Abstract
OBJECTIVE
We sought to determine prevalence and likelihood of venous thromboembolism (VTE) among women with and without polycystic ovary syndrome (PCOS).
STUDY DESIGN
We performed a cross-sectional analysis using Thomson Reuters MarketScan Commercial databases for the years 2003 through 2008. The association between VTE and PCOS among women aged 18–45 years was assessed using age-stratified multivariable logistic regression models.
RESULTS
Prevalence of VTE per 100,000 was 374.2 for PCOS women and 193.8 for women without PCOS. Compared with women without PCOS, those with PCOS were more likely to have VTE (adjusted odds ratio [aOR] 18–24 years, 3.26; 95% confidence interval [CI], 2.61–4.08; aOR 25–34 years, 2.39; 95% CI, 2.12–2.70; aOR 35–45 years, 2.05; 95% CI, 1.84–2.38). A protective association (odds ratio, 0.8; 95% CI, 0.73–0.98) with oral contraceptive use was noted for PCOS women.
CONCLUSION
PCOS might be a predisposing condition for VTE, particularly among women aged 18–24 years. Oral contraceptive use might be protective.
Keywords: polycystic ovary syndrome, prevalence, venous thromboembolism
Venous thromboembolism (VTE) is a chronic condition that includes both deep vein thrombosis (DVT) and pulmonary embolism (PE). Initial presentation might be leg pain, tenderness, shortness of breath, or pleuritic chest pain, or there might be no symptoms.1 VTE is the third most common cardiovascular disease–after myocardial infarction and stroke–among the general population.2,3 The overall annual incidence of VTE is estimated to be 1–2 per 1000 adults per year.4–7 These incident estimates, however, might not represent the entire population because VTE incidence differs by age and race, and slightly by sex. VTE is a multifactorial disease with both inherited and acquired risk factors. In 26–47% of first-time VTE cases, the etiology is unknown4,5,8; a possible predisposing condition not yet assessed is polycystic ovary syndrome (PCOS).
PCOS is the most common endocrine disorder affecting women of reproductive age. Estimates of its prevalence vary and range from 4–6%.9–12 Its exact etiology is unknown, but it is characterized by a heterogeneous presentation of hyperandrogenism, ovulatory dysfunction, and polycystic ovaries.13 PCOS is also associated with insulin-induced elevations of plasminogen activator inhibitor (PAI)-1, which is a potent inhibitor of fibrinolysis.14 While some studies have found PCOS to be associated with coronary heart disease risk factors,15–20 other studies have shown that women with PCOS were 3 times more likely to have a family history of venous thrombosis.21 Mak and Dokras22 hypothesized that women with PCOS likely have an increased baseline risk for developing thrombosis compared with other women in the general population. However, no study has evaluated the prevalence of VTE among women with PCOS compared with women without PCOS. Given the potential for thrombotic disease among women with PCOS, the purpose of this study was to: (1) determine the prevalence of VTE among women with and those without PCOS; and (2) assess the association between PCOS and VTE.
Materials and Methods
Data source
Data for the study were derived from the 2003 through 2008 Thomson Reuters MarketScan Commercial databases.23 These databases comprise longitudinal, deidentified health insurance claims data from large employers and health plans across the United States and contain information on inpatient admissions, outpatient visits, and pharmaceutical claims. The data are derived from multiple US states that are geographically diverse.23 In 2008, >35 million individuals were enrolled, including employees and their dependents who were aged <65 years and geographically distributed throughout the United States.23
This commercial database has met or exceeded requirements of the US Health Insurance Portability and Accountability Act of 1996 and, accordingly, does not require specific patient consent to participate in the study.24
Population
We restricted the analysis to women 18–45 years of age. The upper limit of age was set at 45 years to keep within the age limit used by other prevalence studies.1–4 Women with PCOS were defined by the presence of hyperandrogenism, the presence of ovulatory dysfunction, and/or the presence of polycystic ovaries. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used to identify these conditions in the database. Each condition was defined as follows.
“Clinical hyperandrogenism” was defined as the presence of ICD-9-CM codes for acne (706.0 or 706.1), alopecia (704.0x), or hirsutism (704.1). Elevated testosterone, another characteristic of clinical hyperandrogenism, was not included because no ICD-9-CM codes were available for this laboratory value in the database.
“Ovulatory dysfunction” was defined by the presence of any 1 of the ICD-9-CM codes for the 626 series, which are codes dealing with disorders of menstruation and other abnormal bleeding from the female genital tract: 626.0, 626.1, 626.2, 626.3, 626.4, 626.5, 626.6, 626.7, 626.8, or 626.9.
