Abstract
Transforming growth factor β (TGF-β) is a central mediator of fibrogenesis. TGF-β is upregulated and activated in fibrotic diseases and modulates fibroblast phenotype and function, inducing myofibroblast transdifferentiation while promoting matrix preservation. Studies in a wide range of experimental models have demonstrated the involvement of the canonical ALK5/Smad3 pathway in fibrosis. Smad-independent pathways may regulate Smad activation and, under certain conditions, may directly transduce fibrogenic signals. The profibrotic actions of TGF-β are mediated, at least in part, through induction of its downstream effector, Connective Tissue Growth Factor. In light of its essential role in the pathogenesis of fibrosis, TGF-β has emerged as an attractive therapeutic target. However, the pleiotropic and multifunctional effects of TGF-β and its role in tissue homeostasis, immunity and cell proliferation raise concerns regarding potential side effects that may be caused by TGF-β blockade. This minireview summarizes the role of TGF-β signaling pathways in the fibrotic response.
Keywords: TGF-β, fibrosis, collagen, Smad, MAPK
Introduction
Fibrosis is characterized by excessive accumulation of extracellular matrix in the affected tissue that often results in destruction of its normal architecture and causes significant organ dysfunction. Although fibrotic conditions in various organs have diverse etiologies, fibrosis typically results from chronic persistent inflammation induced by a variety of stimuli, such as chronic infections, ischemia, allergic and autoimmune reactions, chemical insults or radiation injury. In its early stages, inflammation may be beneficial as part of a reparative mechanism following tissue damage; however, defective resolution and impaired containment of the inflammatory reaction may result in accentuated and sustained production of fibrogenic growth factors, chemokines and cytokines (57). The main cellular effectors of fibrosis are the myofibroblasts (19), phenotypically modulated fibroblasts that acquire expression of contractile proteins (such as α-smooth muscle actin) and, when activated, can produce large amounts of matrix proteins. Macrophage and lymphocyte subpopulations play essential regulatory roles in fibrotic conditions by releasing mediators that modulate fibroblast phenotype and by regulating matrix metabolism. Among the mediators involved in tissue fibrosis, Transforming Growth Factor (TGF)-β is considered a key molecule in activation of the fibrotic program. The goal of this minireview is to provide a brief discussion of the role of TGF-β-mediated signaling pathways in the pathogenesis of fibrosis.
The basic biology of TGF-β
The TGF-β superfamily includes the bone morphogenetic proteins (BMP)/GDF (growth differentiation factor)/MIS and the TGF-β/activin/Nodal subfamily, which activate distinct signaling pathways. The TGF-βs are some of the most pleiotropic and multifunctional peptides known. They are secreted by many cell types and are implicated in a wide range of cell functions, critically regulating tissue homeostasis and repair, immune and inflammatory responses, extracellular matrix deposition, cell differentiation and growth (41), (59). In mammals, three structurally similar isoforms of TGF-β, designated TGF-β1, 2 and 3, are encoded by three different genes (41), (25). Although each isoform is expressed in a distinct tissue-specific manner under control of a unique promoter (25), all three isoforms signal through the same cell surface receptors and have similar cellular targets. TGF-β1 is the prevalent isoform and is almost ubiquitously found in mammalian tissues, whereas the other isoforms are expressed in a more limited spectrum of cells and tissues. Although the in vitro functions of the three isoforms are similar, their in vivo effects are distinct. Loss-of-function experiments in mice have demonstrated that each TGF-β isoform plays an independent role in embryonic development highlighting their non-compensated functions. Moreover, although all three isoforms are expressed in fibrotic tissues, the development of tissue fibrosis is primarily attributed to TGF-β1 (3).
