Abstract
Chemosensitizers can improve the therapeutic index of chemotherapy and overcome treatment resistance. Successful translation of chemosensitizers depends on the development of strategies that can preferentially deliver chemosensitizers to tumors while avoiding normal tissue. We hypothesized that nanoparticle (NP) formulation of chemosensitizers can improve their delivery to tumors which can in turn improve their therapeutic index. To demonstrate the proof of principle of this approach, we engineered NP formulations of two chemosensitizers, the PI3-kindase inhibitor wortmanin (Wtmn) and the PARP inhibitor olaparib. NP Wtmn and NP olaparib were evaluated as chemosensitizers using lung cancer cells and breast cancer cells respectively. We found Wtmn to be an efficient chemosensitizer in all tested lung-cancer cell lines reducing tumor cell growth between 20 and 60% compared to drug alone. NP formulation did not decrease its efficacy in vitro. Olaparib showed less consistent chemosensitization as a free drug or in NP formulation. NP Wtmn was further evaluated as a chemosensitizer using mouse models of lung cancer. We found that NP Wtmn is an effective chemosensitizer and more effective than free Wtmn showing a 32% reduction in tumor growth compared to free Wtmn when given with etoposide. Importantly, NP Wtmn was able to sensitize the multi-drug resistant H69AR cells to etoposide. Additionally, the combination of NP Wtmn and etoposide chemotherapy did not significantly increase toxicity. The present study demonstrates the proof of principle of using NP formulation of chemosensitizing drugs to improve the therapeutic index of chemotherapy.
Introduction
Chemotherapy is a key component of cancer treatment, and improvement in its therapeutic index can directly translate into increase in survival in cancer patients.1 Because of its importance, there has been long standing interest in the development of novel approaches to improve the therapeutic index of chemotherapy. One strategy is to utilize chemosensitizers, agents that can sensitize tumor cells to chemotherapy, in conjunction with chemotherapy regimens. Although some chemosensitizers only enhance chemotherapy effects in cancer cells, such as poly (ADP ribose) polymerase (PARP) inhibitors in BRCA deficient cancers, other chemosensitizers, such as wortmannin (Wtmn) and 2-morphlin-4-yl-8-phenylhomen-4-one LY294002, affect both tumor cells as well as cells comprising normal tissues.2,3 The sensitization of normal tissues to chemotherapy results in higher treatment toxicity which limits the overall therapeutic index of chemotherapy. Thus, few chemosensitizers have been translated and evaluated clinically. Successful translation of chemosensitizers depends on the development of strategies that can preferentially deliver chemosensitizers to tumors while largely avoiding normal tissue. While this has not been possible with traditional drug delivery techniques, the development of nanoparticle (NP) drug delivery vehicles offers a unique opportunity.
NP therapeutic carriers possess several important characteristics that are well-suited for the delivery of chemosensitizers. First, NPs preferentially accumulate in tumors through the enhanced permeability and retention (EPR) effect, leading to high intratumoral drug concentrations.4,5 A significant increase in therapeutic efficacy can also lead to reductions in chemotherapy doses, which in turn would reduce treatment toxicity. Second, NPs have reduced permeability to normal vasculature and capillaries, thus leading to lower drug dose to normal tissues such as skin, lung, and heart when compared to their small molecule counterparts.6 The advantages of NP biodistribution are illustrated in Fig. 1. Third, many NP platforms allow slow and controlled drug delivery. Such prolonged release can increase the synergistic effects between chemosensitizers and chemotherapy. Because of such unique properties we hypothesized that NP delivery of chemosensitizers can improve the therapeutic efficacy of chemotherapy without increasing its toxicity.
Fig. 1.
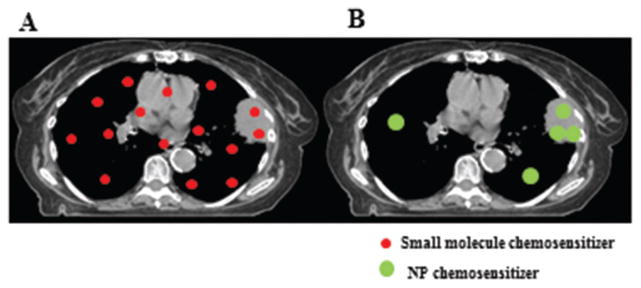
Lung cancer treatment with small molecule chemosensitizers (A) or NP chemosensitizers (B). With small molecule chemosensitizers, there is more normal lung that receives both chemotherapy and chemosensitizer. In contrast, NP chemosensitizers preferrentially accumulate in tumors, which can improve chemosensitizer concentration in tumor while reducing dose to normal lung.
