 78.1 | 81.5 | 80.7 | 81 | 0.733 |
| Race | <0.001 | ||||
| White | 73.1 | 81.0 | 81.4 | 91.0 | |
| Black | 20.8 | 14.4 | 13.3 | 5.4 | |
| Other | 6.1 | 4.6 | 5.3 | 4.0 | |
| Left ventricular ejection fraction, median (IQR) | 23 (18-28) | 25 (20-30) | 25 (20-30) | 25 (20-29) | 0.004 |
| New York Heart Association Class | 0.034 | ||||
| I | 13.6 | 19.1 | 20.2 | 24.4 | |
| II | 60.5 | 55.8 | 52.5 | 43.9 | |
| III | 25.9 | 25.1 | 27.4 | 31.8 | |
| Medical history, % | |||||
| Atrial fibrillation | 6.6 | 8.1 | 14.7 | 20.9 | <0.001 |
| Coronary artery bypass grafting | 26.9 | 41.1 | 48.5 | 54.8 | <0.001 |
| Diabetes | 23.3 | 34.2 | 33.4 | 23.8 | <0.001 |
| Hypercholesterolemia | 65.5 | 46.9 | 42.9 | 9.1 | 0.013 |
| Hypertension | 47.0 | 53.8 | 58.0 | 55.0 | <0.001 |
| Ischemic heart disease | 45.6 | 64.6 | 72.9 | 76.4 | <0.001 |
| Myocardial infarction | 44.1 | 62.1 | 69.8 | 73.1 | <0.001 |
| Peripheral vascular disease | 2.8 | 6.2 | 9.0 | 7.0 | 0.458 |
| Percutaneous coronary intervention | 25.2 | 30.3 | 32.5 | 31.6 | 0.498 |
| Pulmonary disease | 12.8 | 19.4 | 23.7 | 20.3 | <0.001 |
| Smoking | 81.1 | 83.6 | 75.9 | 74.5 | <0.001 |
| Medication, % | |||||
| Angiotensin converting enzyme inhibitor | 91.2 | 86.5 | 81.6 | 76.4 | <0.001 |
| β-blocker | 74.5 | 66.3 | 59.0 | 55.4 | <0.001 |
| Diuretic | 79.6 | 79.1 | 79.2 | 78.2 | 0.100 |
| Antiarrhythmic | 1.8 | 4.0 | 4.3 | 3.1 | 0.200 |
| Laboratory data | |||||
| Serum creatinine, median (IQR) | 1.0 (0.9, 1.2) | 1.1 (1.0, 1.3) | 1.2 (1.1, 1.4) | 1.3 (1.1, 1.6) | <0.001 |
| Left bundle branch block, % | 16.4 | 18.0 | 22.0 | 24.1 | <0.001 |
| QRS duration, median (IQR) | 104 (92-120) | 110 (95-140) | 114 (96-144) | 120 (100-160) | <0.001 |
| Included patients, n | |||||
| MADIT-I | 33 | 58 | 74 | 14 | |
| MUSTT | 24 | 57 | 76 | 25 | |
| MADIT-II | 192 | 321 | 402 | 174 | |
| DEFINITE | 181 | 120 | 114 | 43 | |
| SCD-HeFT | 580 | 499 | 409 | 134 | |
ICD indicates implantable cardioverter-defibrillator and IQR interquartile range.
Data are presented as % unless otherwise indicated and are based on patients with available data.
Tests do not treat age as an ordered categorical variable.
Outcomes
After a median duration of follow-up of 2.6 years, 323 of 1836 (21.3%) ICD recipients and 463 of 1694 non-recipients (30.6%) died. ICD recipients were less likely to die than non-recipients in all age groups (Table 3). Death rates among women receiving usual care were comparable to those of men among patients <55 years of age and lower in older age groups. The reduction in death observed in ICD recipients was comparable between sexes among patients <55 years of age and lower in older age groups (Table 3). Kaplan-Meier estimates of death as a function of time and corresponding hazard ratios from the stratified Cox-Proportional hazards models showed a mortality benefit of ICD therapy in all age groups (Figure 1). In the total study cohort, 1045 (71.4%) ICD recipients and 771 (60.2%) non-recipients were hospitalized one or more times. This trend was consistent across all age groups (Table 3).
