Introduction
Peripheral nerve injuries are common conditions with broad ranging groups of symptoms depending on the severity and nerves involved. Although much knowledge exists on the mechanisms of injury and regeneration, reliable treatments that ensure full functional recovery are scarce. This review aims to summarize various ways these injuries are classified in the light of decades of research on peripheral nerve injury and regeneration.
Peripheral Nerve Anatomy
The peripheral nervous system is comprised of three types of cells: neuronal cells, glial cells, and stromal cells. Peripheral nerves convey signals between the spinal cord and the rest of the body. Nerves are comprised of various combinations of motor, sensory, and autonomic neurons. Efferent neurons (motor and autonomic) receive signals through their dendrites from neurons of the central nervous system, primarily using the neurotransmitter acetylcholine among others. Afferent (sensory) neurons receive their signals through their dendrites from specialized cell types, such as Paccinian corpuscles for fine sensation and others. These signals are sent to the CNS to provide sensory information to the brain and possibly interneurons in the spinal cord when a reflex response is necessary1.
Key roles are played by cells other than neurons in the maintenance and function of the peripheral nerves. Schwann cells ensheath nerves in a layer of myelin and provide trophic support through the release of important neurotrophs such as Nerve Growth Factor (NGF). Myelin improves conduction velocity by limiting the sites of ionic transfer along the axon to the nodes of Ranvier, resulting in a faster, “jumping” action potential propagation that is termed saltatory conduction. The most heavily myelinated fibers are the large motor neurons (Type Aα), followed by afferent muscle spindles (Type Aβ). Nerve conduction velocities in theses neurons are approximately 30-120m/s. Unmyelinated neurons (Type C), such as the sensory neurons involved in transmitting pain and temperature and postganglionic sympathetics are the slowest, conducting at approximately 1-2 m/s (Table 1)2,3.
Table 1.
Nerve Fiber Types and Properties
| Fiber Class | Myelin | Diameter (Mm) | Conduction Velocity (m/s) | Spinal Cord Tract | Location | Function |
|---|---|---|---|---|---|---|
| Aα | + | 6-22 | 30-120 | Ipsilateral dorsal column | Efferent to muscles | Motor |
| Aβ | + | 6-22 | 30-120 | Contralateral spinothalamic tract | Afferent from skin and joints | Tactile, proprioception |
| Aγ | + | 3-8 | 15-35 | Ipsilateral dorsal column | Efferent to muscle spindles | Muscle tone |
| Aδ | + | 1-4 | 5-30 | Contralateral spinothalamic tract | Afferent sensory nerves | Pain, cold, temperature, touch |
| B | + | 1-3 | 3-15 | Preganglionic | Preganglionic sympathetic | Various autonomic functions |
| sC | - | 0.3-1.3 | 0.7-1.3 | - | Postganglionic sympathetic | Various autonomic functions |
| dC | - | 0.4-1.2 | 0.1-2.0 | Contralateral spinothalamic tract | Afferent sensory nerves | Various autonomic functions Pain, warm, temperature, touch |
Modified from: Berde CB and Strichartz GR. Local Anesthetics. Miller:Miller's Anesthesia, 7th ed. Lars (eds) Eriksson I, Fleisher LA, Wiener-Kronish JP, Young WL. 2009.
Miner JR, Paris PM, Yealy DM. Pain Management. Mark: Rosen's Emergency Medicine, 7th ed. (eds) Marx et al. 2010
The non-neuronal cells and connective tissues surrounding neuronal axons provide a complex stromal connective tissue scaffold4 for the nerve and are important in understanding and classifying nerve injuries. Encasing the individual axons is the deepest structural layer, the endoneurium. Surrounding the endoneurium, the perineurium circumferentially bundles axons together to form fascicles. The outermost connective tissue layer of the nerve, the epineurium consists of two parts. Dispersed between fascicles is the epifascicular epineurium while surrounding the nerve trunk proper is the epineural epinerium. Microvessels progressively branch through the nerve according to the structural layers providing blood to the axons. Due to their more peripheral location, epineural vessels are more susceptible to trauma than the deeper vessels of the nerve5.
Classifications of Nerve Injuries
Peripheral nerve injuries pose various challenges to patients, ranging from mild discomfort to life-long impairment. A classification scheme provides a common language for physicians and scientists to effectively discuss nerve pathophysiology (Table 2). Seddon was the first to classify nerve injuries into three categories based on the presence of demyelination and the extent of damage to the axons and the connective tissues of the nerve6. The mildest form of injury is called neurapraxia, defined by focal demyelination without damage to the axons or the connective tissues. Neurapraxia typically occurs from mild compression or traction of the nerve and results in a decrease in conduction velocity. Depending on the severity of demyelination, the effects can range from asynchronous conduction to conduction block, causing muscle weakness. The next level is called axonotmesis, which involves direct damage to the axons in addition to focal demyelination while maintaining continuity of the nerve's connective tissues. The most severe form of injury is called neurotmesis, which is a full transection of the axons and connective tissue layers wherein complete discontinuity of the nerve is observed.
Table 2.
Seddon and Sunderland Classification of Nerve Injury
| Seddon | Sunderland | Injury |
|---|---|---|
| Neurapraxia | Grade I | Focal segmental demyelination |
| Axonotmesis | Grade II | Axon damaged with intact endoneurium |
| Axonotmesis | Grade III | Axon and endoneurium damaged with intact perineurium |
| Axonotmesis | Grade IV | Axon, endoneurium, and perineurium damaged with intact epineurium |
| Neurotmesis | Grade V | Complete nerve transection. |
| Grade VI (MacKinnon & Dellon) | Mixed levels of injury along the nerve |
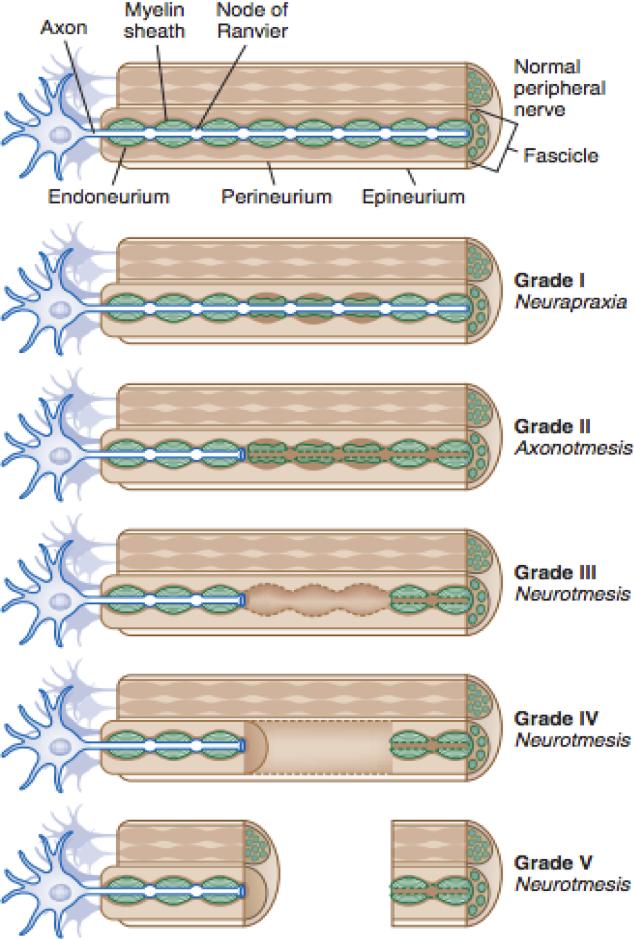
Sunderland later expanded on this classification to distinguish the extent of damage in the connective tissues7. In his classification scheme, Grade I and Grade V corresponded with Seddon's neurapraxia and neurotmesis respectively. However, Grade II-IV are all forms of axonotmesis with increasing amounts of connective tissue damage. In Grade II, axon damage is observed with no damage present in the connective tissue. Grade III involves damage to the endoneurium and Grade IV includes damage to the perineurium (Figure 1). A Grade VI lesion was later introduced by McKennon and Dellon to denote combinations of Grade III-V injuries along a damaged nerve, although its usage has not been widely accepted8. Attempts at simplifying this scheme by classifying nerves as either non-degenerative or degenerative have been proposed by Thomas and Holdroff in 1993, but the clinical significance of this simplification is still questionable9.