To explore our hypothesis and to demonstrate the proof of principle of using NP to deliver chemosensitizers, we utilized the chemosensitizers olaparib and Wtmn as model drugs. Olaparib is a PARP inhibitor and has been shown to sensitize breast cancer cells to chemotherapy.7 Wtmn is a potent inhibitor of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3 K) and related proteins and is known to sensitize different types of tumor cells to treatment.8–10 In this study, we evaluated NP formulations of olaparib and Wtmn as chemosensitizers using breast cancer and lung cancer (both small cell and non-small cell lung cancer) as tumor models. We compared the therapeutic effects of several chemotherapeutics with and without NP chemosensitizers. We also examined the toxicity profile of chemotherapy treatment with and without NP Wtmn.
Experimental
Materials
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-carboxy (polyethylene glycol) 2000 (DSPE–PEG2000–COOH) and the cross-linkable lipid (1-palmitoyl-2-(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine) (PTPC) were obtained from Avanti Polar Lipids (Alabaster, AL). Docetaxel (Dtxl), wortmannin (Wtmn), etoposide and gemcitabine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). Olaparib was purchased from Selleckchem (Houston, TX). PLGA (poly(D,L-lactide-co-glycolide)) with a 50 : 50 monomer ratio, ester terminated, and viscosity of 0.72–0.92 dl g−1 was purchased from Durect Corporation (Pelham, AL). Soybean lecithin consisting of 90–95% phosphatidylcholine was obtained from MP Biomedicals (Solon, OH). 200 Proof Ethanol (Molecular Biology Grade) and Acetonitrile (HPLC Grade) were purchased from Fisher Scientific (Hampton, NH). Dulbeco’s Phosphate Buffer Saline (PBS, 1X) was purchased from Gibco by Life Technologies (Carlsbad, CA). H460, H23 and H69AR cell lines were purchased from UNC’s Lineberger tissue culture facility.
Formulation and characterization of NP Wtmn and NP olaparib
NP Wtmn and NP olaparib were formulated using a previously described method. Briefly, 1.0 mg mL−1 of Lecithin and DSPE-PEG-COOH were separately dissolved in 4% ethanol and mixed in the molar ratio of 7 : 3 (aqueous solution). The mixture was heated at 55.0 °C for about 15 minutes with continuous stirring. Separately, PLGA (10 mg mL−1) and Wtmn (1.0 mg mL−1) were dissolved in acetonitrile while olaparib (3.0 mg mL−1) was dissolved in acetone. For the preparation of NP Wtmn, 100 μL of PLGA and 100 μL Wtmn solutions were mixed together and added dropwise (~1 mL min−1) to the heated aqueous solution. For the preparation of NP Olaparib, 100 μL of PLGA and 100 μL of Olaparib solutions were mixed in 800 μL of acetonitrile and added dropwise (~1 mL min−1) to the heated aqueous solution. Immediately following the addition of the organic solution, the mixture was vortexed for 3 minutes. The NPs were allowed to self-assemble for 1 hour with continuous stirring followed by washing the solution twice using Amicon Ultra-4 (30 kDa) and resuspended in PBS to obtain 1 mg mL−1 of NP concentration.
Drug loading determination
For the determination of percent drug loading, 30 μL NPs samples containing Wtmn or olaparib were collected and mixed thoroughly with 120 μL acetonitrile and left overnight to disrupt the NPs. Each mixture was subjected to HPLC. Drug concentration was determined using standard curves.