“Polycystic ovaries” were defined by ICD-9-CM code 256.4.
Exclusion of other conditions
We excluded women who met the criteria for any of the PCOS phenotypes and had ≥1 of the following conditions: adrenal hyperplasia (255.0 or 255.9), hyperprolactinemia (253.1), or thyroid disorder (244.0, 244.1, 244.2, 244.3, 244.8, 244.9, 242.90, or 242.91).
Combinations of these conditions were used to create 4 mutually exclusive PCOS phenotypes based on the 3 available criterion recommendations (National Institutes of Health, Rotterdam, and Androgen Society). Women with phenotype A, or classic PCOS, were defined by the presence of hyperandrogenism (704.1, 706.0 or 706.1, or 704.0x) and ovulatory dysfunction (626.0, 626.1, 626.2, 626.3, 626.4, 626.5, 626.6, 626.7, 626.8, or 626.9); but polycystic ovaries (256.4), adrenal hyperplasia (255.0 or 255.9), hyperprolactinemia (253.1), and thyroid disorder (244.0, 244.1, 244.2, 244.3, 244.8, 244.9, 242.90, or 242.91) were absent. Definitions of the remaining PCOS phenotypes are found in the Appendix.
Due to the heterogenic nature of PCOS and its complex presentation and definition, we also assessed the prevalence of associated conditions seen among women with PCOS (eg, obesity [278.0, 278.00–278.02, 783.1, or V77.8]; infertility [628.0 or 628.9]; syndrome X or metabolic syndrome [277.7]; and diabetes [250.00, 250.02, 250.90, or 250.92]) to provide additional information on the frequency of these conditions within the different PCOS phenotypes. For all conditions related to PCOS, diagnoses reported on inpatient claims were considered valid; diagnoses based solely on outpatient claims required that the diagnoses were reported on ≥2 claims that occurred >30 days apart.
Women with possible VTE during the study period also were identified using ICD-9-CM codes for DVT (671.3x, 671.4x, 671.5x, 671.9x, 451.11, 451.19, 451.2, 451.81, 451.9, 453.1, 453.2, 453.40–453.42, 453.8, or 453.9), PE (673.2x, 673.8x, 415.11, or 415.19), or both on any inpatient or outpatient claim. To reduce inaccurate reporting of DVT and PE diagnoses, we restricted outpatient diagnoses to those with a relevant Current Procedural Terminology code (Appendix) and who had a filled prescription for an anticoagulant medication within 90 days of the VTE diagnosis; for an inpatient diagnosis, only 1 appropriate ICD-9-CM code for DVT or PE was required. Diagnosis of VTE was counted only once, regardless of the number of times it was reported in the claims database.
Unique identification numbers were used to track all women longitudinally over the study period. Women were included if they were enrolled in any health plan, even if no health claims were submitted during the 6-year study period.
Covariates
Information on demographic characteristics and clinical comorbid conditions was captured from the claims database: age, geographic region of residence, pregnancy during the time frame of study, oral contraceptive use, diabetes, obesity, metabolic syndrome, thrombophilia (289.81), and history of a VTE (V12.51). Pregnancy was defined as any inpatient hospital admission for a pregnancy-related diagnosis or procedure (V27.x, 650, 72.0, 72.1, 72.21, 72.29, 72.31, 72.39, 72.4, 72.51–72.54, 72.6, 72.71, 72.79, 72.8, 72.9, 73.22, 73.59, 73.6, 74.0–74.2, 74.4, or 74.99), excluding records with codes or procedures for hydatidiform mole, ectopic pregnancy, other abnormal products of conception, or abortion (630, 631, 632, 633.x, 634.x–638.x, 639.x, 69.01, 69.51, 74.91, or 75.0). Contraceptive use was identified by ≥1 filled oral contraceptive pill (OCP) prescription during the study period; the use of other types of contraceptives such as the patch, the ring, and/or injections was excluded from the analysis.
Data analysis
To calculate VTE prevalence among women with PCOS, the number of VTE cases among women with PCOS was divided by the total number of women among the underlying MarketScan population with PCOS. For the individual phenotypes, prevalence was calculated by dividing the number of women with VTE and the particular PCOS phenotype by the total number of women with that type of PCOS. For women without PCOS, the prevalence of VTE was calculated by dividing the number of women with VTE and no PCOS by the total underlying MarketScan population without PCOS. Differences in the distribution of demographic and comorbid conditions among women with and those without PCOS were assessed using 2-tailed χ2 tests.
