Summary
Standard methods to monitor tuberculosis (TB) treatment response rely on sputum microscopy and culture conversion. Alternatives to these methods are needed for those patients whose sputum tests are smear or culture negative. Here, we examine anti-phospholipid IgM antibody level changes as a biomarker for treatment response in smear positive TB patients. Serum samples were obtained from 40 pulmonary TB patients at the start and end of the intensive phase treatment (IPT) from the CDC-TB Trials Consortium randomized clinical trial in Kampala, Uganda. Samples were screened by ELISA for IgM levels against five phospholipids found in Mycobacterium tuberculosis and host cells. Lipid antigens included cardiolipin (CL), phosphatidyl inositol (PI), phosphatidyl ethanolamine (PE), phosphatidyl choline (PTC), and sphingolipid (SL). Levels of IgM against all phospholipids significantly decreased (p= 0.034, 0.001, 0.008 0.008, 0.040, respectively) following anti-TB drug treatment in patients without lung cavitary disease at baseline. The mean sensitivity of this test in these patients was 83% when the IgM response to a single lipid antigen was used; it was >90% when responses to 2 or more lipids were assessed. In contrast, cavitary TB patients showed an overall IgM increase, with a significant rise against PE (p= 0.025). There was no significant difference in the change in antibody levels between patients who remained culture-positive and those who culture-converted after 40 doses of drug therapy. The measurement of IgM anti-phospholipid antibodies may be a useful biomarker to monitor treatment response in non-cavitary TB patients.
Keywords: Biomarker, IgM, anti-phospholipid, Tuberculosis, Monitoring, B-1 B cell.
1. Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis (MTB) remains a major global health challenge1. Successful TB treatment is critical for preventing further TB transmission to others, minimizing relapse rates, and preventing drug resistance. Therefore, successful treatment requires monitoring response to anti-TB therapy. Current methods to monitor response to treatment include the demonstration of conversion of sputum acid fast bacilli (AFB) smear by microscopy and culture for MTB two months into treatment 2, 3. However, in most TB endemic regions of the world, sputum culture is not routinely performed. The sputum microscopy test is not highly sensitive and is negative in a substantial proportion of baseline sputum samples in new TB patients. Absence of such sputum microscopy and culture results precludes uses of such tests to monitor response to treatment in most TB endemic settings of the world. High TB burden areas require inexpensive, reliable, and rapid tests that do not rely on the detection of tubercle bacilli.
A large portion of the MTB genome is dedicated to the synthesis and catabolism of lipids located in the cell wall. The plasma membrane of MTB is comprised of phospholipids found in other bacteria as well as numerous lipids unique to the genus Mycobacterium; notably, 2-alkys-3-hydroxy fatty acids composed of up to 90 carbons. Other lipid-containing molecules unique to the MTB complex include phenolic glycolipids, poly acyl trehalose and lipooligosaccharides 4. These mycobacterial lipids are distinguished by the immune system in different contexts. Lipoarabinomannan molecules are recognized by Toll-like receptors of the innate immune system. Other lipids such as mycolic acids and glucose monomycolates are presented by CD1 molecules to T lymphocytes 5. Other acidic phospholipids present in the MTB cell wall include cardiolipin (CL), phosphatidylglycerol (PG), phosphatidylinositol (PI) and its mannoside derivatives, in addition to basic phospholipids such as phosphatidylethanolamine (PE) 6. Because some of these lipids have a unique presence in mycobacteria, they may provide a valuable biosignature to be evaluated as potential biomarkers for diagnosis and treatment response 7.