Fig. 1.
Classification of nerve trauma
From: Tsao B, Boulis N, Bethoux F, Murray B. Trauma of the Nervous System, Peripheral Nerve Trauma. In: Daroff: Bradley's Neurology in Clinical Practice, 6th ed. 2012 p 984-1001. (Image courtesy Cleveland Clinic, 2006. Illustrator, David Schumick, BS, CMI.)
Compression Injury
Causes
Compression injuries are not always captured by the commonly used classification schemes. Nonetheless, there is little doubt that the majority of peripheral nerve compressions fall under the general class of neurapraxia, or Grade I nerve injuries, and commonly occur in locations where nerves pass through narrow anatomical openings. The most common sites in the upper extremity are the carpal tunnel and the cubital tunnel. As expected with a Grade I nerve injury, these are defined by focal demyelination at the site of compression with the absence of axonal and connective tissue damage. Compressions can be acute or chronic in nature. Acute compressions, as is seen in radial mononeuropathy, are commonly acquired after a night of external compression ie hanging one's arm over a chair, and typically present with transient paresthesia, numbness, and wrist drop. Complete recovery of the acutely compressed nerve can range from weeks to years. In contrast, chronic compressions, as seen in carpal tunnel syndrome, are progressively worsening conditions that persist without proper intervention. Symptoms may begin with paresthesia and distal numbness, but unlike acute compressions, often progress over time to muscle weakness and muscle wasting, depending on the extent of axonal damage at later stages10.
Pathophysiology
Light and electron microscopy shows that normal nerve morphology and neuromuscular junctions are still present in chronic compression injuries11,12. However, a degraded, thinner myelin sheath is seen, as evidenced by an increased g ratio (a ratio of the axon diameter to the axon plus myelin sheath diameter) and a decreased internodal length (the distance between adjacent nodes of Ranvier). Also observed in the presence of this demyelination are Schwann cell proliferation, dediffentiation, and an increase in Schmidt-Lanterman incisures (SLIs). SLIs are cytoplasmic components of Schwann cells that are thought to maintain the metabolism of the myelin sheath, so an increase in SLIs suggests that Schwann cells are increasing their metabolism to undergo re-myelination in the presence of demyelination, as is typically seen in chronic compression injuries13.
There are various proposed mechanisms that are thought to lead to compression injuries14,103. From an anatomical standpoint, the narrowing of openings leads to increased pressure at that site, compressing blood vessels and leading to nerve ischemia, as occurs with vasculitis and artherosclorotic diseases. Another proposed mechanism is the result of lower pressure, which decreases venous return and can lead to venous stasis. In this state, extraneural edema may form over time with subsequent fibrous and scar tissue around the nerve and eventual intraneural edema. The examination of these two mechanisms begets the question of why the Schwann cells are chiefly affected and not the neurons themselves.
Chronic compression injury was once thought of as a milder form of Wallerian degeneration. This has long been disproven due to the lack of axonal damage in this injury. The role of macrophages and their mitogenic factors, like interleukins 1, 6, 10, and 12, in promoting Schwann cell activity has been proposed but is unlikely since Schwann cells are able to proliferate in the absence of macrophages15. Also, since the subtle gradual damage in chronic compression does not lead to an inflammatory response, macrophages arrive slowly and after much of the Schwann cell proliferation has already occurred, further distancing macrophages from being the likely culprit16.
More recent studies have shown that shear stress alone can induce Schwann cell demyelination, proliferation and re-myelination17. These in-vitro experiments were conducted in highly controlled chambers where gas concentrations, pressure, and solutes in the medium were regulated and monitored in real time. In such controlled conditions, the application of shear stresses leads to altered Schwann cell protein expression with key changes attributed to altered expression of integrin B417.
Much of the in vivo work for studying compression neuropathy has come from the usage of biologically inert, polymeric silicone silastic tubes, more commonly used for surgical implants and prosthesis. These tubes have been used to create reliable models of compressive neuropathy in many animal models, such as rats29, rabbits30, and mice32, and have also been used to study double crush in vivo 31. The compression neuropathy models are typically assessed by nerve conduction studies, which in these models reveal a gradual decline in nerve conduction velocities in the absence of neuronal or muscular damage, which would be evident if an altered compound muscle action potential (CMAP) was seen but was not14,31,103.
Double Crush
Within the realm of compression is another form of injury known as double crush23. Double crush pertains to the increased susceptibility of a nerve to develop a compressive neuropathy when a proximal compressive lesion of the same nerve is found. This phenomenon was first defined by Upton and McComas in 1973 after an observation that 81/115 patients with carpal or cubital tunnel also had a neural lesion at the neck24. The reverse form of double crush, aptly named reverse double crush, was later described by Dahlin and Lundborg in 1990 after observing patients with an ulnar nerve entrapment at the wrist later developing a similar proximal injury at the elbow25. The morphology of the lesion seen in double crush is identical to lesions seen in chronic compression with the unique additive effect of multiple sites with otherwise subthreshold levels of pressure contributing to a suprathreshold effect on the nerve. Interruption of axonal traffic and flow may prove an explanation for how two minor areas of compression can collude to form an effect that would mimic a greater compressive insult at a single site. An article by Wilbourn in 1997 discusses these models, their limitations, and points out a need for further research and a clinical overuse of the term “double crush” in cases that do not fit the specific parameters described by Upton and McComas and Dahlin and Lundborg26. The existence of double crush has been confirmed in various studies under subacute compression27,28 and severe compression29, using animal nerves in vitro and in vivo.
Crush and Transection Injury
Causes
Crush injuries can cause many different degrees of neural damage that can represent any of the class of the schemes described by Seddon6 or Sunderland7. Moreover, most of these injuries probably often represent mixed injuries of the sort suggested by Dellon and MacKinnon21.
Crush injuries typically occur from an acute traumatic compression of the nerve from a blunt object, such as a bat, surgical clamp or other crushing object that does not result in a complete transection of the nerve. In contrast, transection injuries, also known as neurotmesis or grade V nerve injuries, have a complete discontinuation of the nerve, commonly due to a laceration from a knife, gunshot, glass shard, etc34. Ballistic injuries are a special case that tends to combine both transection and crush of the nerve from the shockwave that moves through the tissue after the passage of the bullet. This has both a tearing and compressing effect on the nerve even without the actual passage of the projectile through the nerve itself.
