Abstract
Background:
Primary intraosseous cavernous hemangiomas (PICH) of the skull represent an infrequent bone tumor. Although some rare cases of PICHs located in the skull base have been published, to our concern only three cases have been reported in the English literature of PICHs arising within the clivus.
Case Description:
We present the case of a patient presenting an isolated abducens paresis due to a rare PICH of the clivus showing also an unusual destruction of the inner table as well as infiltration of the dura mater. Due to this uncommon infiltrative pattern of an otherwise expected intraosseous tumor, a cerebrospinal fluid (CSF)-fistula occurred while performing a transnasal biopsy. The patient recovered successfully without need of lumbar drainage or re-surgery. Additionally, intratumoral decompression was sufficient to relief the abducens paresis.
Conclusions:
Our case provides new and meaningful information about clinical features as well as growth pattern of these rare clival tumors. We also discuss the importance of knowing these peculiarities before surgery in order to plan the optimal operative management as well as to avoid complications while approaching PICHs localized in such a delicate cranial region.
Keywords: Clivus, cavernous hemangioma, cerebrospinal fluid leakage
INTRODUCTION
Primary intraosseous cavernous hemangiomas (PICH) comprehend only between 0.7 and 1% of all bone tumors.[19] They can be found in almost all bones, but they tend to affect more frequently the spine and calvarial bones.[9] Although sporadically cases of PICH affecting the skull base have been described, up to the present only three cases have been reported of PICH arising from the clivus.[9,29,30] Few experiences in respect to their clinical evolution, added to the complexity of a surgical approach to the tumors in this location, can constitute a challenge for the surgeon. We present a case of a clival PICH with meningeal involvement. This feature, as well as some aspects of the clinical evolution, make our case unique with important implications regarding surgical management and post-operative concerns.
CASE REPORT
A 62-year-old female patient consulted because of diplopia, headache, and gait disbalance. From previous clinical antecedents it was remarkable a right carotid-ophtalmic aneurysm, which had been successfully clipped 14 years ago. Despite a hyperthyroid-related osteoporosis, the patient had no other remarkable clinical history. At the presentation in our emergency department, the woman was awake, fully orientated and pupilles were isocoric with normal light response. There were no signs of meningeal irritation or sight disturbances. The patient had only a left abducens paresis. Otherwise was cranial nerve and the remaining neurological examination inconspicuous. Initial computed tomography (CT) images showed a large, erosive process infiltrating the sphenoidal body, left petrosal tip, and cavernous sinus, as well as extending into the clivus [Figure 1].
Figure 1.
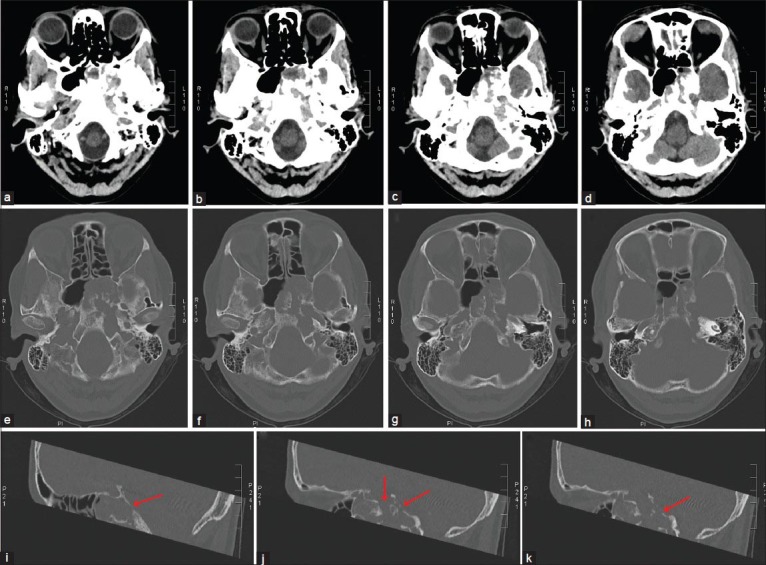
Axial (a-h) and sagittal (i, j, k) reconstructed CT images showing a clear involvement of bone structures within the skull base. It is remarkable that the tumor showed infiltration and destruction of the tabula interna in many sites (arrows), (i, j, k) which corresponds to the extension and adhesion to the dural layer
There was no evidence of brainstem or cerebellar infarction or intracranial bleeding. Magnetic resonance imaging (MRI) scans confirmed the presence of a large tumor with the above described characteristics [Figure 2].