Bivariate analyses were used to compare the distribution of demographic and potential clinical risk factors for VTE among women with and those without PCOS. Multivariate logistic regression analyses also were used to assess the likelihood of a VTE diagnosis among women with and those without PCOS. The final multivariable regression model had only those variables that have been shown to be associated with VTE risk and changed the estimate of the association between VTE and PCOS by >10%. These variables, which we adjusted for, were age, pregnancy during study period, OCP use, region, obesity, and diabetes. We also used logistic regression models to test for interactions between PCOS and the previously cited variables. Age was found to be an effect modifier, so we stratified the analysis by 3 age groups (18–24, 25–34, and 35–45 years). Even though OCP use was not found to be an effect modifier, we stratified the analysis by OCP use because, in 2003, Seaman et al25 demonstrated an increased risk of VTE among women with acne, hirsutism, or PCOS who used cyproterone acetate in combination with ethinyl estradiol. All data were analyzed and managed using software (SAS, version 9.2; SAS Institute Inc, Cary, NC). All analyses were conducted at a priori significance level (P < .05).
Results
Prevalence of VTE
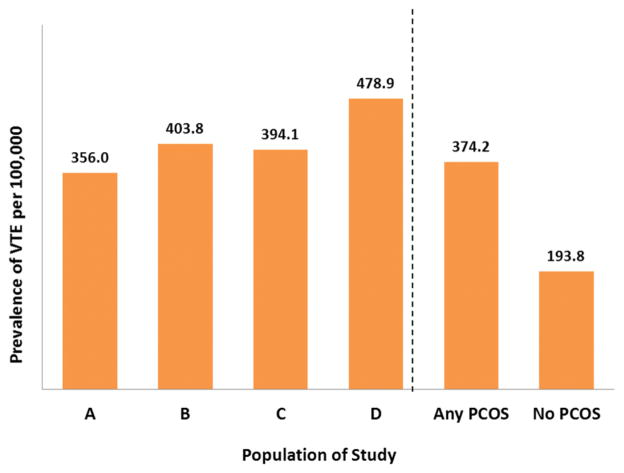
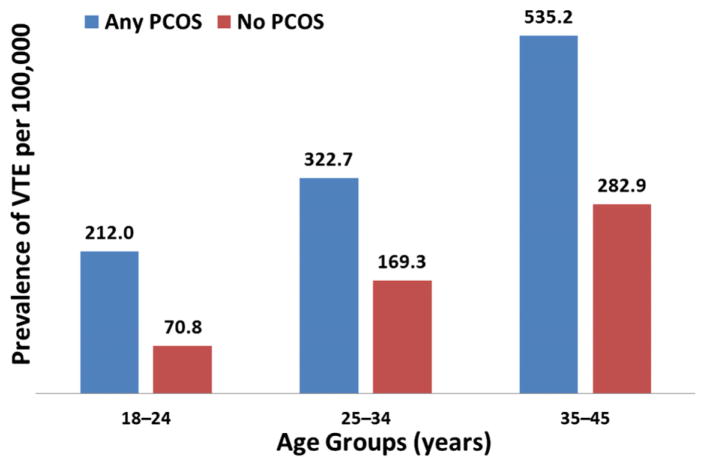
A total of 12,171,830 women were eligible to be in the study; of these, 23,941 (0.20%) had VTE; among the group with VTE, 14,321 (59.8%) had DVT only, 4967 (20.7%) had PE only, and 4653 (19.4%) had both VTE and PE. The overall prevalence of VTE among the PCOS population was 374.2 per 100,000, while the prevalence of VTE among women without PCOS was 193.8 per 100,000 (Figure 1). Among the phenotypes, women with phenotype D had the highest prevalence of VTE, at 478.9 per 100,000 (Figure 1). Figure 2 displays the prevalence of VTE per 100,000, stratified by age, among women with and those without PCOS. For both cohorts, the prevalence of VTE was highest among women 35–45 years of age (Figure 2).
FIGURE 1. Prevalence of VTE during study period, 2003 through 2008.
A, Women with clinical hyperandrogenism; and menstrual or ovulatory dysfunction, or both. B, Women with hyperandrogenism and polycystic ovaries. C, Women with menstrual or ovulatory dysfunction, or both; and polycystic ovaries. D, Women with clinical hyperandrogenism; menstrual or ovulatory dysfunction, or both; and polycystic ovaries. Any PCOS = Women with any polycystic ovary syndrome (PCOS) phenotype (A–D). No PCOS = Women without any PCOS phenotype (A–D).
VTE, venous thromboembolism.