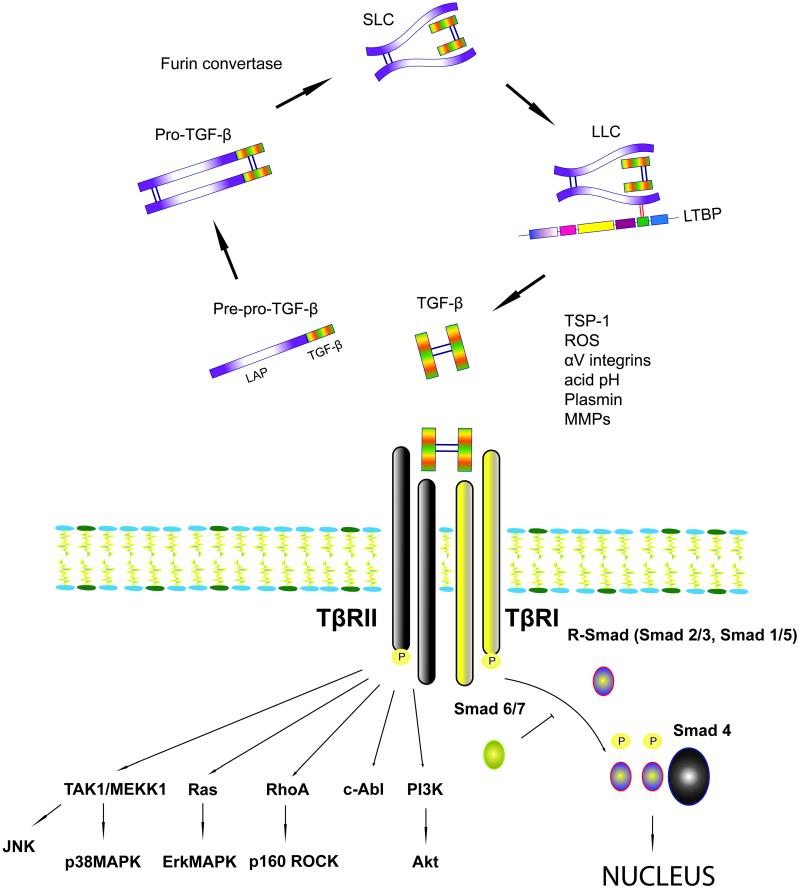
The mechanisms of TGF-β activation (Figure 1)
Figure 1.
Activation of TGF-β requires liberation of the TGF-β dimer from an inactive latent complex with the LAP (see text). TGF-β signals through Smad-dependent and Smad-independent pathways. Activation of the canonical ALK5/Smad3 pathway plays a central role in the pathogenesis of fibrosis. Smad-independent pathways may regulate Smad signaling or exert direct fibrogenic actions.
TGF–β is produced by many cell types as an inactive latent complex, consisting of a C-terminal mature TGF-β and an N-terminal latency-associated peptide (LAP); this complex is unable to associate with its receptors. TGF-β is cleaved from LAP before it is secreted by the cell by a plasma membrane-bound furin convertase (27). After cleavage mature TGF-β remains attached to LAP by noncovalent bonds forming the Small Latent Complex (SLC). This complex is bound to the Latent TGF-β-Binding Protein (LTBP) by disulfide bonds, forming a larger complex called Large Latent Complex (LLC). In connective tissue, it is the LLC that is secreted and bound to the extracellular matrix (ECM) components, such as elastin fibrils and fibronectin-rich pericellular fibers (51). The attachment of mature TGF-β to the binding proteins shields its active epitopes and prevents interactions with the TGF-β receptors. In most tissues significant amounts of latent TGF-β are “stored” in the matrix; thus, activation of TGF-β signaling is primarily regulated by conversion of latent TGF-β to active TGF-β. Due to the high affinity between TGF-β and its receptors, activation of only a small fraction of this latent TGF-β generates maximal cellular response (2). In order for latent TGF-β to become activated and function at adjacent and neighboring cells, the LLC must be liberated from the matrix. Several proteases, including plasmin, mast-cell chymase and thrombin, release LLC from the ECM (2). Release of bioactive TGF-β also requires disruption of the noncovalent bonds attaching it to the LAP. Liberation of active TGF-β from the LAP:TGF-β complex is a poorly understood process that may occur in a protease-dependent manner and involves conformational changes of the LAP. The LAP structure is important to maintain its function; conformational modification of the LAP results in disruption of the interaction between LAP and TGF-β, thus releasing active TGF-β. The inducible matricellular protein thrombospondin (TSP)-1 acts as a TGF-β activator by binding to the LSKL sequence in the LAP, thus altering its conformation and making TGF-β accessible to its receptor (33). Other factors that may cause such structural modifications include hydroxyl radicals from reactive oxygen species and integrins (5). αVβ6 integrin was the first integrin identified to activate TGF-β1 in the absence of proteolytic cleavage by binding an RGD motif in the LAP (32). On the other hand αVβ8 integrin was shown to activate TGF-β1 through a membrane type 1 matrix metalloproteinase (MT1-MMP)-dependent mechanism (31). A variety of proteases have been described to target the LAP:TGF-β complex, inducing liberation of TGF-β. Proteases including plasmin, matrix metalloproteinase (MMP)-2 and MMP-9 are known to cleave latent TGF-β, coupling matrix degradation with activation of a molecule that has a primary role in preserving matrix integrity and stability (2), (20). Moreover a mildly acidic environment can denature the LAP inducing TGF-β activation (27).