Figure 2.
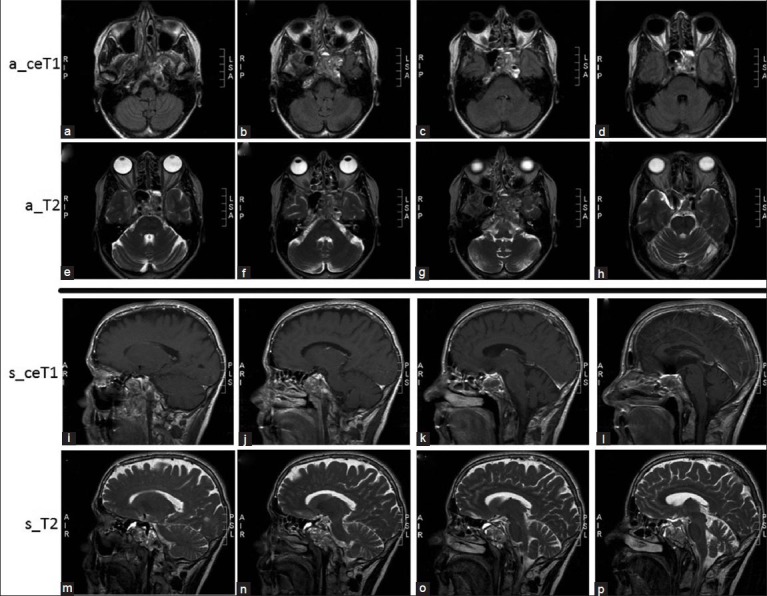
Axial (a_) and sagittal (s_) contrast enhanced T1-weigthed (ceT1) and T2-weighted MRI images showing a heterogeneous lesion involving the clivus, (a, b, j, k, n, o) sphenoidal bone, (c, d, g, h, k, o) sphenoidal wing, (b, i) as well as extending to the left petrosal apex and cavernous (b, d, g, h, i, m) sinus
The petrocavernosal left internal carotid was completely surrounded by the tumor [Figure 3]. The mass grew within the clivus, infiltrated the ethmoidal holes, epipharynx and extended up to the left petrooccipital synchondrosis. The signal was homogenic hyperintense in T1-weighted images and T2 was homogenealy hyperintense.
Figure 3.
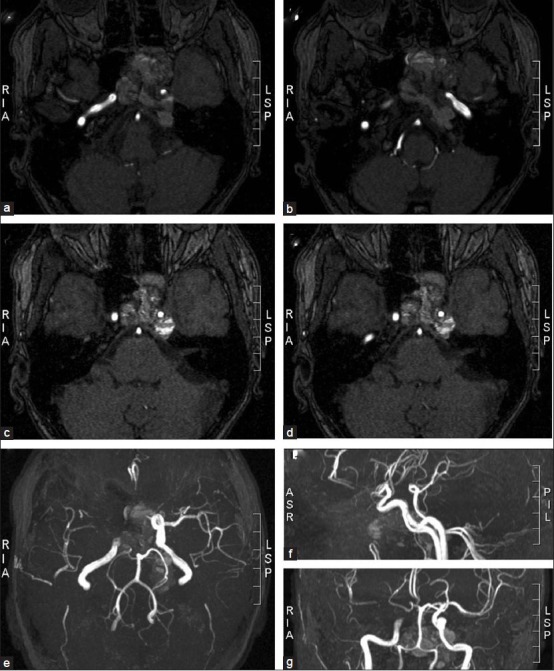
MRI angiographic scans show the relationship between the tumor and major vessels of the skull base. The intracavernous and supraclinoidal segment of the left internal carotid was surrounded and compressed by the tumor (c, d, e, g). However, sufficient blood supply to the left medial and anterior brain arteries provided by the right carotid circulation through the Willis circle (e, f, g), as well as a slow tumor growth enabling collateralization, can explain the lack of neurological deficits besides the ones produced by a direct tumor compression of cranial nerves
There was evidence in both CT and MRI of garland-like calcifications and areas of hyperostosis. Nevertheless the tumor showed a clear erosive growth with an expansive destruction of the described bone structures [Figure 1] and there was evidence of possible dural adhesions to the tumor [Figure 2]. It was decided to perform a biopsy and partial excision through a transnasal transesphenoidal approach in order to clarify the histological nature of the tumor and plan the following therapeutic strategy. The procedure was performed under navigation guidance [Figure 4a]. At opening the esphenoidal sinus it was demonstrated a brown-green tissue with multiple hemorrhagic areas and a trabecular aspect [Figure 4b]. Both trabecular and compact bone of the clivus was eroded. The tumor reached the predural space and infiltrated the duramater, preventing a clear recognition of this structure. Despite a careful dissection, following tumor excision exposed the subarachnoidal space and cerebrospinal fluid (CSF) flowed through a small perforation [Figure 4c and d]. The gap was sealed by using muscle graft [Figure 4e], fibrinogen-fibrin-based sponge (TachoSil®) and reabsorbable gelatine (Mabagelan®) applied in a multilevel pattern.
Figure 4.
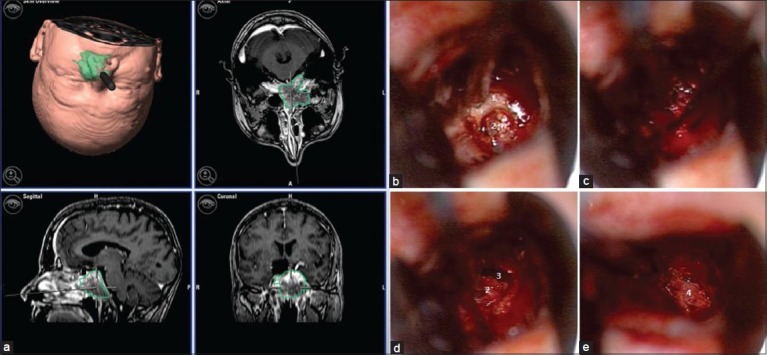