Lipid molecules induce an antibody response by B-1 B cells, which represent about 5% of the entire B cell population 8. B-1 B cells have little or no immunological memory and are located mainly in the pleural and peritoneal cavities. Interestingly, B-1 B cells express high levels of IgM and do not require T cells for proliferation; they self-renew from pre-existing B-1 cells 7–8. Phospholipids are the major antigens that stimulate B-1 B cells 9. Recently, B-1 B cells have been shown to migrate from the peritoneal cavity to the lungs in mice infected with Mycobacterium bovis bacillus Calmette-Guerin (BCG) 10. IgM anti-phospholipid antibodies produced by B-1 B cells have self and poly-reactive properties11,13–14. This characteristic contributes to their rapid clearance from the bloodstream. Thus, the IgM antibodies produced by B-1 B cells contribute to the innate immunity of the host and their induction requires continued stimulation by lipid antigens generated by replicating mycobacteria during TB as well as host cell turn-over.
We thus propose that the B-1 B cell-produced IgM antibody response to lipids may serve as a biomarker for monitoring TB treatment. We hypothesize that a decrease over 2 months of serum IgM antibody level to MTB phospholipids may serve as a marker of favorable response to treatment and end-point assessment in clinical trials of new anti-TB drug regimens.
2. Materials and methods
2.1 Patient specimens
Serum samples were obtained at baseline (before initiation of drug therapy) and at the end of the intensive phase of treatment (IPT) from 40 HIV-negative patients with acid-fast bacilli (AFB) smear and culture-confirmed pulmonary tuberculosis (PTB). These patients were enrolled in a study of the Center for Disease Control and Prevention –Tuberculosis Trials Consortium (CDC-TBTC) randomized clinical trial conducted in Kampala, Uganda. The patient cohort was equally composed of two groups: 19 culture-positive patients (slow responders) and 21 culture-negative patients (fast responders) at the end of 40 doses of IPT anti-TB combination, which corresponds to eight weeks of treatment (5 doses per week). Patients were further categorized according to disease severity, based on the extent of findings on pre-treatment chest radiographs (limited, moderate, and extensive based on a validated grading scheme; and whether or not cavitary lesions were present: cavity or no cavity12). Serum samples were screened for levels of IgM antibodies against five phospholipids extracted from bovine sources available commercially (Avanti Polar Lipids, Alabama, USA).
2.2 Enzyme-linked immunosorbent assay (ELISA)
The phospholipid antigens included cardiolipin (CL), phosphatidylinositol (PI), phosphatidylethanolamine (PE), phosphatidylcholine (PTC), and sphingolipid (SL). Lipids were diluted to 10mg/ml in ethanol and 50 µl of the solutions were dried overnight in flat bottom well polystyrene ELISA plates (Fisher Scientific, USA). ELISA plates were blocked with 100 µl of 3% low fatty acid bovine serum albumin (BSA) (USBiologicals, USA) and washed with phosphate buffered saline (PBS) pH 7.4. Frozen serum samples were thawed twice and diluted 1:100 in 3% low fatty acid BSA. The diluted sample was added to the plate and incubated for one hour at room temperature (RT), followed by three washes with PBS. Then, 100 µl of 1:5,000 goat-derived anti-human IgM labeled with horse radish peroxidase (HRP) (Thermo Scientific, Ill) diluted in PBS pH 7.4 was added, followed by incubation at RT for 1 hr and washed again with PBS. Finally, 100 µl of tetramethylbenzidine substrate (TMB) (Thermo Scientific Pierce, Illinois, USA) was added and the reaction was stopped immediately with 50 µl of sulfuric acid 1M.
Reactions were read within ten minutes at 450 nm in a spectrophotometer (Cambridge Technologies, Massachusetts). The results were read out as the average of optical densities (O.D.) of triplicate assays. Standards curves for our in-house ELISA were prepared with polyclonal human IgM (Thermo Scientific Pierce, Illinois, USA), which yielded a correlation coefficient R2 of 0.99. The concentration of IgM in serum samples (in ng/ml) was calculated based on this standard curve. High, medium, and low IgM level control samples were included in each assay. Assays with control sample results outside two standard deviations were discarded and repeated.