In vitro cytotoxicity
In a 96-well plate, 1 × 104 H460, H23 or H69AR cells were plated 24 hours prior to treatment with different concentrations of drugs (Etoposide (200 nM), Gemcitabine (2.0 nM) or Docetaxel (5.0 nM)). Fresh media was added prior to the drug treatment and incubated with the drugs for 24 hours. Chemosensitizer (free Wtmn or NP Wtmn) was added to the H460, H23 (5.0 μM) or H69AR (10 μM) cells without washing. Similarly, 5.0 μM of olaparib (free or NP) was added to Hs578 T or HCC38 cells following the 24 hour drug treatments. The cells were further incubated with the two therapeutics for 6.0 hours, bringing the total drug treatment to 30 hours. The cells were subsequently washed with PBS and allowed to grow for another 24 hours. Cell viability was analyzed using an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. Briefly, the cells were washed with PBS, followed by the addition of a 120 μL mixture of their respective culture medium containing 20% MTS reagent (Promega) and 1% phenazine methosulfate (PMS) as the electron coupling reagent (Promega), directly to culture wells. H460 cells were incubated for about 30 minutes, H23 cells were incubated for about 45 minutes and H69AR cells were incubated for about 2 hours, after which the plates were read at the absorbance value of 490 nm using a 96-well plate reader (BioTek, Synergy 2). The difference in the timing is reflective of the cellular activity of the different cell lines.
In vivo tumor efficacy
H69AR cells (1 × 106) in 200 μL 1 : 1 serum free RPMI-1640 and matrigel were subcutaneously inoculated into right flank of about 8 week-old male nude (nu/nu) mice. Ten days after inoculation, the mice were randomly distributed into different groups for subsequent treatment. Mice (n = 6–7 per group) were administered either saline, free or NP Wtmn (100 μg kg−1), free Etop (20 mg kg−1), or simultaneous injection of free Wtmn and Etoposide (100 μg kg−1 Wtmn, 20 mg kg−1 etoposide) or NP Wtmn and Etoposide (100 μg kg−1 Wtmn, 20 mg kg−1 etoposide) dosed via a tail vein injections. The tumor volumes were measured every two or three days and relative change in tumor volume was calculated using the relation V/Vo, where V is the volume calculated and Vo is the initial volume on day 0 (ten days after the inoculation).
Hepatotoxicity study
Free or NP Wtmn was injected i.v. via tail vein at a dose of 100 μg kg−1 into nude mice (n = 5 per group) with or without 10 mg kg−1 of Etoposide. Blood was collected from the mice 24 hours post-injection via submandibular bleed. The blood samples were centrifuged at 3000 rpm for 10 minutes to separate the plasma. The plasma was then submitted to the Animal Clinical Laboratory Core Facility at UNC School of Medicine for analysis of AST and ALT levels, which analyzed the samples using an automated chemical analyzer (VT 350, Ortho Clinical Diagnostics, Rochester, NY). Paired t-test was performed for statistical analysis.
Western-Blot
H460 cells were seeded overnight and then treated with chemotherapeutics (etoposide (200 nM), gemcitabine (2 nM), or docetaxel (5 nM) with saline, free, or NP Wtmn (10 μM) for six hours. Cell lysates were then collected in RIPA (25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) buffer. Protein concentration was measured by bicinchonic acid protein assay (Pierce). Primary antibodies were p-AKT (Ser473) (Cell Signaling), p-DNA PK (Ser2056) (Abcam), p-mTOR (Ser2448) (Cell Signaling), and β-actin (13E5) (Cell Signaling) at dilutions indicated by manufactures. Secondary antibodies were α-mouse IgE HRP-linked antibody (Cell Signaling) or α-rabbit IgE HRP-linked antibody (Cell Signaling).
Statistical analysis
To statistically compare the cell survival results for the in vitro toxicity studies we performed two-way ANOVAs using variables of chemotherapy (saline, docetaxel, gemcitabine, or etoposide) and chemosensitizers (saline, free or NP Wtmn). Post-hoc analyses were then performed using Tukey’s test to determine significant differences between groups when significant interactions were found on ANOVAs. Multiplicity adjusted P values are reported. ANOVA and Tukey’s tests were performed using Prism Graphpad software, version 6.05, La Jolla, CA.
For in vivo studies, AUC was calculated for each cohort. We then statistically compared tumor growth curves with Wilcoxon rank-sum test (using Van der Waerden normal scores). Two-sided P values are reported. These analyses were performed using R statistical software, version 3.1.1.