Microsurgical tumor biopsy performed with neuronavigation (a) through a transnasal transesphenoidal approach. After osteotomy of the esphenoidal sinus (b), the tissue showed a strong tendency to bleed (c). The destruction of the tabula interna and adhesions to the dura made the opening of subarachnoidal space almost unavoidable (d) and muscle (e) was used to close the dural defect. Esphenoidal bone (1), tumor (2), subarachnoidal space (3), and muscle graft (4)
The further histopathological evaluation revealed a cavernous hemangioma. The immediate postoperative evolution was favorable. The patient developed no additional neurological deficits and even the left abducens paresis regressed. On first instance, there was no evidence of CSF fistula and the patient was discharged 5 days later. However, on re-admission 2 days later because of rhinoliquorrhoea, which stopped spontaneously without need of lumbar drainage or any other invasive measurement. The same phenomenon occurred 4 weeks later. Although the presence of CSF was confirmed by positive traces of beta transferin, the rhinoliquorrhoea ceased without any intervention. An otorhinolaryngologist examination including a meticulous rhinopharyngoscopy revealed no evidence of an active CSF fistula and no more episodes of rhinoliquorrhoea have occurred up to the present.
DISCUSSION
General overview of cavernous hemangiomas of the skull
Among all primary bone tumors, PICH account for only 0.7–1% of all bone tumors.[19] Although they can occur in almost all bones, the most frequent localization is the spine (30–50%) and skull (20%).[9] PICH of the cranium account for 0.2% of all benign skull tumors[16,18,31] and affect mostly frontal and parietal bones.[10,11,12,17,18,19,20,25,26,31] PICH within the skull base constitutes an extreme uncommon feature and the structure affected in the vast majority of cases is the petrous bone.[2,3,4,8,14,15] These tumors have been anecdotally described in other skull base bones, such as the sphenoidal wing,[5,16] sphenoid body,[27] and occipital condyle.[7] To date, only three cases have been reported of PICH of the clivus.[9,29,30]
Because of their clinical evolution, radiological features, and complexity of a surgical approach the management of skull base PICH differs significantly from those arising in cranial vault bones. Furthermore, lack of cases in the literature on skull base PICH compared with calvarial PICH constitute an additional challenge for surgeons, at the moment, for deciding the proper management of these tumors.