Mechanism of recovery
When an end-organ becomes denervated, reinnervation can occur in two ways: through collateral branching of intact axons or by regeneration of the injured axon35. In injuries where 20-30% of the axons are damaged, collateral branching is the primary mechanism of recovery. This begins in the first 4 days following injury and will continue for about 3-6 months, until recovery occurs. As may be expected, an increase in motor unit size is observed and the remaining innervated muscle hypertrophies in an attempt to compensate the initial denervation of other sections of the muscle. Over time, however, the muscle eventually atrophies as fibers without innervation shrink and outpace the ability of remaining muscle fibers to expand34. There are more axonal branches that sprout than the actual number of nerves that end up eventually innervating a target-organ36. Those branches that do not receive neurotrophic factors from the target-end organ undergo a pruning process and are destined to degenerate37,38.
In injuries affecting greater than 90% of the axon population within a nerve, axonal regeneration is the primary means for recovery39. To achieve full recovery, the nerve must undergo three main processes: Wallerian degeneration (the clearing process of the distal stump), axonal regeneration, and end-organ reinnervation. Failure of any of these processes can contribute to the poor functional outcome commonly observed in patients with peripheral nerve injuries.
Wallerian Degeneration
Axons that incur traumatic damage will undergo Wallerian degeneration to create a microenvironment conducive for axonal regrowth and reinnervation. This process takes place within the first week after injury when the typical markers of axonal damage occur: the loss of cell membrane integrity and the breakdown of axonal cytoskeleton. Initially, swelling occurs at both ends of the damaged neuron from futile continuation of retrograde and anterograde axonal transport40.
Changes at the Distal Stump
The axonal changes at the distal end of the severed nerve eventually lead to the breakdown of the nerve stump to make way for a newly regenerating axon. The hallmark of this phase is the granular disintegration of the cytoskeleton41. This occurs after a sudden inflow of extracellular ions, primarily Ca+ and Na+, leads to a cascade of events resembling apoptosis, which serves to recruit macrophages using signals elaborated from Schwann cells. It has been shown that the distal nerve stump is capable of transmitting an action potential hours after transection, a fact used to study synaptic transmission and muscle function in isolated nerve-muscle preparations42-44. More recently, mRNA of brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) were found to be up-regulated while neurotrophin-3 and ciliary neurotrophic factor (CNTF) were down-regulated in the distal stumps of transected tibial nerves. Chronically denervated distal nerve stumps will maintain these changes 6 months after injury if regeneration has not occurred45.
Changes at the Proximal Stump
The changes in the proximal stump vary based on the location of the injury relative to the neuronal body and the severity of the injury. The breakdown of the proximal stump is limited and typically only progresses to the first node of Ranvier. However if the site of injury is very close to the neuronal body, apoptosis may occur46. In severe injuries, the proximal portion of the nerve will undergo chromatolysis - changing the genetic motive of the cell to alter its focus to the regeneration phenotype. During this process, proteins associated with growth such as GAP-43, tubulin and actin are upregulated and neurofilaments (involved in maintaining axonal diameter) are downregulated47-49. Endogenous neuroprotectants such as heat-shock protein-27 (HSP27) are upregulated perhaps to promote the survival of the damaged neuron50,51. When the axon eventually reaches its target-organ and neuronal maturation is required, the gene expression of the neuron will revert back from a regenerative state to a maintenance state.
Schwann cells and Macrophages
Schwann cells are primary mediators in triggering many of the events in Wallerian degeneration and changes in their protein expression at the site of injury are key to axon regeneration. In the absence of axonal contact, Schwann cells convert to a non-myelinating behavior - downregulating the expression of several proteins such as PMP22, Krox-2052, P0 and connexin-3253. Synthesis is arrested and differentiation is promoted with production of C-jun54, and neurotrophic factors such as NGF and CNTF are produced55. Together, these factors contribute to the formation of a pool of new schwann cells under the protection of endogenous compounds such as erythropoietin56. These changes are later progressed by mitogens released from the proximal stump neurons such as ATP and neuregulin57,58 which together with acetylcholine help mature the new Schwann cells to a myelinating phenotype59.
Schwann cells clear debris through phagocytosis and by recruiting macrophages60. This effect is dependent on the protein, MAC-2, which supports Schwann cell phagocytosis61. Schwann cells are also the source of monocyte chemoattractant protein-1 (MCP-1) which works to recruit macrophages62.
Macrophages, attracted by schwann cells, rapidly arrive at the site of nerve injury63 – a process dependent on the breakdown of the blood-nerve barrier. Macrophages also rescue the precious cholesterol in damaged nerves and produce apolipoprotein E, among other lipoproteins64-66. Finally, macrophages themselves produce factors that further promote Schwann cell proliferation64,67.
After the clearance of myelin debris, the dedifferentiated Schwann cells proliferate on the remaining endoneurial tubes of the extracellular matrix (ECM). Collectively, these are known as the bands of Bungner and the hollow tube that is formed provides a path for the regenerating axon to regrow68. Greater success in reinnervation from a regenerative axon is observed when the endoneurial tube is intact and neuroma formation is favored without the tube which shrinks with time if unpopulated by an axon within four months34,39. It is the accumulation of Na+ channels in ectopic axons unable to find tubes which may result in neuropathic pain.
Nerve Regeneration
Although the alteration in the genetic expression of the neuron from a quiescent state to a regenerative state occurs concurrently with the events of Wallerian degeneration, axonal regeneration itself begins after Wallerian degeneration is completed69. At the distal tip of the proximal bud, a growth cone is formed. It has been found that calcium plays a role in the proximal stump to promote growth cone formation70. Hours after injury, the growth cone sends out filapodia to sample the microenvironment. On their way to the distal nerve stump, the filopodia are initially randomly oriented but gain direction once actin and myosin expression is upregulated within the cell body49,71,72.
The mobility of the growth cone is dependent on the presence of particular receptors in its membrane. It can be attracted or repulsed by contact-mediated or chemical means known as neurotrophism73,74. Examples of guidance molecules are semaphorins, ephrins, netrins, and slits. Inhibitory guidance molecules like Collapsin-1 promote growth cone collapse. Neurotrophins like BDNF lessen the susceptibility of growth cones promoting nerve regeneration75. The rate of regeneration may very depending on location along the neuron in which proximal segments may see an increase of 2-3mm/day while more distal segments may progress at a rate of 1-2mm/day.
The path of the growth cone may be disrupted by scar tissue and growth cones release proteases and plasminogen activators to clear its path. This is also to clear any cell-cell or cell-matrix interactions from non-neuronal cells that are hindering its path70.
Schwann cells play a substantial role in promoting axonal regeneration, as they are the main source of neurotrophic factors, which generally interact with tyrosine kinase receptors to alter the gene expression profile of the neuron to promote regeneration. This message is relayed through a retrograde transport76. NGF has a low level expression within healthy nerves, but is upregulated in Schwann cells during injury. NGF promotes growth and proliferation of Schwann cells and is also found on the receptors of Schwann cell lining bands of Bungner to provide trophism to the outgrowing axon39. Many neurotrohic factors have been discovered, with functions ranging from improving cell survival through mechanisms of apoptosis prevention, to promoting regenerating factors in the neurons and in Schwann cells (Table 3)77. Furthermore, Schwann cells produce neurite-promoting factors, which are incorporated in the ECM such as fibronectin and laminin. Growth cones utilize these proteins for adhesion to the basal lamina of the endoneurial tubes78.
Table 3.