FIGURE 2. Prevalence of VTE stratified by age.
Any PCOS = Women with any PCOS phenotype. No PCOS = Women without any PCOS phenotype.
PCOS, polycystic ovary syndrome; VTE, venous thromboembolism.
The characteristics of women with and those without PCOS are shown in Table 1. Statistically significant differences existed in percentage distributions in VTE frequencies (P <.0001), age (P <.0001), region (P < .0001), presence of metabolic syndrome (P = .0002), obesity (P <.0001), pregnancy (P <.0001), and diabetes (P < .0001). Other significant differences were noted between having inherited thrombophilia (P < .0001) and having used OCPs (P < .0001) (Table 1). There was no significant difference in having a history of VTE (P = .243) between women with and those without PCOS (Table 1).
TABLE 1.
Characteristics of women with and without polycystic ovary syndrome
| Variables | PCOS, n = 192,936 (%)b | No PCOS, n = 11,978,894 (%)b | P valuea |
|---|---|---|---|
| VTE frequencies | 0.37 | 0.19 | < .0001 |
| Age categories, y | < .0001 | ||
| 18–24 | 20.6 | 24.5 | |
| 25–34 | 45.3 | 32.6 | |
| 35–45 | 34.7 | 42.9 | |
| Geographic regionc | < .0001 | ||
| Northeast | 10.3 | 9.8 | |
| North Central | 23.0 | 21.6 | |
| South | 47.5 | 45.4 | |
| West | 18.7 | 22.7 | |
| Unknown | 0.4 | 0.6 | |
| Comorbidities | |||
| Metabolic syndrome | 0.11 | 0.09 | .0002 |
| Obesity | 0.6 | 1.3 | < .0001 |
| Pregnancy | 0.4 | 7.4 | < .0001 |
| Diabetes | 0.3 | 1.4 | < .0001 |
| History of VTE | .2428 | ||
| Yes | 0.034 | 0.029 | |
| Inherited thrombophilia | < .0001 | ||
| Yes | 0.021 | 0.055 | |
| OCP use | < .0001 | ||
| Yes | 49.7 | 24.3 | |
OCP, oral contraceptive pills; PCOS, polycystic ovary syndrome; VTE, venous thromboembolism.
χ2 test comparing proportion between women with and without PCOS;
Might not add up to 100% secondary to rounding;
Final n differed due to missing data from each region.
Okoroh. Polycystic ovary syndrome and venous thromboembolism. Am J Obstet Gynecol 2012.
Table 2 displays the age-stratified association between the PCOS phenotypes and VTE. We found that regardless of age and PCOS phenotype, the odds of having VTE were higher for women with PCOS compared with women without PCOS. We also observed that women 18–24 years of age with PCOS had the highest odds of having VTE (adjusted odds ratio [OR], 3.26; 95% confidence interval [CI], 2.61–4.08) when compared with women 18–24 years of age without PCOS. Lastly, we observed that women 18–24 years of age with phenotype B (hyperandrogenism and polycystic ovaries) had the highest probability of having VTE (adjusted OR, 5.23; 95% CI, 2.88–9.51) when compared with women of the same age without PCOS (Table 2).
TABLE 2.
Association between polycystic ovary syndrome types and venous thromboembolism stratified by age groups
| PCOS types | Age groups | ||
|---|---|---|---|
| 18–24 y | 25–34 y | 35–45 y | |
| aOR (95% CI)a | |||
| No PCOSb | Reference | Reference | Reference |
| Any PCOSc | 3.26 (2.61–4.08) | 2.39 (2.12–2.70) | 2.05 (1.84–2.28) |
| Phenotype Ad | 3.05 (2.29–4.07) | 2.25 (1.91–2.65) | 1.98 (1.75–2.25) |
| Phenotype Be | 5.23 (2.88–9.51) | 2.25 (1.35–3.74) | 1.87 (1.11–3.18) |
| Phenotype Cf | 2.87 (1.80–4.58) | 2.35 (1.91–2.89) | 2.18 (1.73–2.73) |
| Phenotype Dg | 3.11 (1.29–7.49) | 3.06 (2.06–4.54) | 2.37 (1.49–3.78) |
aOR, adjusted odds ratio; CI, confidence interval; PCOS, polycystic ovary syndrome.
Multivariate logistic regression model was adjusted for pregnancy, oral contraceptive pill use, region, diabetes, and obesity;
Women without any PCOS phenotype;
Women with any PCOS phenotype;
Women with clinical hyperandrogenism and menstrual or ovulatory dysfunction, or both;
Women with hyperandrogenism and polycystic ovaries;
Women with menstrual or ovulatory dysfunction, or both, and polycystic ovaries;
Women with clinical hyperandrogenism; menstrual or ovulatory dysfunction, or both; and polycystic ovaries.