Table 3.
Outcomes According to Age at Enrollment*
| Age at Enrollment (years) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <55 | 55-64 | |||||||||||
| Usual care | ICD | Usual care | ICD | |||||||||
| Total | Men | Women | Total | Men | Women | Total | Men | Women | Total | Men | Women | |
| Event | (n=483) | (n=374) | (n=109) | (n=527) | (n=415) | (n=112) | (n=527) | (n=433) | (n=94) | (n=528) | (n=427) | (n=101) |
| Death | 17.4 (84) | 17.4 (65) | 17.4 (19) | 8.2 (43) | 8.4 (35) | 7.1 (8) | 26.4 (139) | 27.9 (121) | 19.2 (18) | 18.4 (97) | 18.7 (80) | 16.8 (17) |
| ≥1 rehospitalization | 54.4 (200) | 53.4 (150) | 57.5 (50) | 66.2 (272) | 64.6 (210) | 72.1 (62) | 59.9 (240) | 58.3 (193) | 67.1 (47) | 69.7 (294) | 68.6 (231) | 74.1 (63) |
| 65-74 | >75 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | ICD | Usual care | ICD | |||||||||
| Total | Men | Women | Total | Men | Women | Total | Men | Women | Total | Men | Women | |
| Event | (n=520) | (n=421) | (n=99) | (n=555) | (n=447) | (n=108) | (n=164) | (n=134) | (n=30) | (n=226) | (n=182) | (n=44) |
| Death | 33.5 (174) | 35.9 (151) | 23.2 (23) | 22.9 (127) | 20.8 (93) | 31.5 (34) | 40.2 (66) | 44.0 (59) | 23.3 (7) | 24.8 (56) | 26.4 (48) | 18.2 (8) |
| ≥1 rehospitalization | 62.3 (238) | 60.1 (188) | 72.5 (50) | 76.1 (343) | 75.7 (274) | 77.5 (69) | 72.1 (93) | 74.8 (80) | 59.1 (13) | 75.6 (136) | 76.7 (115) | 70 (21) |
Data are presented as % (n).
Figure 1.
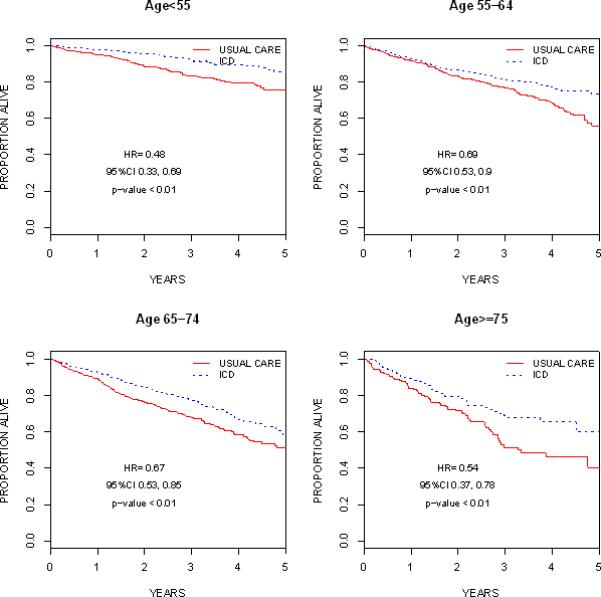
Unadjusted Kaplan-Meier Survival Curves by Age Groups. Hazard ratios, 95% CIs and p-values are based on a stratified Cox-regression model by trial.