Summary of the changes in the molecular expression in axotomized neurons after peripheral nerve injury.
| Lesion | Location | References | ||
|---|---|---|---|---|
| Neurotrophic factors/receptors | ||||
| BDNF | ↑ | Transection | DRG | Kashiba and Senba (1999) |
| ↑ | CCI | DRG | Obata et al. (2003) | |
| ↑ | Crush | DRG | Ernfors et al. (1993) | |
| ↑ | Ligation | DRG | Fukuoka et al. (2001) | |
| ↑ | Transection | MN | Gu et al. (1997) | |
| ↑ | Crush | DRG | Tonra et al. (1998) | |
| ↑ | Spinal ligation | DRG | Shen et al. (1999) | |
| Trk | = | Crush | DRG | Ernfors et al. (1993) |
| Ret (GDNF receptor) | ↑ | Transection | MN | Hammarberg et al. (2000) |
| = | Transection | DRG | Bennett et al. (2000) | |
| GFRalpha2 | ↓ | Transection | DRG | Bennett et al. (2000), Hoke et al. (2000) |
| GFRalpha1, GFRalpha3 | ↑ | Transection | DRG | Bennett et al. (2000) |
| Ret and GDNFalpha1 | ↑ | Crush | DRG/MN | Naveilhan et al. (1997) |
| NT-3 | = | Spinal ligation | DRG | Shen et al. (1999) |
| = | Transection | SC | Funakoshi et al. (1993) | |
| trkB | ↑ | Transection | MN | Hammarberg et al. (2000) |
| ↑ | Crush | SC/DRG | Ernfors et al. (1993) | |
| NGF | = | Transection | MN | Gu et al. (1997) |
| ↑ | Spinal ligation | DRG | Shen et al. (1999) | |
| NGF-R | ↑ | Crush | MN | Ernfors et al. (1989) |
| 16 kDa pancreatitis-associated protein (Reg-2) | ↑ | Transection | DRG | Averill et al. (2002) |
| Transcriptional factors | ||||
| c-fos | ↑ | Transection | MN | Gu et al. (1997) |
| ↑ | Transection | DH | Kajander et al. (1996) | |
| ↑ | CCI | DH | Kajander et al. (1996) and Ro et al. (2004) | |
| c-jun | ↑ | Transection | DRG | Jenkins and Hunt (1991), Broude et al. (1997) and Kenney and Kocsis (1997) |
| ↑ | Crush | MN | Jenkins and Hunt (1991) | |
| Signal transd. and activ. of transc. 3 (STAT3) | ↑(A) | Transection | DRG | Qiu et al. (2005) |
| Activating transcription factor 3 (ATF-3) | ↑ | Transection | MN/DRG/DH | Tsujino et al. (2000) |
| NFκB | ↑ | Transection | DH | Pollock et al. (2005) |
| ↑(A) | PNSL/CCI | DRG | Ma and Bisby (1998) | |
| Isl 1 | ↑ | Crush | DRG | Vogelaar et al. (2004) |
| DRG11, LmX1b, Pax3 | = | Crush | DRG | Vogelaar et al. (2004) |
| Others | ||||
| TNF-alpha receptor 1 (p55) | ↑ | Crush | DRG | Ohtori et al. (2004) |
| Nectin-3 | ↑ | Transection | MN | Zelano et al. (2006) |
| P38 mitogen-activated protein kinase (MAPK) | ↑(A) | CCI | DRG | Obata et al. (2004a) |
| Monocyte chemoattractant protein-1 (MCP-1) | ↑ | CCI | DRG/MN/DH | Zhang and De Koninck (2006) |
| Fibroblast growth factor 2 (FGF-2) | ↑ | Ligation | DRG | Madiai et al. (2003) |
| Glutamate transporter EAAC1 | ↓ | CCI | DH | Wang et al. (2006) |
| cAMP response element binding protein (CREB) | ↑(P) | CCI | DH | Miletic et al. (2004) |
All the injuries were performed on the sciatic nerve in murine adult animals. The changes indicated are mainly based on studies on mRNA expression or immunoreactiviy; some are based on phosphorylation (P) or activity (A). Measurements were performed in sensory neurons of dorsal root ganglia (DRG) or motoneurones (MN) of the ventral horn; in some cases, determinations were described as expression in dorsal horn (DH) or spinal cord (SC). Note that for each molecule, references are grouped according to type of injury, as in some cases the expression varies depending on the injury model or the cell type. The arrows (↑: increase; ↓: decrease) represent up- or down-regulation.
Infiltrating macrophages, after phagocytosing myelin, also promote nerve regeneration through the secretion of IL-1, which induces the expression of NGF in Schwann cells and increases the NGF-receptor density on Schwann cells. This feed-forward mechanism leads to the secretion of mitogens to further trigger Schwann cell proliferation79. Macrophages, however, also secrete IL-1 receptor antagonist, which decreases regrowth of myelinated and unmyelinated axons when applied by an implantation tube in mice with transected sciatic nerves80.
When the growth cone reaches the endoneurial tube, it has a better chance of reaching the end-organ. Maturation must occur before the functional connection is complete. The maturation process includes remyelination, axon enlargements, and finally, functional re-innervation. The axonal outgrowth produces ATP and acetylcholine, which promote the change of the Schwann cell's phenotypes from non-myelinating to myelinating59.
Shortcomings for recovery
Although peripheral nerve injuries are not life threatening, they can cause a considerable decline in the patient's quality of life81. This motivates further investigation in finding ways to optimize recovery. Re-innervation is not synonymous with complete functional recovery. It is the emerging understanding of these along with the key role of clinically relevant factors, which stands to complete recovery from the exception to the rule. Key elements of neuroregeneration are gap distance, Wallerian degeneration, axon guidance specificity, and end-organ viability.
The presence of an intact endoneurial tube more often leads to a better outcome in nerve regeneration. Thus Grade II lesions, which confer damage to the axons alone without any damage to the surrounding connective tissue, have optimal conditions for axonal regrowth. Grade III and IV lesions however, not only have a disrupted endoneurial tube making it difficult to form appropriate bands of Bungner, they also have increased scar tissue formation that can be a considerable deterrent to the growth cone, leading to disorganized outgrowth.
The more distal the injury to the neuron, the more likely it is to recover with the very proximal lesions, close to the neuronal cell bodies, often triggering programmed neuronal cell death. Gap length is negatively correlated with successful regeneration – linking the size of the injury to the fidelity of the axonal outgrowth.
During nerve recovery, the axonal outgrowth becomes remyelinated by the resident Schwann cells. However, this myelination is generally much thinner than normal, with predictable electrical consequences82. In a recent study, using a mouse sciatic nerve crush model, it was found that axonal neuregulin-1 (NRG1) type III and I restored normal levels of myelination when overexpressed in transgenic mice. Unexpectedly, denervated Schwann cells also expressed NRG-1 type I as a paracrine/autocrine signal suggesting that full remyelination of regenerated axons may not occur due to simply insufficient stimulation by neuronal growth factors from Schwann cells83.