Okoroh. Polycystic ovary syndrome and venous thromboembolism. Am J Obstet Gynecol 2012.
In Table 3, we present the adjusted relationships between PCOS phenotypes and VTE when stratified by age and OCP use. We found that women with PCOS still had increased odds of VTE compared with women without PCOS, regardless of age and OCP use. However, when we looked at the unadjusted effect of OCP use on VTE prevalence only among the cohort of women with PCOS (not shown in tables), we observed a protective effect for VTE (OR, 0.8; 95% CI, 0.73–0.97).
TABLE 3.
Association between PCOS types and venous thromboembolism stratified by age and OCP use
| PCOS type | Age groups | ||
|---|---|---|---|
| 18–24 y | 25–34 y | 35–45 y | |
| OCP use = yes,a aOR (95% CI)b | |||
| No PCOSc | Reference | Reference | Reference |
| Any PCOSd | 2.89 (2.17–3.85) | 1.98 (1.66–2.37) | 1.79 (1.51–2.12) |
| Phenotype Ae | 3.11 (2.22–4.36) | 1.69 (1.33–2.16) | 1.83 (1.51–2.22) |
| Phenotype Bf | 4.58 (2.17–9.66) | 1.53 (0.68–3.41) | 1.41 (0.58–3.39) |
| Phenotype Cg | 1.07 (0.40–2.87) | 2.24 (1.66–3.03) | 1.65 (1.12–2.44) |
| Phenotype Dh | 3.23 (1.20–8.64) | 2.89 (1.74–4.82) | 1.69 (0.80–3.57) |
| OCP use = no,i aOR (95% CI)b | |||
| No PCOSc | Reference | Reference | Reference |
| Any PCOSd | 3.76 (2.63–5.38) | 2.70 (2.29–3.18) | 2.18 (1.90–2.50) |
| Phenotype Ae | 2.60 (1.47–4.59) | 2.79 (2.24–3.48) | 2.02 (1.71–2.38) |
| Phenotype Bf | 6.37 (2.36–17.23) | 3.02 (1.57–2.84) | 2.20 (1.14–4.26) |
| Phenotype Cg | 5.26 (3.09–8.94) | 2.36 (1.78–3.13) | 2.54 (1.91–3.36) |
| Phenotype Dh | 2.36 (0.33–16.82) | 3.05 (1.63–5.70) | 3.03 (1.67–5.51) |
aOR, adjusted odds ratio; CI, confidence interval; OCP, oral contraceptive pills; PCOS, polycystic ovary syndrome.
Group of women who had ≥1 filled oral contraceptive prescription during study period;
Multivariate logistic regression model was adjusted for pregnancy, region, diabetes, and obesity;
Women without any PCOS phenotype;
Women with any PCOS phenotype;
Women with clinical hyperandrogenism and menstrual or ovulatory dysfunction, or both;
Women with hyperandrogenism and polycystic ovaries;
Women with menstrual or ovulatory dysfunction, or both, and polycystic ovaries;
Women with clinical hyperandrogenism; menstrual or ovulatory dysfunction, or both; and polycystic ovaries;
Group of women who did not have ≥1 filled oral contraceptive prescription during study period.
Okoroh. Polycystic ovary syndrome and venous thromboembolism. Am J Obstet Gynecol 2012.
Comment
To our knowledge, this is the first study to assess the prevalence of VTE among women with PCOS. We found that the prevalence of VTE was higher among women with PCOS compared with women without PCOS. Similar to findings in other studies, our findings revealed the absolute prevalence of VTE to be highest among older women (34–45 years of age), regardless of PCOS status.3,4,6,7,26 Unexpectedly, however, we found that women 18–24 years of age had the highest likelihood of having a VTE diagnosis, meaning that the relative odds of VTE among those who had PCOS compared with those who did not have PCOS was greatest among women 18–24 years of age, even after adjusting for known risk factors (pregnancy, OCP use, region, diabetes, and obesity). In the multivariable logistic regression models, women with PCOS, regardless of phenotype, also had a higher probability of VTE compared with women without PCOS. In addition, we found that women with phenotype B (hyperandrogenism and polycystic ovaries), who were 18–24 years of age, had the highest prevalence of VTE. These findings raise questions about the role of PCOS among younger women and their risk for VTE development.