PICHs of the cranial vault manifest in most of the cases as clinically indolent palpable masses. They tend to grow very slowly and, conform they expand, patients complain of headache.[17,31] However, due to their tendency to grow in an extracranial pattern, neurological deficits are evidenced only in rare cases of calvarial PICH with intracranial expansion.[21,23,32]
By contrast, PICH comprising orbital or skull base bones can be associated to a higher morbidity due to their tendency to involve novel neurovascular structures during their growth. Orbital PICH have been described to produce progressive proptosis, diplopia, and visual loss in some severe cases.[1,13,24]
PICH of petrosal bone commonly impair facial nerve function,[4,6,8] although lower cranial nerve involvement has been also described in a PICH of the petrosal bone compressing the jugular foramen.[8] A rare case of PICH localized in the petrosal bone has also been reported, which manifested with a spontaneous bleeding and subsequent epidural hematoma.[15] Moreover, localization within the sphenoid body has been associated to compression-induced optic atrophy.[27]
Nevertheless, some large PICH of the skull base can grow without disturbing cranial nerve function. In the case reported by Liu et al. of a sphenoid wing PICH, the indication for surgical removal was due to the increase in severity of headache along a period of many years of follow up, but the tumor did not produce cranial nerve deficits.[4] Similarly, the case of occipital condyle PICH published by García-Marín et al. was associated with neck pain and torticollis, but not with neurological impairment.[7]
Regarding the only few cases of clival PICH, these tumors can course with minimal symptomatic manifestation or, in other cases, they may be related to severe progressive cranial nerve deficits including Garcin syndrome, characterized by a dysfunction of cranial nerves from the V to XII.[9] Interestingly, our case is the first describing an isolated abducens paresis produced by a PICH of the clivus. Additionally, the fact that the pareses remitted during the early postoperative follow up is also remarkable, considering that no mass reduction was attempted during the surgery. We propose that probably opening the tumor cavity relieved the intratumoral pressure and therefore the compressive effect on the abducens nerve.
With respect to the radiological features, it is important to remark that the typical and almost pathognomonic characteristics of vertebral intraosseous hemangiomas with honeycomb or polka-dotted pattern on CT are often absent in PICH.[9,16,28,29,30] MRI images are also unspecific, with heterogeneous signal in both T1- and T2-weighted sequences depending on the amount of venous flow and fatty transformation within the tumor.[9,29,30] Moreover, PICH cannot be evidenced on angiography, making this study useless to clarify the diagnostic preoperatively.
Additionally, it is accepted that intraosseous cavernous hemangiomas respect in all cases the cortical bone layer and this feature distinguishes these lesions in radiological studies from bone malignancies.[16] Nevertheless, another unique characteristic in the case is that the internal cortical layer was not respected, presenting the tumor adhesions to the duramater. This feature is unique in our case and suggests that PICH of the clivus could be difficult to distinguish preoperatively from malignant infiltrating neoplasms, such as chordoma.
Assessing a surgical management of cavernous hemangiomas of the clivus and other deep skull base structures: Relevance of being aware about possible dural adhesions and risk of CSF leakage
Due to the fact that PICH have the tendency to grow slowly but they do not show involution, it is suggested that symptomatic PICH should be removed en bloc when possible, because the recurrence after en bloc resection is extreme rare.[16,22]
Although this issue seems to be clear in cases of PICH localized in the cranial vault, whether en bloc or even a partial resection of a skull base PICH should be performed remains unclear. As mentioned earlier, the low incidence of PICH located deeply in the skull base, such as in clivus, makes it difficult to assess and predict the clinical evolution of these tumors with or without surgery.