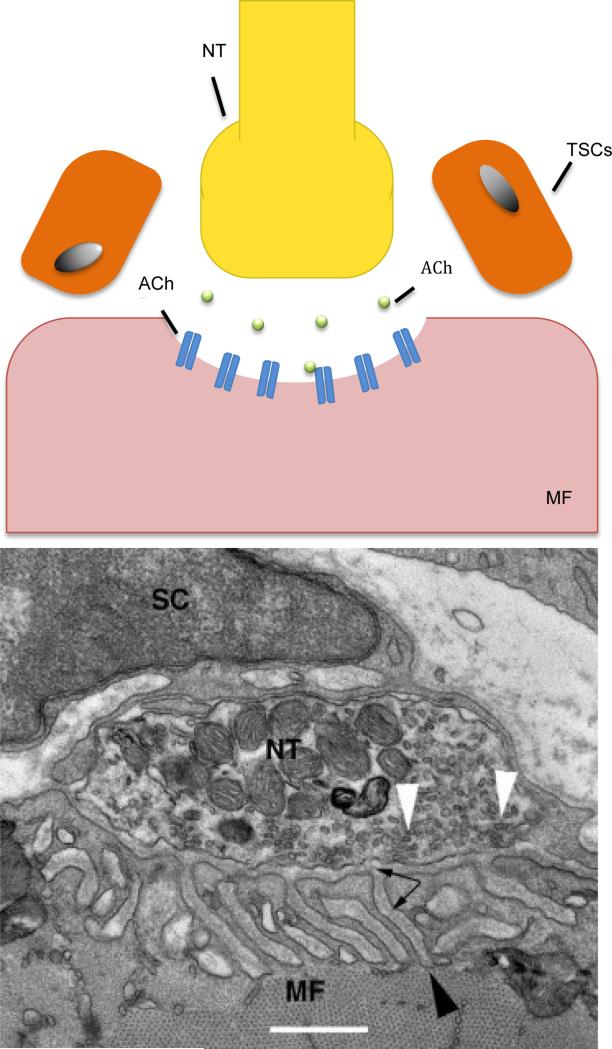
Even if the regenerated axon is able to reach the target, maturation is only possible if the end-organ is maintained. This includes the stabilization of the neuromuscular junction (NMJ) in the case of motoneurons84. The NMJ is comprised of the terminal end of the motor axon, terminal Schwann cells (TSCs) and the end plate of muscle fibers containing acetylcholine receptors (AChR)85 (Figure 2). The presence of the axon provides trophic support to the end-plate and a long degenerated axon leads to dispersion of AChR clusters. Agrin, a glycoprotein released by the distal nerve terminal is suggested to be the key regulator of cluster formation86. In agrin-deficient mutant mice, poor cluster formation and synaptogenesis is observed following nerve injury87. Taken together, these data suggest that supplementing agrin at the NMJ may preserve ACh-R clusers and may lead to better preservation of end-organ. Aside from dispersion, AChR turnover shortens by a factor of ten when an axon is not present at the end plate88.
Fig. 2.
The Neuromuscular Junction
1. Illustration and TEM of the neuromuscular junction. From: Lee Y, Thompson WJ. Chapter 54 - The Vertebrate Neuromuscular Junction. Muscle, 2012;2:775-787.
Muscle fibers undergo atrophy as early as 3 weeks after denervation, with collagen deposits forming in the endomysium and the perimysium. However the structural architecture of the muscle and the end-plate integrity can be maintained for up to 1 year69,89. After 2 years, irreversible muscle fibrosis has occurred along with muscle degeneration, leading to a permanent loss of functional muscle tissue. Sensory end-organs such as Paccinian corpuscles, Meissner corpuscles and Merkel cells can last up to 2-3 years, so sensory function can still be recovered even after muscle function is permanently lost33. The time for growth supportive phenotypes of Schwann cells associated with target tissues are also limited90. Nerve transfers have been used to try and prevent denervation atrophy, but full strength and muscle function are seldom fully recovered91.
Experimental strategies
Various models of nerve injury are used to develop viable treatments for nerve injury. In vitro models of neuronal survival include cell culture, tissue engineered 3D cultures, organotypic cultures and glial cell cultures whereas in vivo models most often involve injury to the sciatic nerve of varied species.
In vitro Models
Neurons from the dorsal root ganglion are the most commonly used neurons in neuronal cell culture experiments. Animals only yield a small number of cells however, which can be prohibitive92. A common cultured cell line is the rat pheochromocytoma cell line (PC12), which has neuroendocrine function and can be induced to differentiate into adult sympathetic neurons using NGF93. Cell cultures are commonly used for in vitro studies of the direct effects of drugs, substrates, and growth factors on neurons. Neurons co-cultured with glial cells and other cell-types have been useful in elucidating the interactions between those cells.
Culturing neural cells has also provided information on the dependence of neural cells on an appropriate microenvironment for optimal recovery. Tissue engineered 3D cultures utilize an artificial tissue based ECM to study neurite growth. Contact guidance cues can be added to study axonal path taking and guidance through the endoneurial tube94,95. Organotypic cultures utilize the natural environment of the neurons in such a way that the tissue cytoarchitecture and interactions between cell types are preserved96,97.
In vivo – nerve injury models
Animal models of crush and laceration injuries can be created to follow clinically relevant lesions and allow for greater study of molecular and cellular processes within particular nerve types (ie mixed, motor, sensory). Rodents are the most commonly used animals due to their inexpensive housing costs and similar distribution of nerve trunks to humans. Also, there is a large availability of genetic, cellular and systemic physiology in rodent models including transgenic animals98.
The most common injury model is the sciatic nerve. Although sciatic nerve injuries in humans are rare to due the deep anatomical location within the lower extremity, the animal model provides a plethora of information regarding recoveries of particular nerve types and their potential for recovery as they attempt to reach endoneurial tubules. Femoral nerve injuries in the rat are relevant in the study of particular nerve types because it has one exclusively motor branch and another, which is exclusively sensory 99,100.
Median nerve injuries are important as they are common in clinical practice but in practice, assays of median nerve function are prohibitively difficult in rodents. Some work focuses on electrophysiological techniques along with measures of grasping for functional outcome in rats101 and more recently, in mice using 12/0 sutures for repair after injury102.
Outcome measurements of in vivo models
Proper outcome measurements in the assessment of functional nerve recovery are critical to define conclusive findings. Outcome measurements include: counting the number of particular structures such as axons, neurons, reinnervated end-plates and reinnervated motor units, assessing nerve and muscle evoked potentials through electrophysiological studies and evaluating muscle contractile force and weight. Behavioral studies also exist particularly in assessing sciatic nerve function through walking track analysis and sensory hypersensitivity function testing. Axon count and muscle contractile forces may be the most valid assessment during the early phases of axon regeneration. At this phase, sprouts regenerate asynchronously and reach their end-target at different times so may provide misleading information using evoked potentials or other forms of assessment103.
Conclusion
The peripheral nerve can be injured in a variety of ways and most injuries are a mixture of previously described mechanisms. The nerve can be assessed in a number of ways clinically but the basic and translational sciences behind new treatments are only now being elucidated. The clinical correlations of these processes when better understood promise to guide treatment decision in the future.
Synopsis/Key Points.
Peripheral nerve injuries are common and can be very debilitating leading to poor quality of life. Available treatments remain suboptimal. Injuries range in severity from mild compression to severe crush and lacerations.
Classification schemes describing the extent of injury provide clinicians and scientists with a language to correlate nerve pathophysiology with patient symptoms and prognosis.
In vivo injury models particularly the sciatic nerve of rodents have extensively been used to study the effectiveness of both surgical and medical treatments for nerve injury using appropriate outcome measurements.
Experiments using cell cultures have elucidated interactions among nerve cell constituents and revealed the complex interplay between factors in the injury micro environment.
Taken together, advancements in the understanding of nerve injury and recovery continue to provide new avenues for surgeons to explore future prospective therapies.
Footnotes
The authors of this article have no disclosures.