The nature of our study design makes it difficult to elucidate the reasons for this finding. However, we do speculate that since fertility is optimized between 18–25 years of age, women with PCOS within this age range would likely have elevated production of estrogen and androgens. This boost in hormone production would likely lead to an increase in the derangement with luteinizing hormone, sex hormone–binding globulin, and hyperinsulinemia noted in PCOS.27 Hyperinsulinemia28 and lower sex hormone–binding globulin29 have been shown to impair fibrinolysis by directly enhancing the secretions of PAI-1.28 PAI-1 is the principal inhibitor of fibrinolysis and may contribute to the development of thrombosis.28,29 From this logic, we speculate that the women in our study population with PCOS between the ages of 18–24 years may have increased levels of PAI-1 compared with the other age groups and thus have the highest probability of VTE. Clinical studies are needed to test this hypothesis. These findings also demonstrate the complex nature of PCOS and its related clinical consequences. For instance, increased cardiovascular risks has been found among women with phenotype A (hyperandrogenism and ovulatory dysfunction) or classic PCOS15–20; on the other hand, Wiltgen and Spritzer30 showed that hirsute ovulatory patients with polycystic ovaries (phenotype B) had a lower prevalence of cardiovascular risk factors than those with the classic PCOS phenotype. One would then expect that the highest probability of VTE also would be found among women with phenotype A and the lowest probability among women with phenotype B. However, our findings indicated the opposite.
Another interesting finding was the protective effect of OCP use on VTE among women with PCOS. We believe this finding makes physiologic sense because the estrogenic component of oral contraceptives suppresses the elevated levels of luteinizing hormone among women with PCOS and, thus, ovarian androgen production. In addition, the estrogen component boosts the making of sex hormone–binding globulin by the liver, thereby reducing the amount of plasma testosterone available to bind to androgen receptors.27 We believe this reduction in androgens likely improves the impaired fibrinolysis present among women with PCOS.
The major strength of this study was in the use of a database that includes a large proportion of the privately insured women in the US population. Also, the group studied was geographically diverse and included women seen both in an inpatient and an outpatient setting, which offered a better estimate of the prevalence of VTE, given that a substantial proportion of patients with VTE are managed in the outpatient setting.31
A principal limitation of the study related to the purpose of administrative claims databases. Because information in such databases is collected for billing rather than for diagnostic purposes, misclassification of cases might occur due to incomplete or inappropriate coding. For instance, we found lower than expected prevalences for obesity and diabetes among women with PCOS, which may be due to poor reliability of the ICD-9-CM codes for these conditions. In 2008, Quan et al32 assessed the validity of International Statistical Classification of Diseases, 10th Revision and ICD-9-CM at 4 teaching hospitals in Alberta, Canada, by evaluating agreement between the codes and chart data for 32 clinical conditions. Sensitivity value for obesity was 24.6%, 77.7% for diabetes without chronic conditions, and 63.6% for diabetes with chronic conditions. Poor agreement for conditions such as obesity was postulated to relate to the fact that these conditions may not be explicitly mentioned by a health care provider in clinical notes and may not affect length of stay, health care, or therapeutic treatment. Moreover, coders may intentionally not code these conditions due to the limited amount of time given to code each chart.32 To handle the possibility of such misclassification, we applied a restriction on how VTE was diagnosed and used an algorithm to define PCOS. Despite these efforts, misclassification still might have been present. For example, since the presence of polycystic ovaries is not an absolute requirement in defining PCOS, an ultrasound may have only been performed in a minority of women; this would have resulted in an underestimation of women with phenotypes B, C, and D.
MarketScan data are obtained from employer-based insurance. Insurance heterogeneity could have resulted in variations in the distribution in other risk factors between the exposed and nonexposed groups that could not have been controlled for in our analysis. However, our findings were comparable with those of studies using clinical information to estimate the prevalence of VTE among women.6,33,34
Ageno et al35 in 2006 and Ay et al36 in 2007 showed that metabolic syndrome was a risk factor for developing VTE and, thus, could play a role in VTE among women with PCOS. Despite our large sample size, we did not have enough women with PCOS, metabolic syndrome, and VTE to surmise on a possible connection. This likely was due to the difficulty in defining metabolic syndrome in an administrative data set and the manner in which it was defined in this study. Lack of demographic information also was a limitation of this study in that we could not report on the racial makeup of our study population.