2.3 Statistical analyses
All statistical analyses were conducted with STATA (Version 11, STATA Corp., Texas). The two-sample Wilcoxon rank-sum test was used to compare lipid-specific IgM concentration differences between fast and slow responders (at baseline and at the end of IPT). We performed a step-wise procedure to test for the following variables suspected to be biologically relevant to IgM antibody response during treatment: age, gender, body mass index (BMI), bilateral abnormalities, AFB smear status at baseline, cavitary disease, and disease severity. Variables were added to the basic model in a stepwise fashion. Variables were eliminated if they failed to achieve significance at the 5% level from the full model to obtain the best fit and simplest model that included cavity classification and disease severity as significant variables. The Wilcoxon signed-rank nonparametric test was used to compare lipid-specific antibody differences between groups for cavity and disease severity variables.
The Committee for Protection of Human Subjects at UC Berkeley (UCB#2010-06-1752) approved this sub-study. The protocol for the parent clinical trial (conducted by the TB Trials Consortium sponsored by the U.S. Centers for Disease Control and Prevention; ClinicalTrials.gov number, NCT00694629) was approved by the Center for Disease Control and Prevention IRB and the local IRBs at all participating sites. All patients gave informed consent for participation.
3. Results
The CDC-TBTC sample set was composed of 40 HIV-negative patients (29 males and 11 females) with smear and culture-confirmed pulmonary TB. The baseline characteristics of the study subjects are shown in Table 1. The average age was 29 years (range 19 to 53 years of age). Seventy-three percent of these patients were non-smokers, non-alcoholics, and non-drug users (Table 1). Patients with negative culture after 2 months of treatment showed a higher pretreatment BMI. On chest radiographs, two patients were diagnosed to have limited disease, 22 had moderate disease, and 16 had extensive disease. Twenty-three (57%) had cavitary lesions. Two patients were diagnosed with MDR-TB at the third month of anti-TB treatment (Table 3).
Table 1.
Demographic and clinical characteristics of TB patients who responded early (sputum culture negative at 8 weeks of treatment) and late (sputum culture positive at 8 weeks).
| Culture negative at 8 weeks (n = 21) |
Culture positive at 8 weeks (n = 19) |
p value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age; median (IQR) | 26.0 (6.5) | 30.0 (4.0) | 0.007 |
| Male gender | 13 (62%) | 16 (84%) | 0.085 |
| Social characteristics | |||
| Smoking history | 4 (19%) | 5 (25%) | 0.255 |
| Past alcohol use | 0 (0%) | 1 (5%) | 0.475 |
| Past drug use (injection) | 0 (0%) | 0 (0%) | 1 |
| Past drug use (non-injection) | 0 (0%) | 1 (5%) | 0.475 |
| Clinical characteristics | |||
| BMI; median (IQR) | 19.8 (2.7) | 18.2 (2.8) | 0.192 |
| HIV infected | 0 (0%) | 0 (0%) | 1 |
| Any co-morbid condition | 1 (5%) | 0 (0%) | 0.525 |
| Presence of cavity on chest x-ray | |||
| Any cavity | 12 (57%) | 11 (58%) | 0.250 |
| Bilateral cavitation | 1 (5%) | 2 (11%) | 0.364 |
| Cavity classification on chest x-ray | |||
| Absent | 9 (43%) | 8 (42%) | 0.250 |
| Present < 4cm | 5 (24%) | 4 (21%) | 0.289 |
| Present ≥ 4cm | 7 (33%) | 7 (37%) | 0.253 |
| Extent of Chest x-ray involvement | |||
| Limited | 0 (0%) | 2 (11%) | 0.219 |
| Moderate | 15 (71%) | 7 (37%) | 0.024 |
| Extensive | 6 (29%) | 10 (52%) | 0.080 |
| Any bilateral abnormalities | 9 (43%) | 12 (63%) | 0.113 |
| AFB smear | |||
| 2+ | 5 (24%) | 0 (0%) | 0.031 |
| 3+ | 6 (29%) | 6 (32%) | 0.264 |
| 4+ | 10 (47%) | 13 (68%) | 0.108 |
| Days to detection; median (IQR) | 5.84 (2.54) | 5.98 (2.60) | 0.538 |
| End of treatment (EOT) assessment at 6 months | |||
| Cured | 21 (100%) | 17 (90%)a | 0.219 |
| Decrease in IgM response tob | |||
| CL | −16.5% | 8.2% | 0.123 |
| PE | 2.5% | 9.7% | 0.419 |
| PI | −2.5% | 3.9% | 0.782 |
| PTC | −11.3% | −3.6% | 0.860 |
| SL | −10.1% | 1.4% | 0.557 |
Two patients who had multidrug-resistant TB were placed on a 2-year treatment course. The sputum of one became culture-negative after 6 months of retreatment, while that of the other was negative throughout the entire retreatment. Both had non-cavitary disease.