TGF-β signaling: Smad-dependent and Smad-independent pathways (Figure 1)
Members of the TGF-β superfamily elicit signaling through distinct combinations of transmembrane type I (TβRI) and type II receptors (TβRII) (28). Type I and type II receptors are serine/threonine kinases that form a heteromeric complex. In response to ligand binding to the type II receptor, a stable complex with the type I receptor is formed allowing its transphosphorylation and thus activation of the type I receptor kinases. Among the seven known mammalian type I receptors termed ALK1-7 (activin receptor-like kinase), ALK5 is expressed on many different cell types and is utilized by TGF-β1 for signaling (38). In endothelial cells TGF-β1 signals through a second type I TGF-β receptor, ALK-1(18). There are additional receptors for TGF-β, including the type III receptors β-glycan and endoglin that serve as accessory co-receptors and may modulate signaling through type I and II receptors.
The canonical signaling pathway for TGF-β involves the Smad family of transcriptional activators (44). The eight mammalian Smads can be grouped into three functional classes: the receptor activated Smads (R-Smads, Smad1, 2, 3 ,5 and 8), the common mediator Smad (Co-Smad, Smad4) and the inhibitory Smads (I-Smads, Smad6 and 7). The R-Smads, Smad2, and Smad3 are phosphorylated directly by ALK5, whereas Smad1, Smad5 and Smad8 are activated by ALK1 (49). Subsequently the R-Smads form complexes with the co-Smad, Smad4, and translocate to the nucleus, where they activate, or repress, gene transcription depending on the recruitment into transcriptional complexes of coactivators such as p300, CBP, AP-1 and Sp1 or corepressors such as c-Ski, SnoN, transforming growth inhibiting factor or smad nuclear-interacting protein-1 (13). The I-Smads serve as negative regulators: Smad6 and Smad7 antagonize TGF-β signaling by binding to type I receptor (Smad7) or by competing with activated R-Smads for binding to Co-Smad4 (Smad6). Moreover inhibitory Smads recruit the E3 ubiquitin-protein ligases Smurf1 and Smurf2 that target Smad proteins for proteasomal degradation thereby terminating Smad-mediated signaling. Smad6 expression is induced by Smads1 and 5, whereas Smad7 expression is triggered by Smad3 therefore providing autoinhibitory feedback loops that suppresses TGF-β-mediated effects.
In addition to activation of Smad-dependent cascades, TGF-β can also signal in a noncanonical fashion. Members of the mitogen-activated protein kinase (MAPK) family encompass serine/threonine-specific protein kinases that respond to extracellular mitogenic and stress stimuli and regulate differentiation, proliferation, cell survival and apoptosis. TGF-β can activate all three known MAPK pathways: extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK) and c-Jun-N-terminal kinase (JNK). Signaling through these pathways may further regulate Smad proteins, but also mediates Smad-independent TGF-β responses. Depending on the cell type examined, ERK activation either increases (16) or decreases (23) Smad signaling. In contrast, p38 MAPK (17) and JNK (60) usually potentiate TGF-β/Smad-induced responses. Other MAPK-mediated TGF-β responses may be Smad-independent, such as TGF-β-induced G0/G1 cell cycle arrest that is transduced by p38 MAPK with no involvement of Smad proteins (42). In addition to Smad and MAPK, TGF-β has been shown to activate PI3 kinase/Akt, Abelson nonreceptor tyrosine kinase (c-Abl), and Rho GTPase pathways and cooperate with Wnt and Notch signaling cascades (9).