Our findings add to the limited literature on the relationship between the hypercoagulable state of PCOS and thrombosis development. While several studies have shown that women with PCOS are more prone to atherosclerotic issues,16–20,37 we show here, as Mak and Dokras22 suggested, that women with PCOS have a higher probability of having VTE compared with women without PCOS.22 We acknowledge that our findings should be interpreted with caution and the relationship between PCOS and VTE should be evaluated further in prospective studies because our data were obtained from administrative data.
VTE and arterial thrombosis have been seen as distinct disease entities because of their differing anatomic locations, risk factors, and clinical presentations. However, a number of studies have shown that idiopathic VTE is associated with an increased risk of subsequent cardiovascular events and that patients with idiopathic VTE have an increased prevalence of asymptomatic atherosclerotic lesions.38–40 Because PCOS is associated with atherosclerotic disease, a relationship with venous diseases needs to be considered. Our findings, therefore, do have connotations that might be relevant in clinical settings. The implications of our study for clinicians are that young women with unexplained VTE should be examined for the presence of PCOS. Similarly, women with PCOS should be identified early so prevention strategies can be implemented to lower their risks for cardiovascular conditions and VTE.
Supplementary Material
Acknowledgments
We thank Deidre Thomas, MLS, from the Centers for Disease Control and Prevention’s Public Health Library and Information Center for providing literature search assistance and Ijeoma Azonobi, MD, MPH, from the Centers for Disease Control and Prevention’s Division of Blood Disorders for assistance with manuscript preparation.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors report no conflict of interest.
Presented orally at the 60th Annual Epidemic Intelligence Service Conference, Atlanta, GA, April 11–15, 2011.
References
- 1.Raskob GE, Silverstein R, Bratzler DW, Heit JA, White RH. Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med. 2010;38(Suppl):S502–9. doi: 10.1016/j.amepre.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Giuntini C, Di Ricco G, Marini C, Melillo E, Palla A. Pulmonary embolism: epidemiology. Chest. 1995;107(Suppl):3–9S. doi: 10.1378/chest.107.1_supplement.3s. [DOI] [PubMed] [Google Scholar]
- 3.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5:692–9. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson FA, Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism: the Worcester DVT study. Arch Intern Med. 1991;151:933–8. [PubMed] [Google Scholar]
- 5.Nordström M, Lindblad B, Bergqvist D, Kjellström T. A prospective study of the incidence of deep-vein thrombosis within a defined urban population. J Intern Med. 1992;232:155–60. doi: 10.1111/j.1365-2796.1992.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 6.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., III Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 7.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 8.White RH, Zhou H, Romano PS. Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Ann Intern Med. 1998;128:737–40. doi: 10.7326/0003-4819-128-9-199805010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–11. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 11.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–8. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 12.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 13.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 14.Glueck CJ, Wang P, Fontaine RN, Sieve-Smith L, Tracy T, Moore SK. Plasminogen activator inhibitor activity: an independent risk factor for the high miscarriage rate during pregnancy in women with polycystic ovary syndrome. Metabolism. 1999;48:1589–95. doi: 10.1016/s0026-0495(99)90250-0. [DOI] [PubMed] [Google Scholar]
- 15.Wild RA, Grubb B, Hartz A, Van Nort JJ, Bachman W, Bartholomew M. Clinical signs of androgen excess as risk factors for coronary artery disease. Fertil Steril. 1990;54:255–9. doi: 10.1016/s0015-0282(16)53699-1. [DOI] [PubMed] [Google Scholar]
- 16.Guzick DS, Talbott EO, Sutton-Tyrrell K, Herzog HC, Kuller LH, Wolfson SK., Jr Carotid atherosclerosis in women with polycystic ovary syndrome: initial results from a case-control study. Am J Obstet Gynecol. 1996;174:1224–32. doi: 10.1016/s0002-9378(96)70665-8. [DOI] [PubMed] [Google Scholar]
- 17.Birdsall MA, Farquhar CM, White HD. Association between polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med. 1997;126:32–5. doi: 10.7326/0003-4819-126-1-199701010-00005. [DOI] [PubMed] [Google Scholar]