Decrease in IgM antibody levels are expressed as percent change. Negative values indicate decrease.
The IgM anti-phospholipid antibody response was highly variable among these patients. A global analysis of our sample set showed heterogeneous results for all five anti-phospholipid IgM levels, regardless of culture conversion status (Table 1). A comparison of the decrease in IgM antibody levels between TB patients who remained culture positive and those who culture converted after 40 doses of IPT, revealed no significant differences using a two-sample t- test (Table 1).
The multi-variable model included age, gender, body mass index, bilateral abnormalities, smear status at baseline, cavity disease and extent of chest disease on radiographs. This model revealed no contribution of the variables to changes in antibody levels upon treatment completion. Thus, we opted to use a simpler model using “cavity classification” as a proxy for disease severity and adjusted for “age”. The “cavity classification” variable showed a uniform distribution across three categories, including no cavity (n= 17), small cavity (n= 9), and large cavity (n= 14). This simpler model allowed us to study the IgM anti-phospholipid antibodies as a biomarker for TB treatment response, despite the variability of the antibody levels within our sample set.
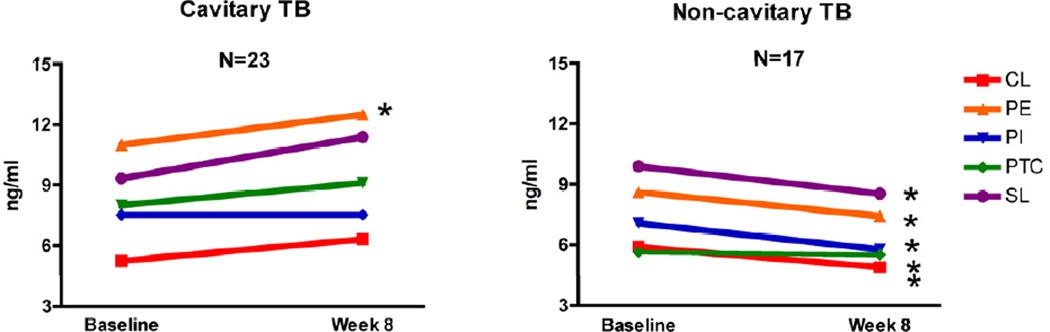
Analysis of this simpler model consistently revealed IgM anti-phospholipids antibodies to decrease among TB patients with no lung cavities. Specifically, the mean IgM concentration decreased significantly against four of the five phospholipids, including PE, PI, PTC and SL (paired t-student p= 0.012, 0.012, 0.022, and 0.043, respectively). The distribution of delta change among all patients was scattered due to the presence of outliers in the cavity classification categories (data not shown). The median values of antibody change were analyzed with a two-sample Wilcoxon rank-sum test. This revealed a significant decrease for all five lipids, CL, PI, PE, PTC, and SL in non-cavitary TB patients (Wilcoxon rank-sum test p= 0.034, 0.008, 0.001, 0.008, 0.040 respectively, see figure 1). The antibody level reduction was different for each lipid. Specifically, we observed a 22.4%, 8.2%, 16.5%, 22.6% and 17.5% serum concentration reduction of IgM anti-CL, PE, PI, PTC and SL, respectively (data not shown). For cavitary TB, IgM antibody levels showed increase in most patients. A significant increase was observed only against PE (p= 0.025, 95% C.I.−2.287, −0.113).
Figure 1.