References
- 1.Jobe MT, Martinez SF. Canale & Beaty: Campbell's Operative Orthopaedics. 12th ed. Elsevier; Philadelphia: 2013. Peripheral Nerve Injuries. pp. 3063–3065. Ch 62D. [Google Scholar]
- 2.Berde CB, Strichartz GR. Local Anesthetics. In: Eriksson I, Fleisher LA, Wiener-Kronish JP, Young WL, editors. Miller's Anesthesia. 7th ed. An Imprint of Elsevier; Churchill Livingstone: 2009. p. 917. [Google Scholar]
- 3.Miner JR, Paris PM, Yealy DM. Marx, et al., editors. Pain Management. Mark: Rosen's Emergency Medicine. 7th ed. 2010.
- 4.Geuna S, Raimondo S, Ronchi G, et al. Histology of the peripheral nerve and changes occurring during nerve regeneration. Int. Rev. Neurobiol. 2009;87:27–46. doi: 10.1016/S0074-7742(09)87003-7. [DOI] [PubMed] [Google Scholar]
- 5.Rydevik B, Lundborg G. Permeability of intraneural microvessels and perineurium following acute, graded experimental nerve compression. Scand J PLast REconstr Surg. 1977;11:179–87. doi: 10.3109/02844317709025516. [DOI] [PubMed] [Google Scholar]
- 6.Seddon HJ. Three types of nerve injury. Brain. 1943;66:237. [Google Scholar]
- 7.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491–516. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 8.Mackinnon SE, Dellon AL. Surgery of the peripheral nerve. Thieme; New York: 1988. [Google Scholar]
- 9.Thomas PK, Holdorff B. Neuropathy due to physical agents. In: Dyck PJ, Thomas PK, editors. Griffin JW editiors. Peripheral neuropathy. 3rd ed. W.B. Saunders; Piladelphia: 1993. p. p990. [Google Scholar]
- 10.Netscher D, Murphy K, Fiore NA. Hand Surgery: Ch. 70 Nerve compression syndrome. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, editors. Townsend: Sabiston Textbook of Surgery. 19th ed. Elsevier; 2012. pp. 1982–1984. [Google Scholar]
- 11.Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol. 2003;461:174–186. doi: 10.1002/cne.10692. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Rummler LS, Palispis W, et al. Local down-regulation of myelin-associated glycoprotein permits axonal sprouting with chronic nerve compression injury. Exp Neurol. 2006;200:418–429. doi: 10.1016/j.expneurol.2006.02.134. [DOI] [PubMed] [Google Scholar]
- 13.Ludwin SK, Maitland M. Long-term remyelination fails to reconstitute normal thickness of central myelin sheaths. J Neurol Sci. 1984;64:193–198. doi: 10.1016/0022-510x(84)90037-6. [DOI] [PubMed] [Google Scholar]
- 14.Tapadia M, Mozaffar T, Gupta R. Compressive neuropathies of the upper extremity: update on pathophysiology, classification, and electrodiagnostic findings. JHS. 2010;35A:668–677. doi: 10.1016/j.jhsa.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray M, Palispis W, Popovich PG, van Rooijen N, Gupta R. Macrophage depletion alters the blood-nerve barrier without affecting Schwann cell function after neural injury. J Neurosci Res. 2007;85:766–777. doi: 10.1002/jnr.21166. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R, Channual JC. Spatiotemporal pattern of macrophage recruitment after chronic nerve compression injury. J Neurotrauma. 2006;23(2):216–26. doi: 10.1089/neu.2006.23.216. [DOI] [PubMed] [Google Scholar]
- 17.Frieboes LR, Gupta R. An in-vitro traumatic model to evaluate the response of myelinated cultures to sustained hydrostatic compression injury. J Neurotrauma. 2009;26:2245–2256. doi: 10.1089/neu.2009.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinik A, Mehrabyan A, Colen L, Boulton A. Focal entrapment neuropathies in diabetes. Diabetes Care. 2004;27:1783–1738. doi: 10.2337/diacare.27.7.1783. [DOI] [PubMed] [Google Scholar]
- 19.Bales JG, Meals R. Peripheral neuropathy of the upper extremity: medical comorbidity that confounds common orthopedic pathology. Orthopedics. 2009;32:758–765. doi: 10.3928/01477447-20091001-02. [DOI] [PubMed] [Google Scholar]
- 20.Stamboulis E, Vassilopoulos D, Kalfakis N. Symptomatic focal mononeuropathies in diabetic patients: increased or not? J Neurol. 2005;252:448–452. doi: 10.1007/s00415-005-0672-8. [DOI] [PubMed] [Google Scholar]
- 21.Dellon AL, Mackinnon SE, Seiler WA. Susceptibility of the diabetic nerve to chronic compression. Ann Plast Surg. 1988;20:117–119. doi: 10.1097/00000637-198802000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura T, Hirata H, Tsujii M, et al. Pathomechanism of entrapment neuropathy in diabetic and nondiabetic rats reared in wire cages. Histol Histopathol. 2008;23:157–166. doi: 10.14670/HH-23.157. [DOI] [PubMed] [Google Scholar]
- 23.Molinari WJ, III, Elfar JC. The Double Crush Syndrome. Brief. JHS Article. 2013 doi: 10.1016/j.jhsa.2012.12.038. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Upton AR, McComas AJ. The double crush in nerve entrapment syndromes. Lancet. 1973;2:359–62. doi: 10.1016/s0140-6736(73)93196-6. [DOI] [PubMed] [Google Scholar]
- 25.Dahlin LB, Lundborg G. The neurone and its response to peripheral nerve compression. J Hand Surg [Br] 1990;15:5–10. doi: 10.1016/0266-7681_90_90040-b. [DOI] [PubMed] [Google Scholar]
- 26.Nemoto K. An experimental study on the vulnerability of peripheral nerve. J Jpn Orthop Assoc. 1983;57:1773–86. [PubMed] [Google Scholar]
- 27.Nemoto K, Matsumoto N, Tazaki K, et al. An experimental study on the “double crush” hypothesis. J Hand Surg [Am] 1987;12:552–9. doi: 10.1016/s0363-5023(87)80207-1. [DOI] [PubMed] [Google Scholar]
- 28.Dellon AL, Mackinnon SE. Chronic nerve compression model for the double crush hypothesis. Ann Plast Surg. 1991;26:259–64. doi: 10.1097/00000637-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien JP, Mackinnon SE, MacLean AR, et al. A model of chronic nerve compression in the rat. Ann Plast Surg. 1987;19:430–435. doi: 10.1097/00000637-198711000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Diao E, Shao F, Liebenberg E, et al. Carpal tunnel pressure alters median nerve function in a dose-dependent manner: a rabbit model for carpal tunnel syndrome. J Orthop Res. 2005;23:218–223. doi: 10.1016/j.orthres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Gupta R, Nassiri N, Hazel A, Bathen M, Mozaffar T. Chronic nerve compression alters Schwann cell myelin architecture in a murine model. Muscle Nerve. 2012;45(2):231–41. doi: 10.1002/mus.22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki Y, Shirai Y. Motor nerve conduction analysis of double crush syndrome in a rabbit model. J Orthop Sci. 2003;8:69–74. doi: 10.1007/s007760300011. [DOI] [PubMed] [Google Scholar]
- 33.Tsao B, Boulis N, Bethoux F, Murray B. Daroff: Bradley's Neurology in Clinical Practice. 6th ed. 2012. Trauma of the Nervous System, Peripheral Nerve Trauma. pp. 984–1001. [Google Scholar]
- 34.Zochodne DW, Levy D. Nitirc Oxide in damage, disease and repair of the peripheral nervous system. Cell Mol Biol (Nosi-le-grand) 2005;51:255–67. [PubMed] [Google Scholar]