- 18.Mather KJ, Kwan F, Corenblum B. Hyperinsulinemia in polycystic ovary syndrome correlates with increased cardiovascular risk independent of obesity. Fertil Steril. 2000;73:150–6. doi: 10.1016/s0015-0282(99)00468-9. [DOI] [PubMed] [Google Scholar]
- 19.Talbott EO, Guzick DS, Sutton-Tyrrell K, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20:2414–21. doi: 10.1161/01.atv.20.11.2414. [DOI] [PubMed] [Google Scholar]
- 20.Orio F, Jr, Palomba S, Spinelli L, et al. The cardiovascular risk of young women with polycystic ovary syndrome: an observational, analytical, prospective case-control study. J Clin Endocrinol Metab. 2004;89:3696–701. doi: 10.1210/jc.2003-032049. [DOI] [PubMed] [Google Scholar]
- 21.Atiomo WU, Fox R, Condon JE, et al. Raised plasminogen activator inhibitor-1 (PAI-1) is not an independent risk factor in the polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2000;52:487–92. doi: 10.1046/j.1365-2265.2000.00946.x. [DOI] [PubMed] [Google Scholar]
- 22.Mak W, Dokras A. Polycystic ovarian syndrome and the risk of cardiovascular disease and thrombosis. Semin Thromb Hemost. 2009;35:613–20. doi: 10.1055/s-0029-1242715. [DOI] [PubMed] [Google Scholar]
- 23.Adamson DM, Chang S, Hansen LG. Health research data for the real world: the MarketScan databases. New York, NY: Thomson Reuters; 2008. [Google Scholar]
- 24.Petri H, Maldonato D, Robinson NJ. Data-driven identification of co-morbidities associated with rheumatoid arthritis in a large US health plan claims database. BMC Musculoskelet Disord. 2010;11:247. doi: 10.1186/1471-2474-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seaman HE, de Vries CS, Farmer RD. The risk of venous thromboembolism in women prescribed cyproterone acetate in combination with ethinyl estradiol: a nested cohort analysis and case-control study. Hum Reprod. 2003;18:522–6. doi: 10.1093/humrep/deg120. [DOI] [PubMed] [Google Scholar]
- 26.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(Suppl):I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 27.Speroff L, Fritz MA, editors. Clinical gynecologic endocrinology and infertility. Philadelphia, PA: Lippincott; 2005. Anovulation and the polycystic ovary; pp. 465–98. [Google Scholar]
- 28.Stegenga ME, van der Crabben SN, Levi M, et al. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes. 2006;55:1807–12. doi: 10.2337/db05-1543. [DOI] [PubMed] [Google Scholar]
- 29.Mannerås-Holm L, Baghaei F, Holm G, et al. Coagulation and fibrinolytic disturbances in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:1068–76. doi: 10.1210/jc.2010-2279. [DOI] [PubMed] [Google Scholar]
- 30.Wiltgen D, Spritzer PM. Variation in metabolic and cardiovascular risk in women with different polycystic ovary syndrome phenotypes. Fertil Steril. 2010;94:2493–6. doi: 10.1016/j.fertnstert.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Spencer FA, Emery C, Joffe SW, et al. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism: the Worcester VTE study. J Thromb Thrombolysis. 2009;28:401–9. doi: 10.1007/s11239-009-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quan H, Li B, Saunders LD, et al. IMECCHI Investigators. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–41. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White RH, Zhou H, Romano PS. Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Ann Intern Med. 1998;128:737–40. doi: 10.7326/0003-4819-128-9-199805010-00006. [DOI] [PubMed] [Google Scholar]
- 34.Deitelzweig SB, Lin J, Johnson BH, Schulman KL. Venous thromboembolism in the US: does race matter? J Thromb Thrombolysis. 2011;31:133–8. doi: 10.1007/s11239-010-0503-3. [DOI] [PubMed] [Google Scholar]
- 35.Ageno W, Prandoni P, Romualdi E, et al. The metabolic syndrome and the risk of venous thrombosis: a case-control study. J Thromb Haemost. 2006;4:1914–8. doi: 10.1111/j.1538-7836.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- 36.Ay C, Tengler T, Vormittag R, et al. Venous thromboembolism–a manifestation of the metabolic syndrome. Haematologica. 2007;92:374–80. doi: 10.3324/haematol.10828. [DOI] [PubMed] [Google Scholar]
- 37.Glueck CJ, Morrison JA, Goldenberg N, Wang P. Coronary heart disease risk factors in adult premenopausal white women with polycystic ovary syndrome compared with a healthy female population. Metabolism. 2009;58:714–21. doi: 10.1016/j.metabol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Becattini C, Agnelli G, Prandoni P, et al. A prospective study on cardiovascular events after acute pulmonary embolism. Eur Heart J. 2005;26:77–83. doi: 10.1093/eurheartj/ehi018. [DOI] [PubMed] [Google Scholar]
- 39.Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348:1435–41. doi: 10.1056/NEJMoa022157. [DOI] [PubMed] [Google Scholar]
- 40.Prandoni P, Ghirarduzzi A, Prins MH, et al. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost. 2006;4:1891–6. doi: 10.1111/j.1538-7836.2006.02058.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.