Plot of the median change in IgM anti-phospholipid antibody levels after 40 doses of anti-TB drug therapy. Antibody levels were determined by ELISA; results are shown as concentrations (ng/ml). The panels compare the antibody level decrease in cavitary TB patients vs. non-cavitary TB patients for each of the five phospholipids including, cardiolipin (CL); phosphatidyl ethanolamine (PE); phosphatidyl Inositol (PI); phosphatidyl choline (PTC); sphingolipid (SL).
We evaluated our biomarker test’s ability to identify successful treatment response in non-cavitary TB patients. We defined a positive biomarker test as a decrease in IgM anti-phospholipid antibodies and a negative test as an increase or no change in IgM anti-phospholipid antibodies. The outcome was successful treatment response. We excluded the MDR-TB cases from this analysis. The sensitivity values were 93.3%, 66.7%, 80.0%, 93.3% and 80.0% for each anti-CL, PE, PI, PTC and SL IgM, respectively, with an overall sensitivity of 82.6% (Table 2). The sensitivity was 93.3% in non-cavitary disease patients when the decrease in IgM response was assessed against 2 or more of the phospholipids (CL and PTC, or CL, PTC, and PI).
Table 2.
Sensitivity of anti-lipid IgM level decrease after 8 weeks of treatment among non-cavitary TB patientsa as a marker of favorable treatment response.
| Decrease in IgM |
Sensitivity % |
|
|---|---|---|
| CL | 14/15 | 93.3 |
| PE | 10/15 | 66.7 |
| PI | 12/15 | 80.0 |
| PTC | 14/15 | 93.3 |
| SL | 12/15 | 80.0 |
Number of non-cavitary TB patients who showed a decrease in IgM anti-phospholipid antibody levels. Values for sensitivity were determined for each lipid by contingency table analysis. Multidrug resistant TB patients were excluded. Cardiolipin (CL); phosphatidyl ethanolamine (PE); phosphatidyl Inositol (PI); phosphatidyl choline (PTC); sphingolipid (SL).
4. Discussion
The standard method to monitor response to TB treatment includes demonstration of sputum smear microscopy and culture conversion after 2 months of IPT. Of course, this requires the first sputum sample to be smear or culture test positive. In most TB endemic regions of the world, sputum culture is not done. Sputum microscopy for AFB has low sensitivity. In this study, after 8 weeks of treatment, sputum of many patients tested AFB positive and 19 patients tested culture positive (Table 1). Among those whose sputum converted at 8 weeks, all were cured at the end of treatment (EOT), while among those who did not convert, 90% were cured at the EOT, indicating poor correlation of these tests to monitor treatment response (Table 1). Thus, for patients in whom such sputum sample conversion information is not available, alternative ways to monitor treatment response are needed. We show here that changes in serum IgM anti-phospholipid antibodies appear to be a useful marker to monitor treatment response in TB patients, especially in those with non-cavitary disease. This IgM decrease may reflect decrease in bacterial burden and healing of lung lesions in these patients.
In contrast, in patients with cavitary disease, IgM levels tended to show an increase, although the significant increase was observed only against one of the phospholipids (phosphatidylethanolamine) after 40 doses of IPT. This increase may reflect liquefied caseum containing high levels of MTB bacilli and their lipid debris, as well as host cells’ phospholipids released during the inflammatory process 13, 14. This increase might be transient during treatment, but additional studies assessing antibody levels at later time points are needed to support this suggestion.
The pathophysiology of cavity formation and healing during TB is not completely understood 15. Evidence from human cavities suggests that Th2 cytokines, such as IL-4 and TNFα, may play a role in cavity formation 16. These Th2 cytokines may antagonize host defense and cause tissue necrosis in TB patients’ lungs 17. Early work by Yamamura et al. showed that a lipid-protein mixture from heat-killed bacilli sensitized and elicited cavities in white male rabbits 18. Interestingly, this study demonstrated that mycobacterial proteins alone were able to produce granulomas, but not cavities. More recently, it has been shown that mycobacterial lipids act as adjuvants. Mycobacterial proteins combined with synthetic adjuvants were able to elicit cavity formation 19. Once the cavity breaks open and communication with the bronchial tree is established, more oxygen enters allowing the bacilli to multiply further inside the cavity 20. Routine cultures have measured 107– 109 bacilli within the liquefied material of a single cavity.