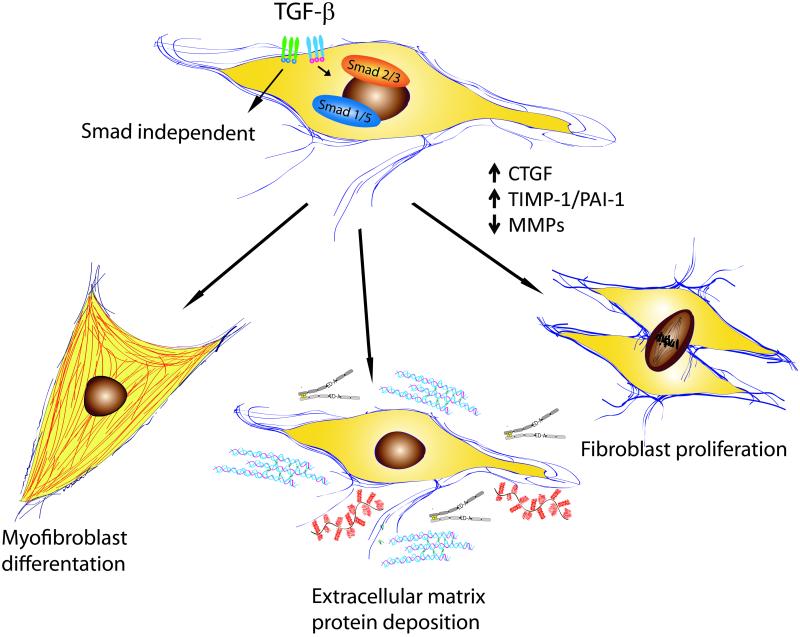
Effects of TGF-β on fibroblast phenotype and function (Figure 2)
Figure 2.
Effects of TGF-β on fibroblast phenotype and function.
TGF-β1 is a crucial regulator of fibroblast phenotype and function. Upon TGF-β stimulation, fibroblasts are activated and undergo phenotypic transition into myofibroblasts, the key effector cells in fibrotic states. The myofibroblast phenotype is characterized by formation of gap junctions and by the acquisition of a contractile apparatus with associated contractile proteins, such as α-SMA and non-muscle myosin (52). TGF-β-induced α-SMA synthesis requires Smad3 (10), but also involves FAK, JNK, TAK and PI3 kinase/Akt pathways (26), (50), (43), (4). In healing wounds myofibroblasts are required for tissue repair; however, in pathologic conditions activated myofibroblasts become the cellular effectors of fibrosis. The origin of fibroblasts in fibrotic tissues remains controversial. The traditional view is that activated myofibroblasts in fibrous tissue derive from phenotypically modulated resident fibroblasts. However, emerging evidence suggests that in many fibrotic conditions, fibroblasts may originate from other cellular sources, such as pericytes and bone marrow-derived progenitor cells. In addition to mesenchymal cells, myofibroblasts may derive from epithelial origin in a process termed epithelial–mesenchymal transition (EMT) (22). This phenomenon is characterized by downregulation of epithelial marker proteins such as E-cadherin and cytokeratins, upregulation of mesenchymal markers (such as vimentin and α-SMA), basement membrane degradation and migration to the interstitial compartment. TGF-β plays an important role in EMT through effects involving Smad signaling (58). Moreover, activation of the Ras-Erk MAPK pathway, p38 MAPK and JNK signaling, as well as Rho GTPase signaling and the PI3 kinase/Akt pathway may be involved in TGF-β-induced EMT (58). Extensive evidence suggests that EMT plays an important role in renal, pulmonary and hepatic fibrosis (21), (56). Moreover, endothelial-mesenchymal transition (EndMT), a form of EMT that occurs during cardiac development, is involved in fibrotic remodeling of the pressure-overloaded myocardium (61). Phenotypic transition of endothelial cells to fibroblasts requires TGF-β/Smad signaling (61).
In addition to its role in myofibroblast transdifferentiation, TGF-β promotes matrix preservation and deposition by enhancing matrix protein synthesis and by altering the balance between matrix-preserving and matrix-degrading signals. TGF-β potently stimulates type I collagen gene transcription in a Smad3-dependent manner. In addition TGF-β may be implicated in postranslational modification of collagen by increasing its stability through enhanced cross-linking. TGF-β also exerts matrix-preserving actions by suppressing the activity of MMPs and by inducing synthesis of protease inhibitors, such as Plasminogen Activator Inhibitor-1 (PAI-1) and Tissue Inhibitor of Metalloproteinase (TIMPs) (41), (29). Activation of the Smad3 signaling pathway appears to be important in mediating TGF-β-induced extracellular matrix protein synthesis and TIMP upregulation (54).
TGF-β signaling pathways in fibrosis
Animal model experiments have suggested an important in vivo role for TGF-β in the pathogenesis of fibrotic conditions (37), (24). TGF-β induction and activation is consistently observed in experimental models of tissue fibrosis. TGF-β overexpression in various tissues induces marked fibrotic changes. Transient overexpression of active TGF-ß1 by adenoviral vector gene transfection in rat lungs induces severe and progressive fibrosis (45). Postnatal induction of TGF-β signaling in fibroblasts of transgenic mice with inducible TβRI activation recapitulates clinical, histologic, and biochemical features of scleroderma (46). Hepatic expression of mature TGF-β1 in transgenic mice under the control of the murine albumin promoter results in prominent hepatic fibrosis and multiple extrahepatic fibrotic and inflammatory lesions (39). In the heart cardiac-specific expression of a constitutively active mutant TGF-β1 results in atrial, but not ventricular, fibrosis (34). TGF-β inhibition attenuated hepatic (35), renal (15) and cardiac fibrosis (48) in various animal models, highlighting the role of TGF-β in a wide range of fibrotic conditions.