Factors associated with death in univariate fashion are shown (Table 4). Fitting Weibull survival regression models that included continuously valued age, ICD therapy and their interaction, without or with adjustment for baseline characteristics, we found that point estimates for ICD therapy efficacy compared with usual care were consistent with a survival benefit across the spectrum of age. However, the absence of a survival benefit could not be ruled out above age 70 (Figure 2 for adjusted model; results for the unadjusted model are similar and thus omitted in the figure). Further, the impact of ICD therapy on survival is attenuated with increasing age (two-sided posterior tail probability of no interaction is 0.02 in the unadjusted model and < 0.01 in the adjusted model). By contrast, when examining rehospitalizations there was no evidence of an interaction between age and ICD treatment (two-sided posterior tail probability of no interaction is 0.58 in the unadjusted model and 0.44 in the adjusted model). In sensitivity analyses, using a quadratic model on age, there was evidence of an interaction between age with ICD treatment on the endpoint of death but not on rehospitalization. The final model for the primary outcome of death inclusive of other adjustment covariates with and without the interaction term using the linear term for age is shown (Table 5).
Table 4.
Factors Associated with Death in Univariate Analysis
| Factor | Hazard Ratio (95% Confidence Interval) |
|---|---|
| ICD | 0.64 (0.55, 0.73) |
| Age | 1.04 (1.03, 1.04) |
| Male sex | 1.22 (1.01, 1.47) |
| Race | |
| Black | 0.98 (0.69, 1.37) |
| White | 0.84 (0.62, 1.13) |
| History of diabetes | 1.67 (1.43, 1.94) |
| History of coronary artery bypass grafting | 1.29 (1.11, 1.51) |
| Left ventricular ejection fraction | 0.59 (0.46, 0.77) |
| New York Heart Association Class | |
| II | 1.12 (0.88, 1.43) |
| III | 2.39 (1.88, 3.03) |
| QRS duration | 1.36 (1.17, 1.57) |
| Angiotensin-converting enzyme inhibitor use | 1.29 (1.11, 1.51) |
| β-blocker use | 0.60 (0.52, 0.70) |
Figure 2.
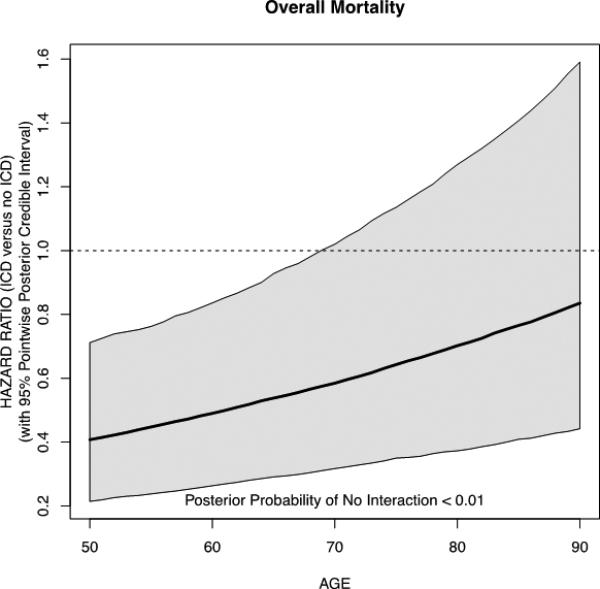
Adjusted Hazard Ratio of Mortality According to Age
Table 5.