Results and discussion
NP Wtmn is an effective chemosensitizer in vitro
Wtmn- and olaparib-encapsulated PLGA nanoparticles of about 35 nm diameter were prepared via nanoprecipitation.11 The lipid-coated PLGA nanoparticles contain 2.0 and 1.3 wt/wt% of encapsulated Wtmn and olaparib, as determined by quantitative HPLC. Fig. 2 shows representative TEM images for both NP formulations. Encapsulation efficiency was 20% for Wtmn and 3% for olaparib.
Fig. 2.
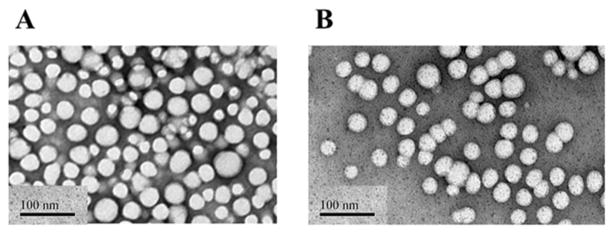
Representative TEM images of NP Wtm (A) and NP olaparib (B). The black scale bar in the left lower corner of each image denotes a distance of 100 nm.
To evaluate NP Wtmn as a chemosensitizer we utilized lung cancer as a disease model because it represents a disease that can greatly benefit from improvements in the efficacy of chemotherapy. Three lung cancer cell lines, 2 non-small cell cancer lines (H460 and H23), and a multidrug resistant small cell cancer line (H69AR), were chosen for in vitro evaluations. NP Wtmn’s efficacy as a chemosensitizer was determined using these tumor cells treated with or without chemotherapeutics commonly used in the treatment of lung cancer, including etoposide, docetaxel (Dtxl), or gemcitabine. Fig. 3 shows cell viability of three different lung cancer cell lines treated with 200 nM etoposide, 2.0 nM gemcitabine or 5.0 nM docetaxel and free or NP Wtmn (5.0 μM for H460 and H23 and 10 μM for H69AR). The addition of Wtmn improved the efficacy of chemotherapeutics. Two-way ANOVA showed a main effect of drug, chemosensitizer, and a significant interaction (P < 0.001 for all 3 cell lines) between chemotherapy and chemosensitizing agent in both NSCLC lines (H460 and H23). As seen in Fig. 3a, H460 cells show about 10–20% reduction in cell survival (compared to control) when treated with Wtmn or chemotherapy alone (P < 0.001 for both). However, the combination of Wtmn and chemotherapy significantly improved cytotoxicity producing 60–80% reductions in cell survival. Similar findings were observed in H23 cells (Fig. 3b). More importantly, our results showed that NP Wtmn is at least as effective as free Wtmn as a chemosensitizer in vitro. In H460 cells and H23 cells, NP Wtmn resulted in similar or significantly lower survival rates than free Wtmn in combination with all three chemotherapeutics. Wtmn also functioned as a chemosensitizer in the SCLC H69AR cells (Fig. 3c). As expected, these cells were chemoresistant and there was no significant main effect of chemotherapy on ANOVA. However, they were sensitive to Wtmn and the combination of Wtmn and chemotherapy was more effective than chemotherapy alone.
Fig. 3.
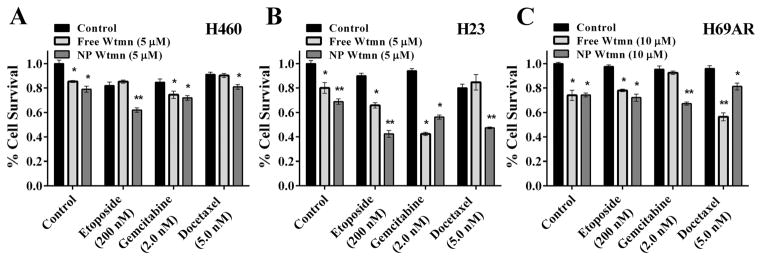
Effects of NP formulation of Wtmn on in vitro chemosensitivity in human lung cancer cell lines. Survival of H460 (A), H23 (B), and H69AR (C) lung cancer cell lines treated with gemcitabine, docetaxel, or etoposide following treatment with saline, (black bars), free (light grey bars) or NP Wtmn (dark grey bars) was determined using clonogenic survival assays. * indicates significantly less survival (P < 0.05) than control. ** indicates significantly less surival (P < 0.05) than both groups. N = 6–12 per treatment group.