Which signaling pathways are implicated in TGF-β-induced fibrosis? Extensive evidence suggests that the canonical ALK5/Smad3 pathway is critically involved in the pathogenesis of fibrosis in many tissues. Oral administration of a small molecular weight selective inhibitor of the kinase activity of ALK5 inhibited fibrogenesis in a rat model of progressive TGF-β1-induced pulmonary fibrosis (6). Moreover, Smad3 null mice exhibit attenuated fibrosis in a wide range of experimental models. Smad3 null mice are resistant to bleomycin-induced pulmonary fibrosis (62). Similarly, dermal fibrosis following irradiation (14), renal interstitial fibrosis produced by unilateral ureteral obstruction (40) and cardiac fibrosis (7), (10) are all attenuated in Smad3-deficient animals.
Although the central role of Smad3 in TGF-β-induced fibrous tissue deposition is widely recognized, a growing body of evidence suggests that noncanonical TGF-β signaling through the Smad1 cascade, or via activation of Smad-independent pathways, may play an important role in certain fibrotic conditions. In a mouse model of scleroderma-like fibrosis due to forced expression of ALK5, activation of a fibrotic gene program was dependent on Smad1 and Erk1/2, and not on Smad2/3 (36). Moreover, several distinct TGF-β-induced Smad-independent pathways have been implicated in the pathogenesis of fibrosis in various tissues. Activation of c-Abl kinase was involved in TGF-β-mediated renal (55) and pulmonary (8) fibrosis. Both in vitro and in vivo findings have suggested that p38 MAPK may play a role in the pathogenesis of renal fibrosis acting downstream of TGF-β (47). The significance of interactions between Smad-dependent and Smad-independent pathways in mediating the fibrogenic actions of TGF-β remains poorly understood.
At least some of the pro-fibrotic effects of TGF-β are mediated through upregulation of its downstream effector Connective Tissue Growth Factor (CTGF). TGF-β induces the expression of CTGF via a functional Smad3 binding site in the CTGF promoter, which in turn stimulates myofibroblast differentiation and collagen synthesis (12). On the other hand, CTGF binds directly to TGF-β, and enhances its activity resulting in increased binding to TβRI and TβRII (1). In vivo studies have suggested that CTGF potentiates TGF-β-mediated fibrogenic actions. When injected into the subcutaneous tissue of newborn mice, TGF-β or CTGF alone induced only transient granulation tissue formation (30). Application of both CTGF and TGF-β was required for sustained fibrotic response (30).
Concluding remarks
The critical involvement of TGF-β in tissue fibrosis suggests that TGF-β-mediated pathways may be attractive therapeutic targets for the treatment of patients with fibrotic conditions. Several distinct strategies for TGF-β inhibition are available, including the administration of anti-TGF-β neutralizing antibodies, or soluble TβRs, and the use of small molecule TβR kinase inhibitors. Because the Smad3 pathway appears to play an essential role in TGF-β-mediated fibrosis, Smad3 inhibition through administration of endogenous inhibitors (such as Smad7), or through the use of small molecule inhibitors (such as halofuginone) may hold promise. The functional pleiotropy of TGF-β, its important homeostatic functions and its crucial role in regulation of the immune system, cell proliferation and tissue repair raise serious concerns regarding potentially catastrophic adverse effects of TGF-β inhibitors (11), (53). However, the relatively modest toxicities associated with anti-TGF-β approaches in early clinical trials may be explained by incomplete inhibition of TGF-β signaling, and provide encouragement for further evaluation of these therapeutic approaches in patients with fibrotic conditions (53).
Acknowledgments
DECLARATION OF INTEREST:
Dr Frangogiannis’ laboratory is supported by NIH grants R01 HL-76246 and R01 HL-85440 and by the Wilf Family Cardiovascular Research Institute. Dr Dobaczewski is the recipient of an American Heart Association Founders’ affiliate post-doctoral grant.