Factors Associated with Death in Multivariable Analysis
| Multivariable Model Without Interaction Term | Multivariable Model With Interaction Term | |
|---|---|---|
| Factor | Hazard Ratio (95% Posterior Credible Interval) | Hazard Ratio (95% Posterior Credible Interval) |
| Age | 1.03 (1.02-1.04) | 1.02 (1.01-1.03) |
| Male sex | 1.23 (1.02-1.50) | 1.21 (0.98-1.46) |
| Race | ||
| White | 0.71 (0.55-0.97) | 0.69 (0.54-1.01) |
| Black | 0.98 (0.73-1.37) | 0.95 (0.70-1.40) |
| Left ventricular ejection fraction | 0.62 (0.48-0.80) | 0.62 (0.48-0.78) |
| New York Heart Association Class | ||
| II v. I | 1.05 (0.82-1.34) | 1.07 (0.86-1.39) |
| III v. I | 2.04 (1.67-2.62) | 2.05 (1.67-2.71) |
| Ischemic disease | 1.52 (1.25-1.92) | 1.58 (1.28-1.97) |
| Diabetes mellitus | 1.44 (1.23-1.67) | 1.45 (1.22-1.68) |
| Medication | ||
| β-blocker | 0.69 (0.60-0.81) | 0.69 (0.59-0.79) |
| Angiotensin converting enzyme inhibitor | 0.92 (0.74-1.12) | 0.93 (0.77-1.16) |
| QRS duration | 1.20 (1.03-1.39) | 1.21 (1.03-1.41) |
| ICD therapy | ||
| DEFINITE | 0.61 (0.39-0.96) | 0.48 (0.30-0.79) |
| MADIT-I | 0.48 (0.28-0.81) | 0.37 (0.22-0.61) |
| MADIT-II | 0.59 (0.45-0.78) | 0.44 (0.31-0.59) |
| MUSTT | 0.37 (0.20-0.64) | 0.27 (0.14-0.49) |
| SCD-HeFT | 0.73 (0.60-0.89) | 0.58 (0.45-0.74) |
| Overall | 0.54 (0.30-0.95) | 0.41 (0.21-0.71) |
| Heterogeneity in Treatment Effect (Standard Deviation) | 0.57 (0.38, 1.04) | 0.59 (0.38, 0.99) |
| Age and ICD therapy interaction | - | 1.02 (1.01-1.03) |
Discussion
Our analysis has 3 main findings. First, after pooling data from 5 clinical trials and adjusting for patient demographics, medical comorbidities, and laboratory values, point estimates indicate the survival benefit of ICD therapy persists across the age spectrum. Second, the survival advantage conferred by ICD therapy is attenuated with advancing age. However, the number of patients older than 75 years was modest, and this may have impacted corresponding efficacy estimates and the observed attenuation of ICD survival benefit. Third, there is no evidence that age influences the odds of rehospitalization after ICD placement.
In a secondary evaluation of MADIT-II, the ICD was associated with a reduction in mortality among patients > 75 years of age comparable to that of younger patients.19 By contrast, two meta-analyses indicate that ICD survival benefit persists but becomes less striking with older age.20, 21 The latter two studies were limited by the use of trial-level estimates of study outcomes. Patient-level analysis allows for adjustment for differences in comorbidities and medical therapies and continuous rather than categorical valuation of relevant covariates such as age. While the former aspect is central to the assessment of the independent relationship of covariates with outcomes, the latter minimizes potential loss of information. These two study design features as well as assessment of the age-related risk of re-hospitalization set the current analysis apart from those preceding it.