Olaparib is a demonstrated chemosensitizer which has been used in clinical trials for patients with BRCA mutated breast and ovarian cancers.12–14 We chose breast cancer as a disease model to study NP olaparib as a chemosensitizer. Breast cancer cell lines, Hs578 T and HCC38, were treated with NP or free olaparib in combination with chemotherapeutics. As seen in Fig. 4a, Hs578 T cells show varied results. Hs578 T cells were sensitive to both olaparib and chemotherapy showing 10–30 percent decreases in survival to either (P < 0.001 for main effects of chemotherapy, sensitizer, and interaction). However, olaparib only showed a chemosensitizing effect when given with gemcitabine but not with etoposide or docetaxel. In HCC38 cells, free olaparib had significant chemosensitizing effects for each of the chemotherapeutics. However, NP olaparib did not improve cytotoxicity to any of the chemotherapeutics in these experiments.
Fig. 4.
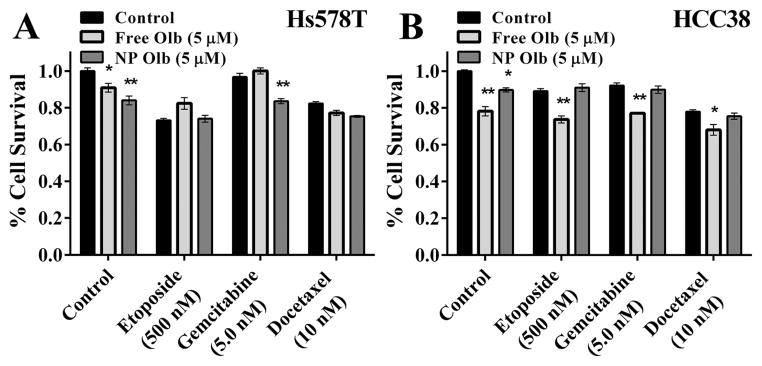
Effects of NP formulation of olaparib on in vitro chemosensitivity in human breast cancer cell lines. Survival of Hs578 T (A) and HCC 38 (B) breast cancer cell lines treated with gemcitabine, docetaxel, or etoposide following treatment with saline (black bars), free (light grey bars) or NP olaparib (dark grey bars) was determined using clonogenic survival assays. * indicates significantly less survival (P < 0.05) than control. ** indicates significantly less surival (P < 0.05) than both groups. N = 6–12 per treatment group.
NP Wtmn is an effective chemosensitizer in vivo
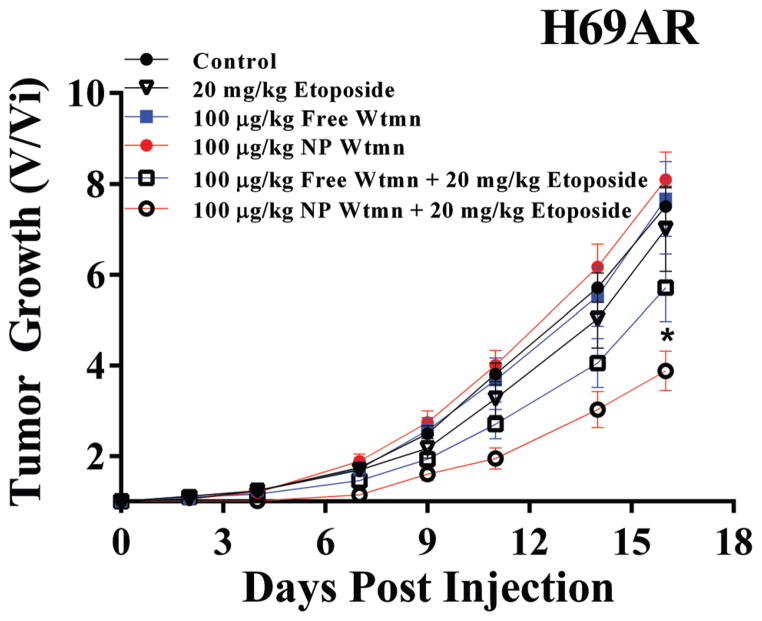
Based on the in vitro results, we focused our in vivo evaluations on NP Wtmn as a chemosensitizer for lung cancer. No in vivo studies were done with breast cancer cells and olaparib as NP olaparib did not appear to be a robust chemosensitzier in vitro. We studied NP Wtmn’s efficacy as a chemosensitizer using mice bearing H69AR xenografts. As seen in Fig. 5, the combination of NP Wtmn and etoposide significantly delayed tumor growth when compared to that of free Wtmn and etoposide or etoposide treatment without chemosensitization. High-dose etoposide alone was ineffective as a treatment. We also confirmed that NP Wtmn was more effective than free Wtmn as a chemosensitizer (P < 0.03).