REFERENCES
- 1.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 3.Ask K, Bonniaud P, Maass K, Eickelberg O, Margetts PJ, Warburton D, Groffen J, Gauldie J, Kolb M. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int J Biochem Cell Biol. 2008;40:484–495. doi: 10.1016/j.biocel.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 5.Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994;93:892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, Protter AA, Gauldie J. Progressive transforming growth factor beta1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med. 2005;171:889–898. doi: 10.1164/rccm.200405-612OC. [DOI] [PubMed] [Google Scholar]
- 7.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 8.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 10.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. Faseb J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- 13.Feng XH, Derynck R. Specificity and Versatility in TGF-Signaling Through Smads. Annu Rev Cell Dev Biol. 2005 doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 14.Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, Major C, Deng C, Russo A, Mitchell JB, Roberts AB. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukasawa H, Yamamoto T, Suzuki H, Togawa A, Ohashi N, Fujigaki Y, Uchida C, Aoki M, Hosono M, Kitagawa M, Hishida A. Treatment with anti-TGF-beta antibody ameliorates chronic progressive nephritis by inhibiting Smad/TGF-beta signaling. Kidney Int. 2004;65:63–74. doi: 10.1111/j.1523-1755.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 16.Funaba M, Zimmerman CM, Mathews LS. Modulation of Smad2-mediated signaling by extracellular signal-regulated kinase. J Biol Chem. 2002;277:41361–41368. doi: 10.1074/jbc.M204597200. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa F, Matsuzaki K, Mori S, Tahashi Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki Y, Nishizawa M, Fujisawa J, Inoue K. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38:879–889. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- 18.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. Embo J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 21.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 25.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Xu SW, Kennedy L, Pala D, Chen Y, Eastwood M, Carter DE, Black CM, Abraham DJ, Leask A. FAK is required for TGFbeta-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype. Mol Biol Cell. 2007;18:2169–2178. doi: 10.1091/mbc.E06-12-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 29.Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med. 2005;117:69–80. doi: 10.1385/1-59259-940-0:069. [DOI] [PubMed] [Google Scholar]
- 30.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 33.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247–255. doi: 10.1053/jhep.2000.9109. [DOI] [PubMed] [Google Scholar]
- 36.Pannu J, Nakerakanti S, Smith E, ten Dijke P, Trojanowska M. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282:10405–10413. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- 37.Pohlers D, Brenmoehl J, Loffler I, Muller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- 39.Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Seay U, Sedding D, Krick S, Hecker M, Seeger W, Eickelberg O. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J Pharmacol Exp Ther. 2005;315:1005–1012. doi: 10.1124/jpet.105.091249. [DOI] [PubMed] [Google Scholar]
- 43.Shi-wen X, Parapuram SK, Pala D, Chen Y, Carter DE, Eastwood M, Denton CP, Abraham DJ, Leask A. Requirement of transforming growth factor beta-activated kinase 1 for transforming growth factor beta-induced alpha-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 2009;60:234–241. doi: 10.1002/art.24223. [DOI] [PubMed] [Google Scholar]
- 44.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 45.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonnylal S, Denton CP, Zheng B, Keene DR, He R, Adams HP, Vanpelt CS, Geng YJ, Deng JM, Behringer RR, de Crombrugghe B. Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 2007;56:334–344. doi: 10.1002/art.22328. [DOI] [PubMed] [Google Scholar]
- 47.Stambe C, Atkins RC, Tesch GH, Masaki T, Schreiner GF, Nikolic-Paterson DJ. The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. J Am Soc Nephrol. 2004;15:370–379. doi: 10.1097/01.asn.0000109669.23650.56. [DOI] [PubMed] [Google Scholar]
- 48.Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest. 120:3520–3529. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 51.Todorovic V, Jurukovski V, Chen Y, Fontana L, Dabovic B, Rifkin DB. Latent TGF-beta binding proteins. Int J Biochem Cell Biol. 2005;37:38–41. doi: 10.1016/j.biocel.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 53.Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5:200–206. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 55.Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. Faseb J. 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- 56.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 57.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida K, Matsuzaki K, Mori S, Tahashi Y, Yamagata H, Furukawa F, Seki T, Nishizawa M, Fujisawa J, Okazaki K. Transforming growth factor-beta and platelet-derived growth factor signal via c-Jun N-terminal kinase-dependent Smad2/3 phosphorylation in rat hepatic stellate cells after acute liver injury. Am J Pathol. 2005;166:1029–1039. doi: 10.1016/s0002-9440(10)62324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 62.Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr., Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L585–593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]