We hypothesize that IgM-secreting B-1 B cells are activated upon treatment by the transiently enhanced inflammation induced by dying MTB in cavitary lesions. Necrotic cells accumulate lipids in the caseum. Lipids from both dead host cells and from MTB in the released caseous material contain sufficient levels of antigens to activate the B-1 B cells and induce IgM anti-phospholipid antibody production. This may explain the increase in IgM anti-phospholipid antibody levels we observed in treated patients with cavitary disease in this study. Indeed, lungs of BCG infected BALB/c mice have shown a significant increase in B-1 B cells at 40 days post infection10. Together, host lipids, mycobacterial lipids, and IL-5 secreted by granulocytes generate the signaling required for full activation of B-1 B cells. Therefore, the increase in IgM anti-phospholipid antibodies observed in cavitary TB patients on IPT might be a reflection of T cell-independent B-1 B cell activation upon cavity liquefaction. Treatment of those with cavitary disease may transiently cause an increase in lipids released from a large burden of dead MTB.
The percentage of TB patients with cavitary disease at initial diagnosis varies by geographical region. Countries with low and mid TB incidence such as Saudi Arabia, Denmark, and Brazil report cavities on the initial chest X-ray in 14%, 42%, and 36% of TB patients, respectively21–23. In contrast, Nigeria, a country with a high prevalence of TB, has reported the presence of cavities in up to 46% of cases 31. Our biomarker IgM anti-phospholipid antibody test will be useful to monitor the treatment of 64–86% of the patients suffering from TB, depending on the cavitary TB prevalence in the region.
The change in IgM anti-phospholipid antibody levels may also serve as a valuable test to monitor TB treatment in HIV-positive patients. As mentioned previously, B-1 B cells do not require T cells for activation. Therefore CD4 and CD8 cell counts will not affect the IgM anti-phospholipid antibody levels produced in patients suffering from HIV. In addition, HIV-positive patients generally do not develop cavitary TB because of a weakened cell-mediated immune response. The IgM anti-phospholipid antibodies in TB-HIV infected patients could be useful in monitoring disease severity.
The proportions of non-cavitary TB patients that showed a decrease in IgM response were 93.3%, 66.7%, 80.0%, 93.3% and 80.0% for IgM anti-CL, PE, PI, PTC and SL, respectively (Table 2). This study shows sensitivity of the IgM ELISA results for favorable initial response but does not show specificity of the response. At eight weeks of treatment, the sensitivity values were all higher than the reported 60–80% sensitivity of the 2-month sputum culture conversion method currently in use to monitor treatment response 24–27. Since a large proportion of sputum cultures from newly diagnosed patients are negative before treatment, the sensitivity of the traditional monitoring test may be actually even lower. The anti-phospholipid IgM ELISA does not require sputum culture results.
This study is limited by the fact that nearly all patients were cured at EOT. Two patients were diagnosed with multidrug-resistant TB (MDR-TB) three months after treatment initiation; both had non-cavitary disease. Drug susceptibility tests showed that patient A had resistance to isoniazid (INH) and rifampicin (RIF), whereas patient B was resistant to INH and streptomycin (STM). After two months of IPT with ethambutol (EMB), pyrazinamide (PZA), INH, RIF or rifapentine (RPT), patient A still had a positive culture with 100–200 CFU in solid media. In contrast, patient B showed negative culture results twice in solid media and a positive result once in liquid media. The IgM anti-phospholipid antibody levels increased in patient A while patient B showed a decrease over the IPT period. These observations support the hypothesis that IgM anti-phospholipids antibody levels reflect the bacterial burden within the lungs of TB patients even though the patients remained culture-positive at the end of IPT. They also suggest, however, that the test needs further refinement for monitoring MDR-TB treatment.