The higher tendency to compromise novel structures due to the slow but constant growing as well as the high curability provided by a complete tumor removal constitutes the main argument to attempt a radical tumor excision when possible. In addition, the concept that PICH grows exclusively intraosseous, respecting the cortical bone layer and without adherences to the meninges, favors the idea of trying to aim a gross tumor removal. Given this condition, a complete tumor resection through an intracranial extradural approach may be feasible, even in tumors located in delicate regions of the skull base.[16] However, if extensive skull base involvement with neurovascular encasement makes a complete resection unfeasible, a subtotal resection may be indicated to relieve symptoms of mass effect.
We demonstrate that PICH of the clivus may present exceptionally not only in involvement of the internal cortical bone layer, but also in adhesion to the dura. Although we report the first case of dural involvement in clival PICH, this feature has been already reported in three cases of PICHs arising from cranial vault bones.[21,23,32] This eventuality has very important implications at the moment of deciding a surgical approach to a PICH arising from the clivus or other bones of the skull base. The adhesion to the dura would constitute an obstacle for the surgeon while attempting a radical tumor removal through an anterior approach to the clivus because of the high risk of CSF leakage and concurrent postoperative meningitis. If an en block resection is planned, other surgical approaches should be taken in consideration, in order to provide a better accessibility for placing a dural graft. Additionally, if a complete tumor removal is intended, the surgeon should try to choose an approach that avoids opening the pharyngeal mucosa in the aim to reduce the risk of meningitis if the dura is to be opened. If a transnasal approach is selected and a high flow CSF leak is observed, some colleagues would recommend closing the dura interposing a pedicled vascularized flap, particularly the nasoseptal flap, due to its demonstrated efficacy in reducing the risk of post operative CSF leak. Finally, if the surgeon decides to perform a diagnostic frameless stereotactic biopsy, the considerations in respect to the depth of tissue excision should be also carefully reviewed in order to avoid possible dural adhesions.
CONCLUSIONS
Skull base PICH and specially clival PICH represent a very infrequent tumor entity, which can manifest from almost no symptoms to multiple cranial nerve impairment. Because of their unspecific radiological characteristics, a frameless stereotactic biopsy may be always considered in lesions involving these structures in order to confirm the diagnosis and exclude other entities. Furthermore, we have shown that some neurological deficits can resolve after opening the tumor cavity, probably due to a reduction of intracavitary pressure and its compressive effect on neural structures.
The need of a radical surgical tumor removal in clival PICH should be always pointed out against the risks and benefits for every individual patient and an attempt to reach a more radical removal should be considered for patients with newly developed symptoms or evidence of tumor progression during the follow up. Moreover, we have evidenced that occasionally dural adhesions may be present and this should not be ignored at the moment of planning a stereotactic biopsy or tumor resection.
Ethical standards and conflict of interest
The manuscript does not contain clinical studies or identity patient data and the authors declare that they have no conflict of interest.
Patient consent
The patient has given consent to the submission of the case report for submission to the journal.
ACKNOWLEDGEMENTS
We thank Dr. Jose Alberto Landeiro MD, PhD and Dr. José Orlando de Melo Júnior MD for the meaningful comments and suggestions.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/4/117/155695
Contributor Information
Lucas Serrano, Email: lucasserrano1986@gmail.com.
Eleftherios Archavlis, Email: Eleftherios.Archavlis@sana.de.
Elke Januschek, Email: elke.januschek@sana.de.
Peter T. Ulrich, Email: peter.ulrich@sana.de.