Fig. 5.
In vivo efficacy of NP Wtmn. Mice were innoculated with flank tumors of H69AR human SCLC cells. They were then treated with saline (black circles) or etoposide (open triangles). To evaluate free Wtmn as a chemosensitizer mice were treated with free Wtmn (closed blue squares) or free Wtmn + etoposide (open blue squares). To evaluate NP Wtmn as a chemosensitizer mice were treated with NP Wtmn (closed red circles) or NP Wtmn + etoposide (open red circles). * indicates significantly different AUC from all other treatment groups. N = 6–7 per treatment group.
NP formulation of Wtmn decreases hepatotoxicity with chemotherapy
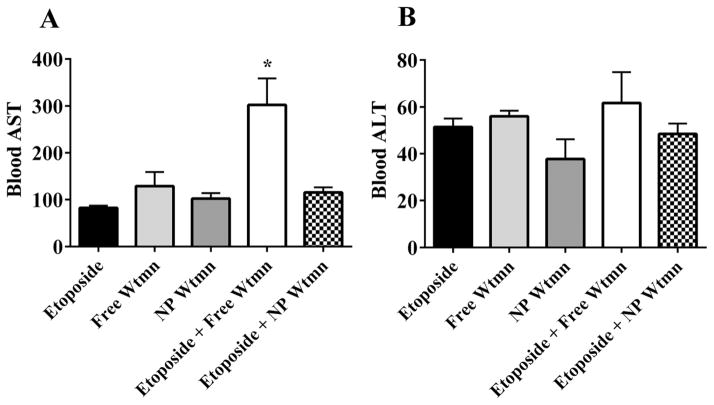
Although our previous work has shown that NP delivery of Wtmn minimized its hepatoxicity,11 hepatotoxicity remains a potential concern when the drug is given in combination with another chemotherapy agent. Hence, we studied the hepatotoxicity profile of Wtmn and etoposide in vivo. Results of hepatotoxicity are seen in Fig. 6. As expected, the treatment regimen of free Wtmn and etoposide showed high levels of hepatotoxicity (AST: 302.5 ± 49.1 (normal range: 40–50 U L−1)) which were significantly greater than all other treatment cohorts (P < 0.05 for all). In contrast, the addition of NP Wtmn to free etoposide did not significantly increase AST or ALT levels compared to etoposide alone.
Fig. 6.
NP formulation of Wtmn reduces hepatotoxicity. Mice were injected with 10 mg kg−1 etoposide, 100 μg kg−1 free or NP Wtmn, or both etoposide and Wtmn. Blood was then collected 24 hours later and AST (A) and ALT (B) levels were analysed. * indicates significantly greater than all other treatment groups. N = 4 per treatment group.
NP Wtmn prevents activation of DNA repair pathways
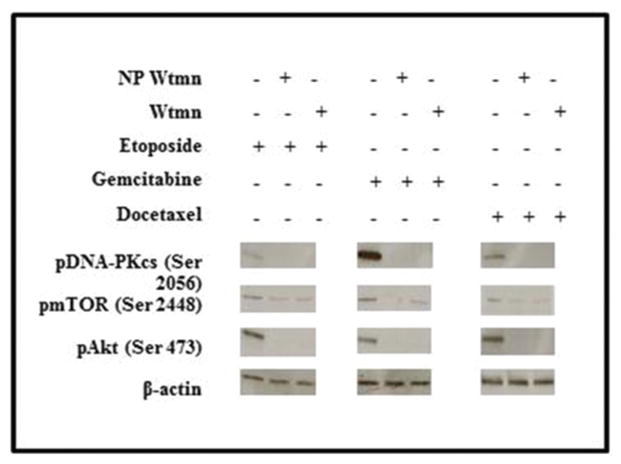
To confirm that the molecular mechanism of the Wtmn NP chemosensitization is indeed through prevention of DNA repair, we also performed western blot analysis of Wtmn targets which are thought to play a key role in DNA repair.15,16 As seen in Fig. 7, H460 cells that were treated with chemotherapy had activation of pDNA-PKcs, pAKT and pmTOR (another important signaling pathway). The activation of pDNA-PKcs and pAKT were inhibited by Wtmn or NP Wtmn. The inhibition from NP Wtmn or free Wtmn was much less for pmTOR, suggesting that the main mechanism of chemosensitization is through the inhibition of pDNA-PKcs and pAKT.