This new test needs to be conducted with a larger sample size that includes patients who do not respond to treatment and also have MDR-TB. If this test can be shown to also have high specificity, it can serve as a low-cost method to monitor response to treatment. The bovine phospholipids used in this study were all readily commercially available and can be used in low concentrations. In most countries, chest x-rays are routinely performed as part of TB diagnostic work up. Thus, the combination of chest x-ray and anti-lipid IgM response results could serve an inexpensive and sensitive approach to monitor response to TB treatment.
Acknowledgements
This project was partly funded by NIH grant R01AI73204-2. Amador Goodridge was funded by the Doctoral Fellowship from SENACYT-Panama 2005–2010. We thank Tianyi Zhang for contributing to ELISA assay performance evaluation as well as Colleen Lynch, Laura Flores, Richard Novak, and Olivera Marjanovic for critical review of the manuscript and valuable suggestions.
Ethical approval:
This study was approved by the Committee for Protection of Human Subjects at UC Berkeley (UCB#2010-06-1752). The protocol for the parent clinical trial (conducted by the TB Trials Consortium sponsored by the U.S. Centers for Disease Control and Prevention; ClinicalTrials.gov number, NCT00694629) was approved by the Center for Disease Control and Prevention IRB and the local IRBs at all participating sites.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors declare no conflicts of interest for this publication.
Contributor Information
Amador Goodridge, Email: agoodridge@indicasat.org.pa.
Carla Cueva, Email: agoodridge@cal.berkeley.edu.
Maureen Lahiff, Email: lahiff@berkeley.edu.
Grace Muzanye, Email: gxm62@case.edu.
John L. Johnson, Email: jlj@case.edu.
Payam Nahid, Email: pnahid@ucsf.edu.
References
- 1.World Health Organization. Global tuberculosis control: a short update to the 2009 report. Geneva: 2009. [Google Scholar]
- 2.CDC. MMWR, editor. Controlling Tuberculosis in United States: Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. Recommendations and reports: CDC. 2005:1–81. [PubMed] [Google Scholar]
- 3.WHO. Treatment of Tuberculosis: Guidelines. Geneve, Switzerland: WHO Press; 2010. [Google Scholar]
- 4.Brennan PJ, Nikaido H. The envelope of mycobacteria. Annual review of biochemistry. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 5.Lang ML, Glatman-Freedman A. Do CD1-restricted T cells contribute to antibody-mediated immunity against Mycobacterium tuberculosis? Infection and immunity. 2006;74:803–809. doi: 10.1128/IAI.74.2.803-809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan PJ, Nikaido H. The envelope of mycobacteria. Annual Review of Biochemistry. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen M, Mattow J, Repsilber D, Kaufmann SH. Novel strategies to identify biomarkers in tuberculosis. Biological chemistry. 2008;389:487–495. doi: 10.1515/bc.2008.053. [DOI] [PubMed] [Google Scholar]
- 8.Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 9.Hardy RR. B-1 B cell development. Journal of immunology (Baltimore, Md: 1950) 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 10.Russo RT, Mariano M. B-1 cell protective role in murine primary Mycobacterium bovis bacillus Calmette-Guerin infection. Immunobiology. 2010;215:1005–1014. doi: 10.1016/j.imbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 12.Falk AOCJ, Pratt PC, Webb WR, Weir JA, Wolinsky E. Chapter 6. Classification of pulmonary Tuberculosis. Diagnostic standards and classification of tuberculosis. 1969:68–76. [Google Scholar]