The current analysis suggests the survival benefit of ICD therapy exists but diminishes with increasing age. Analyses of ICD effectiveness yielded similar results. In the American Heart Association Get With the Guidelines- Heart Failure registry database linked with Medicare claims, receipt of ICD therapy was associated with a lower risk of mortality 3 years after implantation up to 84 years of age (adjusted hazard ratio (HR) 0.65, 95% CI 0.47-0.89 among patients 65 to 74 years; HR 0.80, 95% CI 0.62-1.03 among patients 75-84 years).22 In the Ontario ICD database, mortality also increased with age (2.1, 3.0, 5.4, 6.9, and 10.2 deaths per 100 person-years in ICD recipients aged 18 to 49, 50 to 59, 60 to 69, 70 to 79, and > 80 years, respectively).23 In an analysis of two European registries, the survival benefit of ICD therapy was considerably lower among ICD recipients > 75 years compared with those < 75 years, and their mortality rates were comparable to those of age-matched controls from the general population.24 The decline in the survival benefit of ICD therapy observed in the current analysis is not only biologically plausible but also consistent with existing knowledge of sudden cardiac death. Its incidence rises with increasing age, but the competing risk of non-sudden death becomes proportionally higher.25 The lower fraction of sudden deaths accompanying advancing age corresponds to a reduced number of opportunities for the ICD to improve patient survival. The greater burden of comorbid illness with advancing age observed in the current analysis may underlie the increase in non-sudden death. Advancing age was also accompanied by a reduction in receipt of evidence-based medications, including β-blockers and angiotensin-converting enzyme inhibitors. This has been observed previously26 and represents a potential missed opportunity to improve patient outcomes.27 Our multivariable analysis included the use of these medications, but residual confounding related to the quality of medical care otherwise received may persist and potentially impact the magnitude or directionality of the observed ICD treatment effect. The modest sample size of patients > 75 years of age or residual confounding may also in part explain the observed attenuation in ICD survival benefit.
Previous studies examining the relationship between age and rehospitalization were sparse, conducted in single centers, and divergent. In a retrospective analysis of 65 consecutive patients undergoing ICD placement between 1991 and 1993, age was not associated with readmission.28 In a similar analysis, age >60 years independently predicted rehospitalization for both cardiovascular and arrhythmic causes among 180 patients who underwent ICD placement in the late 1990s.29 A trend between increasing age and rates of cardiovascular rehospitalization was also observed in the Ontario ICD database (17.4%, 13.2%, 14.5%, 17.0%, and 21.1% among patients aged 18 to 49, 50 to 59, 60 to 69, 70 to 79, and > 80 years, respectively; p = 0.035).23 Differences in the clinical practices between trials and real-world settings may in part explain these discrepancies. Other potential explanations include modest sample sizes or residual confounding. An increased risk of rehospitalization among ICD recipients versus non-recipients has been observed previously and may be due to shocks, shock-associated heart failure exacerbations, and post-procedural complications.30, 31
Our data indicate the survival benefit of ICDs is comparable between sexes among patients <55 years of age. It becomes less so with increasing age but nonetheless persists. Notably, the death rate among men was lower than that of women receiving usual care, and thus there may have been fewer opportunities for the ICD to alter the natural course of events by aborting sudden death.32 Randomized or observational analyses with larger sample sizes and higher event rates allowing for adjustment are needed to further inform the ongoing debate regarding ICD utilization in women.
The current analysis suggests age per se should not be a contraindication to ICD placement. Rather, clinical judgment should take into account other factors, including patient preference, periprocedural risk, and comorbidity burden.33, 34 However, randomized data of patients > 75 years of age are limited, and more studies are needed. The absence of an association between age and the likelihood of rehospitalization in the clinical trial setting but not the real-world setting is likewise noteworthy and also deserves further study.
Limitations
The current analysis was retrospective; however, data were collected prospectively. Data were also derived from clinical trials and thus the findings may not be generalizable to less monitored or controlled settings. Data are stratified by sex for descriptive purposes; the study sample size cannot afford testing interactions by sex in addition to by age. More studies on older patients in clinical and real-world settings are needed. Adjustment covariates were limited to those available in all included trials. Our study may be subject to residual or unmeasured confounding by relevant covariates such as frailty, atrial fibrillation, chronic kidney disease, or lung disease. In spite of pooling of data, subgroup sample sizes were modest, particularly among patients > 75 years of age. The power of our study to detect small differences in ICD treatment effect was correspondingly limited, particularly among older age groups. Since the treatment benefit of ICD therapy likely increases with time after ICD placement and the follow-up of the current analysis was short compared to some,35 our ability to discern a survival benefit of ICD therapy may have been correspondingly limited.