Fig. 7.
NP Wtmn inhibits DNA repair. Western blot analyses were used to measure the activation (phosphorylation) of DNA repair proteins DNA-PK, AKT, and mTOR. Activation was inhibited by both free and NP Wtmn.
Discussion
The development of chemosensitizers can improve chemotherapy treatment and overcome chemoresistance, a major obstacle in cancer treatment. A key impediment to the clinical translation of chemosensitizers has been the lack of targeted delivery of these agents to tumors to avoid the sensitization of normal cells to chemotherapy. As discussed above, lipid-polymer NPs like those used in these experiments preferentially accumulate in tumors. We believe advances in NP drug delivery offer a unique opportunity. Our current study aims to demonstrate the proof of principle of using NPs to deliver chemosensitizers.
In this study, we focused on two chemosensitizers: Wtmn and olaparib. Wtmn has broad activity against many signaling molecules (including PI3 K, PI3KK, and DNA-PK)17–19 and can affect both tumor cells and normal tissue cells. In contrast, olaparib mainly inhibits PARP20 and has been shown to have limited systemic toxicity in clinical trials.12–14 By engineering and evaluting NP formulations of both of these chemosensitizers, we aimed to demonstrate that NP delivery improves chemosensitizers’ efficacy without significantly increasing toxicity. In vitro evaluation of NP Wtmn showed that it is an effective chemosensitizer and more effective than free Wtmn when combined with etoposide and Dtxl. Our previous studies have shown that NP formulations typically are less effective than their small molecule counterparts when evaluated in vitro due to the lower intracellular uptake.15,16 Therefore, our in vitro results were highly suggestive of NP Wtmn’s potency as a chemosensitizer. In this study, we surprisingly observed that NP Wtmn was a more effective in vitro chemosensitizer than free Wtmn with docetaxel or etoposide in NSCL lines. The mechanism may be explained by the prolonged release of Wtmn from the NPs resulting in more effective inhibitors of molecular pathways with longer duration within the cell than free Wtmn. It is interesting to note that we did not observe such as effect with gemcitabine. The most encouraging result was that NP Wtmn was able to sensitize a highly drug resistant cell line H69AR to the effects of chemotherapy.
The results on NP olaparib were mixed. In Hs578 T cells we observed that NP olaparib was more effective than free olaparib only when it is combined with gemcitabine. Olaparib did not sensitize the tumor cells to etoposide or docetaxel. In HCC38 cells, free olaparib was an effective chemosensitizer but NP olaparib was not. Our findings showed that NP formulation of olaparib can improve its efficacy as a chemosensitizer in certain selected situations but not broadly as Wtmn. There are several reasons for the lack of efficacy of olaparib. First, despite the high enthusiasm for PARP inhibition as a strategy to improve chemotherapy, preclinical and clinical results have thus far been largely disappointing.12,21 Therefore, NP delivery may not be able to improve the therapeutic efficacy. Wtmn on the other hand is a potent inhibitor of PI3 K and affects a broad range of signaling pathways. Thus, it is a better chemosensitizer. As a result, we focused on the in vivo evaluation of NP Wtmn as a chemosensitizer.