- 13.Converse PJ, Dannenberg AM, Estep JE, Sugisaki K, Abe Y, Schofield BH, Pitt ML. Cavitary tuberculosis produced in rabbits by aerosolized virulent tubercle bacilli. Infection and immunity. 1996;64:4776–4787. doi: 10.1128/iai.64.11.4776-4787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dannenberg AM. Liquefaction and cavity formation in pulmonary TB: a simple method in rabbit skin to test inhibitors. Tuberculosis (Edinburgh, Scotland) 2009;89:243–247. doi: 10.1016/j.tube.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clinical microbiology reviews. 2008;21:305–333. doi: 10.1128/CMR.00060-07. , table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somoskovi A, Zissel G, Zipfel PF, Ziegenhagen MW, Klaucke J, Haas H, Schlaak M, Muller- Quernheim J. Different cytokine patterns correlate with the extension of disease in pulmonary tuberculosis. Eur Cytokine Netw. 1999;10:135–142. [PubMed] [Google Scholar]
- 17.van Crevel R, Karyadi E, Preyers F, Leenders M, Kullberg B-J, Nelwan RHH. van der Meer JWM. Increased Production of Interleukin 4 by CD4+ and CD8+ T Cells from Patients with Tuberculosis Is Related to the Presence of Pulmonary Cavities. Journal of Infectious Diseases. 2000;181:1194–1197. doi: 10.1086/315325. [DOI] [PubMed] [Google Scholar]
- 18.Yamamura Y, Maeda H, Ogawa Y, Hashimoto T. Experimental pulmonary cavity formation by mycobacterial components and synthetic adjuvants. Microbiol Immunol. 1986;30:1175–1187. doi: 10.1111/j.1348-0421.1986.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 19.Helke KL, Mankowski JL, Manabe YC. Animal models of cavitation in pulmonary tuberculosis. Tuberculosis (Edinburgh, Scotland) 2006;86:337–348. doi: 10.1016/j.tube.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Palaci M, Dietze R, Hadad DJ, Ribeiro FKC, Peres RL, Vinhas SA, Maciel ELN, do Valle Dettoni V, Horter L, Boom WH, Johnson JL, Eisenach KD. Cavitary Disease and Quantitative Sputum Bacillary Load in Pulmonary Tuberculosis. J Clin Microbiol. 2007 doi: 10.1128/JCM.01780-07. JCM.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes M, Saad R, Junior, Stirbulov R. Pulmonary tuberculosis: relationship between sputum bacilloscopy and radiological lesions. Rev Inst Med Trop Sao Paulo. 2003;45:275–281. doi: 10.1590/s0036-46652003000500007. [DOI] [PubMed] [Google Scholar]
- 22.Wilcke JT, Askgaard DS, Nybo Jensen B, Dossing M. Radiographic spectrum of adult pulmonary tuberculosis in a developed country. Respir Med. 1998;92:493–497. doi: 10.1016/s0954-6111(98)90297-9. [DOI] [PubMed] [Google Scholar]
- 23.Nyman RS, Brismar J, Hugosson C, Larsson SG, Lundstedt C. Imaging of tuberculosis--experience from 503 patients. I. Tuberculosis of the chest. Acta Radiol. 1996;37:482–488. doi: 10.1177/02841851960373P212. [DOI] [PubMed] [Google Scholar]
- 24.Uzundag Iseri A, Dulkar G, Selcuk Sonmez O, Yilmaz Aydin L, Yilmaz B. Factors that effect sputum culture conversion rate in hospitalized patients with pulmonary tuberculosis who were applied directly observation therapy and non-directly observation therapy. Tuberk Toraks. 2010;58:44–52. [PubMed] [Google Scholar]
- 25.Salihu HM, Aliyu MH, Ratard R, Pierre-Louis BJ. Characteristics associated with reported sputum culture conversion in the era of re-emergent Mycobacterium tuberculosis in the State of North Carolina, 1993–1998. Int J Tuberc Lung Dis. 2003;7:1070–1076. [PubMed] [Google Scholar]
- 26.Alguire PC. Internal medicine essentials for clerkship students 2. ACP Press; 2008. Medicine CDiI. [Google Scholar]
- 27.Liu Z, Shilkret KL, Ellis HM. Predictors of sputum culture conversion among patients with tuberculosis in the era of tuberculosis resurgence. Arch Intern Med. 1999;159:1110–1116. doi: 10.1001/archinte.159.10.1110. [DOI] [PubMed] [Google Scholar]