- 35.Aguayo AJ, Peyronnard JM, Bray GM. A quantitative ultrastructural study of regeneration from isolated proximal stumps of transected unmyelinated nerves. J. Neuropathol. Exp. Neurol. 1973;32:256–270. doi: 10.1097/00005072-197304000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Sanders FK, Young JZ. The indluence of peripheral connection on the diameter of regenerating nerve fibers. J Exp. Biol. 1946;22:203–212. doi: 10.1242/jeb.22.3-4.203. [DOI] [PubMed] [Google Scholar]
- 37.Griffin JW, Hoffman PN. Degeneration and regeneration in the peripheral nervous system. In: Dyck PJ, Thomas P, editors. Peripheral Neuropathy. WB Saunders; Philadelphia: 1993. [Google Scholar]
- 38.Campbell WW. Evaluation and management of peripheral nerve injury. Invited review. Clinical Neurophys. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Lunn ER, Brown MC, Perry VH. The pattern of axonal degeneration in the peripheral nervous system varies with different types of lesion. Neuroscience. 1990;35:157–65. doi: 10.1016/0306-4522(90)90130-v. [DOI] [PubMed] [Google Scholar]
- 40.George R, Griffin JW. The proximo-distal spread of axonal degeneration in the dorsal columns of the rat. J Neurocytol. 1994;23(11):657–667. doi: 10.1007/BF01181641. [DOI] [PubMed] [Google Scholar]
- 41.Miledi R, Slater CR. On the degeneration of rat neuromuscular junctions after nerve section. J. Physiol. 1970;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones R, Vrbova G. Two factors responsible for the development of denervation hypersensitivity. J Physiol. 1974;236(3):517–538. doi: 10.1113/jphysiol.1974.sp010450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charlton MP, Atwood HL. Modulation of transmitter release by intracellular sodium in squid giant synapse. Brain Res. 1977;134(2):367–371. doi: 10.1016/0006-8993(77)91081-2. [DOI] [PubMed] [Google Scholar]
- 44.Michalski B, Bain JR, Fahnestock M. Long-term changes in neurotrophic factor expression in distal nerve stump following denervation and reinnervation with motor or sensory nerve. J Neurochem. 2008;105(4):1244–52. doi: 10.1111/j.1471-4159.2008.05224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dieu T, Johnstone BR, Newgreen DF. Genes and nerves. J Reconstr Microsurg. 2005;21:179–186. doi: 10.1055/s-2005-869824. [DOI] [PubMed] [Google Scholar]
- 46.Tetzlaff W, Bisby MA. Neurofilament elongation into regenerating facial nerve axons. Neuroscience. 1989;29(3):659–666. doi: 10.1016/0306-4522(89)90138-3. [DOI] [PubMed] [Google Scholar]
- 47.Tetzlaff W, Alexander SW, Miller FD, Bisby MA. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J. Neurosci. 1991;11(8):2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997;14(1-2):67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 49.Costigan M, Mannion RJ, Kendall G, et al. Heat shock protein 27:developmental regulation and expression after peripheral nerve injury. J. Neurosci. 2005;21:2051–62. doi: 10.1523/JNEUROSCI.18-15-05891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis SE, Mannion RJ, White FA, et al. A role for HSP27 in sensory neuron survival. J Neurosci. 1999;19(89):45–53. doi: 10.1523/JNEUROSCI.19-20-08945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall S. Mechanisms of repair after traumatic injury. In: Dyck PJ, Thomas PK, editors. Peripheral neuropathy. Elsevier, Saunder; Philadelphia: 2005. pp. 1403–33. [Google Scholar]
- 52.Topilko P, Schneider-Maunoury S, Baron-Van Evercooren G, Levi A, Chennoufi AB, Seitanidou T, et al. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- 53.Parkinson DB, Bhaskaran A, Arthur-Farraj P, et al. c- Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Funakoshi H, Friesen J, Barbany G, et al. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–65. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elfar JC, Jacobson JA, Puzas JE, et al. Erythropoietin accelerates functional recovery after peripheral nerve injury. J Bone Joint Surg Am. 2008;90:1644–1653. doi: 10.2106/JBJS.G.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pellegrino RG, Spencer PS. Schwann cell mitosis in response to regenerating peripheral axons in vivo. Brain Res. 1985;341(1):16–25. doi: 10.1016/0006-8993(85)91467-2. [DOI] [PubMed] [Google Scholar]
- 57.Birchemier C, Nave KA. Neuregulin-1 a key axonal signal that drives Schwann cell growth and differentiation. GLIA. 2008;56(14):1491–1497. doi: 10.1002/glia.20753. [DOI] [PubMed] [Google Scholar]
- 58.Vrbova G, Mehra N, Shanmuganathan H, et al. Chemical communication between regenerating motor axons and Schwann cells in the growth pathway. Eur. J. Neurosci. 2009;30(3):366–375. doi: 10.1111/j.1460-9568.2009.06847.x. [DOI] [PubMed] [Google Scholar]
- 59.Stoll G, Griffin JW, Li CY, Trapp BD. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol. 1989;18:671–683. doi: 10.1007/BF01187086. [DOI] [PubMed] [Google Scholar]
- 60.Reichert F, Saada A, Rotshenker S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. Journal of NeuroScience. 1994;14:3231–3245. doi: 10.1523/JNEUROSCI.14-05-03231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toews AD, Barrett C, Morel P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. Journal of Neuroscience Research. 1998;53:260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 62.Taskinen HS, Roytta M. The dynamics of macrophage recruitment after nerve transection. Acta Neuropathol. 1997;82:412–425. doi: 10.1007/s004010050611. [DOI] [PubMed] [Google Scholar]
- 63.Bruck W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997;7(2):741–752. doi: 10.1111/j.1750-3639.1997.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rothshenker S. Microglia and macrophage activation and the regulation of complement-receptor-3 (CR3/MAC-1)-mediated myelin phagocytosis in injury and disease. J. Mol. Neurosci. 2003;21(1):65–72. doi: 10.1385/JMN:21:1:65. [DOI] [PubMed] [Google Scholar]
- 65.Venezie RD, Toews AD, Morell P. Macrophage recruitment in different models of nerve injury: lysozyme as a marker for active phagocytosis. J of Neuroscience Research. 1995;40:99–107. doi: 10.1002/jnr.490400111. [DOI] [PubMed] [Google Scholar]
- 66.Baichwal J, Bigbee W, Devries GH. Macrophage-mediated myelin-related mitogenic factor for cultured Schwann cells. Neurobiology. 1988;85:1701–1705. doi: 10.1073/pnas.85.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weinberg HJ, Spencer PS. The fate of Schwann cells isolated from axonal contact. J Neurocytol. 1978;7(5):555–569. doi: 10.1007/BF01260889. [DOI] [PubMed] [Google Scholar]
- 68.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:1–7. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 69.Dahlin LB. Nerve injury and repair: from molecule to man. In: Slutsky DJ, Hentz VR, editors. Peripheral nerve surgery: practical applications in the upper extremity. Churchill Livingston, Elsevier; Philadelphia: 2006. pp. 1–22. [Google Scholar]