Using mice bearing H69AR xenografts we demonstrated that NP Wtmn is a potent chemosensitizer and is more effective than free Wtmn. As predicted, this drug resistant lung cancer cell line was highly resistant to etoposide treatment. However, the addition of NP Wtmn sensitized these tumor cells to the effects of etoposide. Since neither Wtmn nor NP Wtmn had any effects on the tumor growth, the function of NP Wtmn was entirely as a chemosensitizer. Such results confirm that chemosensitizers can overcome treatment resistance of tumor cells. Toxicity evaluation showed that the addition of NP Wtmn to etoposide did not significantly increase hepatotoxicity, the main toxicity of Wtmn. Such results suggest that NP Wtmn has clinical translation potential as a chemosensitizer. Lastly, we showed that the mechanism of chemosensitization of NP Wtmn is likely through inhibition of pDNA-PKcs and pAKT pathways.
Conclusions
In conclusion, we have shown that NP formulation of chemosensitizers can improve their therapeutic efficacy. Moreover, NP chemosensitizers can be co-administered with chemotherapy without increasing toxicity. By improving the therapeutic index of systemic chemotherapy it is possible that NP delivery of chemosensitizers could lead to improved clinical outcomes. Future studies could investigate the potential to co-deliver chemosensitizing agents with chemotherapeutics such as gemcitabine or adding NP chemosensitizers with combination chemotherapy regimens. Our results provide proof of principle for NP delivery of chemosensitizers with systemic chemotherapy and provide a basis for further study.
Acknowledgments
This work was supported by the University Cancer Research Fund from the University of North Carolina and R01CA178748-01 from the National Institutes of Health/National Cancer Institute. AZW was also supported by Career Development Award 5-K12-CA120780-01-05 and National Institutes of Health Center for Nanotechnology Excellence Grant 1-U54-CA151652-01.
References
- 1.Citron ML, Berry DA, Cirrincione C, et al. J Clin Oncol. 2003;21:1431. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 2.Shah GM, Robu M, Purohit NK, et al. Front Oncol. 2013;3:279. doi: 10.3389/fonc.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Workman P, Clarke PA, Raynaud FI, et al. Cancer Res. 2010;70:2146. doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobbs SK, Monsky WL, et al. Proc Natl Acad Sci U S A. 1998;95:4607. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain RK, Stylianopoulos T. Nat Rev Clin Oncol. 2011;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda H, Wu J, Sawa T, et al. J Controlled Release. 2000;65:271. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 7.Evers B, Schut E, van der Burg E, et al. Clin Cancer Res. 2010;16:99–108. doi: 10.1158/1078-0432.CCR-09-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welker ME, Kulik G. Bioorg Med Chem. 2013;21:4063. doi: 10.1016/j.bmc.2013.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng SSW, Tsao MS, Chow S, et al. Cancer Res. 2000;60:5451. [PubMed] [Google Scholar]
- 10.Yu HG, Ai YW, Yu LL, et al. Int J Cancer. 2008;122:433. doi: 10.1002/ijc.23049. [DOI] [PubMed] [Google Scholar]
- 11.Karve S, Werner ME, Sukumar R, et al. Proc Natl Acad Sci U S A. 2012;109:8230. doi: 10.1073/pnas.1120508109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledermann J, Harter P, Gourley C, et al. N Engl J Med. 2012;366:1382. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 13.Audeh MW, Carmichael J, Penson RT, et al. Lancet. 2010;376:245. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 14.Tutt A, Robson M, Garber JE, et al. Lancet. 2010;376:235. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 15.Wipf P, Halter RJ. Chem Biol wortmannin. 2005;3:2053. doi: 10.1039/b504418a. [DOI] [PubMed] [Google Scholar]
- 16.Werner ME, Copp JA, Karve S, et al. ACS Nano. 2011;5:8990. doi: 10.1021/nn203165z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenweig KE, Youmell MB, Palayoor ST, et al. Clin Cancer Res. 1997;3:1149. [PubMed] [Google Scholar]
- 18.Hashimoto M, Rao S, Tokuno O, et al. J Raiat Res. 2003;44:151. doi: 10.1269/jrr.44.151. [DOI] [PubMed] [Google Scholar]
- 19.Sakaria JN, Tibbetss RS, Busby EC, et al. Cancer Res. 1998;58:4375. [PubMed] [Google Scholar]
- 20.Menear KA, Adcock C, Barlter R, et al. J Med Chem. 2008;51:6581. doi: 10.1021/jm8001263. [DOI] [PubMed] [Google Scholar]
- 21.Guha M. Nat Nanotechnol. 2011;29:373. [Google Scholar]