Conclusions
In this analysis of 3,530 patients from 5 clinical trials, the survival benefit of ICD therapy is attenuated with increasing age and may be related to an accompanying increase in the burden of comorbid illness. The survival benefit nonetheless appears to persist. Randomized data of patients > 75 years of age are sparse, and this may in part explain the observed attenuation in ICD survival benefit. More studies among older patients are needed. There was no evidence that age modifies the association between ICD treatment and rehospitalization.
Supplementary Material
Acknowledgments
Sources of Funding
Primary funding was provided by the Agency for Healthcare Research and Quality (5 R01 HS018505-03). P.L.H. was funded by NIH T-32 training grant HL069749-09. The funding sources had no role in the design, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.
Footnotes
Disclosures (none unless otherwise noted below)
Drs Hess and Kadish received consulting fees/honoraria from Sanofi-Aventis.
References
- 1.Moss AJ, Hall J, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted cardioverterdefibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall J, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 4.Kadish A, Dyer A, Daubert JP, Quigg R, Estes M, Anderson KP, Calkins H, Hoch D, Goldberg J, Shalaby A, Sanders WE, Schaechter A, Levine JH. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 5.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 6.Epstein AE, Kay GN, Plumb VJ, McElderry HT, Doppalapudi H, Yamada T, Shafiroff J, Syed ZA, Shkurovich S. Implantable cardioverter-defibrillator prescription in the elderly. Heart Rhythm. 2009;6:1136–43. doi: 10.1016/j.hrthm.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Older Americans Key Indicators of Well-Being. Federal Interagency Forum on Aging-Related Statistics. 2010;2010:1–156. [Google Scholar]
- 8.Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, Kapa S, Kremers MS, Lindsay BD, Stevenson LW. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Heart Rhythm. 2013;10:e11–58. doi: 10.1016/j.hrthm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Lee KL, Hafley G, Fisher JD, Gold MR, Prystowsky EN, Talajic M, Josephson ME, Packer DL, Buxton AE. Effect of implantable defibrillators on arrhythmic events and mortality in the Multicenter Unsustained Tachycardia Trial. Circulation. 2002;106:233–238. doi: 10.1161/01.cir.0000021920.73149.c3. [DOI] [PubMed] [Google Scholar]
- 10.The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators A comparison of atiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–83. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 11.Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: The Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102:748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 12.Bigger JT., Jr Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. N Engl J Med. 1997;332:1569–75. doi: 10.1056/NEJM199711273372201. [DOI] [PubMed] [Google Scholar]
- 13.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ. Prophylactic use of implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–8. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 14.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr., Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008. 117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim JG, Chen MH, Sinha D. Bayesian Survival Analysis. Springer-Verlag; New York, NY: 2001. [Google Scholar]
- 16.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10:277–303. doi: 10.1177/096228020101000404. [DOI] [PubMed] [Google Scholar]
- 17.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian Analysis. Third Edition. Taylor & Francis group, LLC; Boca Raton, FL: 2014. [Google Scholar]
- 18.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBugs-- a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 19.Huang DT, Sesselberg HW, McNitt S, Noyes K, Andrews ML, Hall WJ, Dick A, Daubert JP, Zareba W, Moss AJ. Improved survival associated with prophylactic implantable defibrillators in elderly patients with prior myocardial infarction and depressed ventricular function: a MADIT-II substudy. J Cardiovasc Electrophysiol. 2007;18:833–8. doi: 10.1111/j.1540-8167.2007.00857.x. [DOI] [PubMed] [Google Scholar]