- 70.Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J. Cell Scie. 2009;122(20):3595–3604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin LQ, Zhang G, Jamison C, Jr, et al. Axon regeneration in the absence of growth cones: acceleration by cyclic AMP. J. Comp. Neurol. 2009;515(3):295–312. doi: 10.1002/cne.22057. [DOI] [PubMed] [Google Scholar]
- 72.Marx J. Helping neurons find their way. Science. 1995;268:971–973. doi: 10.1126/science.7754391. [DOI] [PubMed] [Google Scholar]
- 73.Goodman CS. Mechanisms and molecules that control growth cone guidance. Annu Rev Neurosci. 1996;19:341–377. doi: 10.1146/annurev.ne.19.030196.002013. [DOI] [PubMed] [Google Scholar]
- 74.Tuttle R, O'Leary DD. Neurotrophins rapidly modulate growth cone response to the axon guidance molecule, collapsin-1. Mol Cell Neurosci. 1998;11:1–8. doi: 10.1006/mcne.1998.0671. [DOI] [PubMed] [Google Scholar]
- 75.Frostick SP, Yin Q. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 76.Hall S. Axonal regeneration through acellular muscle grafts. J Anat. 1997;190:57–71. doi: 10.1046/j.1469-7580.1997.19010057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis JB, Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J Cell Biol. 1990;110:1353–1360. doi: 10.1083/jcb.110.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guenard V, Dinarello CA, Weston PJ, Aebischer P. Peripheral nerve regeneration is impeded by interleukin-l receptor antagonist released from a polymeric guidance channel. Journal of Neuroscience Research. 1991;29:396–400. doi: 10.1002/jnr.490290315. [DOI] [PubMed] [Google Scholar]
- 79.Battison B, Papalia I, Tos P, Geuna S. Peripheral nerve repair and regeneration research: a historical note. Int. Rev. Neurobiol. 2009;87:1–7. doi: 10.1016/S0074-7742(09)87001-3. [DOI] [PubMed] [Google Scholar]
- 80.Schröder JM. Altered ratio between axon diameter and myelin sheath thickness in regenerated nerve fibers. Brain Res. 1972;45:49–65. doi: 10.1016/0006-8993(72)90215-6. [DOI] [PubMed] [Google Scholar]
- 81.Stassart RM, Fledrich R, Velanac V, et al. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16(1):48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- 82.Kang JR, Zamorano DP, Gupta R. Limb salvage with major nerve injury: current management and future directions. J Am Acad Orthop Surg. 2011;19(suppl 1):S28–S34. doi: 10.5435/00124635-201102001-00006. [DOI] [PubMed] [Google Scholar]
- 83.Hughes BW, Kusner LL, Kaminski HJ. Molecular architecture of the neuromuscular junction. Muscle Nerve. 2006;33(4):445–461. doi: 10.1002/mus.20440. [DOI] [PubMed] [Google Scholar]
- 84.Reist NE, Werle MJ, McMahan UJ. Agrin released by motor neurons induces the aggregation of acetylcholine receptors at neuromuscular junctions. Neuron. 1992;8(5):865–868. doi: 10.1016/0896-6273(92)90200-w. [DOI] [PubMed] [Google Scholar]
- 85.Gautam M, Noakes PG, Moscoso L, et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85(4):525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 86.Shyng SL, Salpeter MM. Degradation rate of acetylcholine receptors inserted into denervated vertebrate neuromuscular junctions. J Cell Biol. 1989;108(2):647–651. doi: 10.1083/jcb.108.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kline DG. Clinical and electrical evaluation. In: Kim DH, Midha R, Murovic JA, Spinner RJ, editors. Kline & Hudson's nerve injuries. 2nd ed. Elsevier, Saunders; Philadelphia: 2008. pp. 43–63. [Google Scholar]
- 88.Furey MJ, Midha R, Xu QG, Belkas J, Gordon T. Prolonged target deprivation reduces the capacity of injured motoneurons to regenerate. Neurosurgery. 2007;60(4):723–732. doi: 10.1227/01.NEU.0000255412.63184.CC. [DOI] [PubMed] [Google Scholar]
- 89.Noaman HH, Shiha AE, Bahm J. Oberlin's ulnar nerve transfer to the biceps motor nerve in obstetric brachial plexus palsy: Indications, and good and bad results. Microsurgery. 2004;24(3):182–187. doi: 10.1002/micr.20037. [DOI] [PubMed] [Google Scholar]
- 90.Federoff S, Richardson A. Protocols for Neural cell culture. Humana Press; 2001. [Google Scholar]
- 91.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor”. Proc. Natl. Acad. Sci. U.S.A. 1976;73(7):2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bellamkonda R, Ranieri JP, Aebischer P. Laminin oligopeptide derivatized agarose gels allows three0dimensional neurite extension in vitro. J. Neurosci. 1995;41:501–509. doi: 10.1002/jnr.490410409. [DOI] [PubMed] [Google Scholar]
- 93.Ahmed Z, Brown RA. Adhesion, alignment, and migration of cultured Schwann cells on ultrathin fibronectin fibres. Cell Motil. Cytoskeleton. 1999;42:331–343. doi: 10.1002/(SICI)1097-0169(1999)42:4<331::AID-CM6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 94.Hall SM. The biology of chronically denervated Schwann cells. Ann. N.Y Acad Sci. 1999;883:215–233. [PubMed] [Google Scholar]
- 95.Maniwa S, Iwata A, Hirata H, Ochi M. Effects of neurotrohic factors on chemokinesis of Schwann cells in culture. Scand J Plast Reconstr Surg Hand Surg. 2003;37(1):14–7. doi: 10.1080/alp.37.1.14.17. [DOI] [PubMed] [Google Scholar]
- 96.Paratcha G, Ledda F, Ibanez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2004;113:867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez FJ, Valero-Cabre A, Navarro X. Regeneration and functional recovery following peripheral nerve injury. Drug Discovery Today: Dis Mod. 2004;1(2):177–185. [Google Scholar]
- 98.Brushart TM, Gerber J, Kessens P, et al. Contributions of pathway and neuron preferential motor reinnervation. J. Neurosci. 1998;18:8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Madison RD, Archibald SJ, Brushart TM. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. J. Neurosci. 1996;16:5698–5703. doi: 10.1523/JNEUROSCI.16-18-05698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Papalia I, Tos P, Stagno d'Alcontres F, et al. On the use of grasping test in the rat median nerve model: A reappraisal of its efficacy for quantitative assessment of motor function recovery. J. Neurosci. Methods. 2003;134:75–80. doi: 10.1016/s0165-0270(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 101.Tos P, Ronchi G, Nicolino S, et al. Employment of the mouse median nerve model for the experimental assessment of peripheral nerve regeneration. J Neurosci Methods. 2008;30169(1):119–27. doi: 10.1016/j.jneumeth.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 102.Wood MD, Kemp SWP, Borschel GH, Gordon T. Outcome measures of peripheral nerve regeneration. Annals of Anatomy. 2011;193:321–333. doi: 10.1016/j.aanat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Pham K, Gupta R. Understanding the mechanisms of entrapment neuropathies. Review article. Neurosurg Focus. Feb. 2009;26(2):E7. doi: 10.3171/FOC.2009.26.2.E7. [DOI] [PubMed] [Google Scholar]
- 104.Navarro X, Vivo M, Valero-Cabre Neural plasticity after peripheral nerve injury and regeneration. Progress in Neurobiology. 2007 Jul;82(4):163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]