- 20.Santangeli P, Di Biase L, Russo AD, Casella M, Bartoletti S, Santarelli P, Pelargonio G, Natale A. Meta-analysis: age and effectiveness of prophylactic implantable cardioverterdefibrillators. Ann Intern Med. 2010;153:592–599. doi: 10.7326/0003-4819-153-9-201011020-00009. [DOI] [PubMed] [Google Scholar]
- 21.Kong MH, Al-Khatib SM, Sanders GD, Hasselblad V, Peterson ED. Use of implantable cardioverter-defibrillators for primary prevention in older patients: a systematic literature review and meta-analysis. Cardiol J. 2011;18:503–14. doi: 10.5603/cj.2011.0005. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez AF, Fonarow GC, Hammill BG, Al-Khatib SM, Yancy CW, O'Connor CM, Schulman KA, Peterson ED, Curtis LH. Clinical effectiveness of implantable cardioverter-defibrillators among medicare beneficiaries with heart failure. Circ Heart Fail. 2010;3:7–13. doi: 10.1161/CIRCHEARTFAILURE.109.884395. [DOI] [PubMed] [Google Scholar]
- 23.Yung D, Birnie D, Dorian P, Healey JS, Simpson CS, Crystal E, Krahn AD, Khaykin Y, Cameron D, Chen Z, Lee DS. Survival after implantable cardioverter-defibrillator implantation in the elderly. Circulation. 2013;127:2383–92. doi: 10.1161/CIRCULATIONAHA.113.001442. [DOI] [PubMed] [Google Scholar]
- 24.Brullmann S, Dichtl W, Paoli U, Haegeli L, Schmied C, Steffel J, Brunckhorst C, Hintringer F, Seifert B, Duru F, Wolber T. Comparison of benefit and mortality of implantable cardioverter-defibrillator therapy in patients aged >/=75 years versus those <75 years. Am J Cardiol. 2011;109:712–7. doi: 10.1016/j.amjcard.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Krahn AD, Connolly SJ, Roberts RS, Gent M. Diminishing proportional risk of sudden death with advancing age: implications for prevention of sudden death. Am Heart J. 2004;147:837–40. doi: 10.1016/j.ahj.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Fumagalli S, Valsecchi S, Boriani G, Gasparini M, Landolina M, Lunati M, Padeletti M, Tronconi F, Marchionni N, Padeletti L. Comparison of the usefulness of cardiac resynchronization therapy in three age-groups (<65, 65-74 and >/=75 years) (from the InSync/InSync ICD Italian Registry). Am J Cardiol. 2011;107:1510–6. doi: 10.1016/j.amjcard.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585–96. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 28.Fahy GJ, Sgarbossa EB, Tchou PJ, Pinski SL. Hospital readmission in patients treated with tiered-therapy implantable defibrillators. Circulation. 1996;94:1350–6. doi: 10.1161/01.cir.94.6.1350. [DOI] [PubMed] [Google Scholar]
- 29.Korte T, Jung W, Ostermann G, Wolpert C, Spehl S, Esmailzadeh B, Luderitz B. Hospital readmission after transvenous cardioverter/defibrillator implantation; a single centre study. Eur Heart J. 2000;21:1186–91. doi: 10.1053/euhj.1999.2044. [DOI] [PubMed] [Google Scholar]
- 30.Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, Daubert JP, McNitt S, Andrews ML, Elkin AD. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 31.Goldenberg I, Moss AJ, Hall WJ, McNitt S, Zareba W, Andrews ML, Cannom DS. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113:2810–7. doi: 10.1161/CIRCULATIONAHA.105.577262. [DOI] [PubMed] [Google Scholar]
- 32.Lampert R, McPherson CA, Clancy JF, Caulin-Glaser TL, Rosenfeld LE, Batsford WP. Gender differences in ventricular arrhythmia recurrence in patients with coronary artery disease and implantable cardioverter-defibrillators. J Am Coll Cardiol. 2004;43:2293–9. doi: 10.1016/j.jacc.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–96. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 34.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr., Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death). J Am Coll Cardiol. 2006;48:e247–346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg I, Gillespie J, Moss AJ, Hall WJ, Klein H, McNitt S, Brown MW, Cygankiewicz I, Zareba W. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2010;122:1265–71. doi: 10.1161/CIRCULATIONAHA.110.940148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