REFERENCES
- 1.Banerji D, Inao S, Sugita K, Kaur A, Chhabra DK. Primary intraosseous orbital hemangioma: A case report and review of the literature. Neurosurgery. 1994;35:1131–4. doi: 10.1227/00006123-199412000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Bottrill I, Poe DS. Imaging quiz case 2. Intraosseous cavernous–type hemangioma of the petrous temporal bone. Arch Otolaryngol Head Neck Surg. 1995;121:348. 350. [PubMed] [Google Scholar]
- 3.Bucy PC, Capp CS. Primary hemangioma of bone with special reference to roentgenologic diagnosis. AJR Am J Roentgenol. 1930;23:1–33. [Google Scholar]
- 4.Curtin HD, Jensen JE, Barnes L, Jr, May M. “Ossifying” hemangiomas of the temporal bone: Evaluation with CT. Radiology. 1987;164:831–5. doi: 10.1148/radiology.164.3.3112865. [DOI] [PubMed] [Google Scholar]
- 5.Dickins JR. Cavernous hemangioma of the sphenoid wing. Arch Otolaryngol. 1978;104:58–60. [PubMed] [Google Scholar]
- 6.Friedman O, Neff BA, Willcox TO, Kenyon LC, Sataloff RT. Temporal bone hemangiomas involving the facial nerve. Otol Neurotol. 2002;23:760–6. doi: 10.1097/00129492-200209000-00025. [DOI] [PubMed] [Google Scholar]
- 7.García-Marín V, Ravina J, Trujillo E, González-Feria L. Symptomatic cavernous hemangioma of the occipital condyle treated with methacrylate embolization. Surg Neurol. 2001;56:301–3. doi: 10.1016/s0090-3019(01)00613-9. [DOI] [PubMed] [Google Scholar]
- 8.Glasscock ME, 3rd, Smith PG, Schwaber MK, Nissen AJ. Clinical aspects of osseous hemangiomas of the skull base. Laryngoscope. 1984;94:869–73. doi: 10.1288/00005537-198407000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gologorsky Y, Shrivastava RK, Panov F, Mascitelli J, Signore AD, Govindaraj S, et al. Primary intraosseous cavernous hemangioma of the clivus: Case report and review of the literature. J Neurol Surg Rep. 2013;74:17–22. doi: 10.1055/s-0033-1346980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta SD, Tiwari IN, Pasupathy NK. Cavernous haemangioma of the frontal bone: Case report. Br J Surg. 1975;62:330–2. doi: 10.1002/bjs.1800620421. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman DF, Israel J. Intraosseous frontal hemangioma. Head Neck. 1990;12:160–3. doi: 10.1002/hed.2880120212. [DOI] [PubMed] [Google Scholar]
- 12.Hook SR, Font RL, McCrary JA, Harper RL. Intraosseous capillary hemangioma of the frontal bone. Am J Ophthalmol. 1987;103:824–7. doi: 10.1016/s0002-9394(14)74401-0. [DOI] [PubMed] [Google Scholar]
- 13.Hwang K. Intraosseous hemangioma of the orbit. J Craniofac Surg. 2000;11:386–7. doi: 10.1097/00001665-200011040-00020. [DOI] [PubMed] [Google Scholar]
- 14.Irgens ER. Haemangioma of skull involving right petrous and occipital bone. Arch Otolaryngol. 1939;29:709–12. [Google Scholar]
- 15.Kessler LA, Lubic LG, Koskoff YD. Epidural haemorrhage secondary to cavernous haemangioma of the petrous portion of the temporal bone. J Neurosurg. 1957;14:329–31. doi: 10.3171/jns.1957.14.3.0329. [DOI] [PubMed] [Google Scholar]
- 16.Liu JK, Burger PC, Harnsberger HR, Couldwell WT. Primary Intraosseous Skull Base Cavernous Hemangioma: Case Report. Skull Base. 2003;13:219–28. doi: 10.1055/s-2004-817698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIntyre NG, Brebner DM, Gluckman J. The cavernous haemangioma of the frontal bone. A case report. S Afr Med J. 1977;52:537–8. [PubMed] [Google Scholar]
- 18.Murrone D, De Paulis D, Millimaggi DF, Del Maestro M, Galzio RJ. Cavernous hemangioma of the frontal bone: A case report. J Med Case Rep. 2014;8:121. doi: 10.1186/1752-1947-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naama O, Gazzaz M, Akhaddar A, Belhachmi A, Asri A, Elmostarchid B, et al. Cavernous hemangioma of the skull: 3 case reports. Surg Neurol. 2008;70:654–9. doi: 10.1016/j.surneu.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Pastore FS, De Caro GM, Faiola A, Mauriello A, Giuffrè R. Cavernous hemangioma of the parietal bone. Case report and review of the literature. Neurochirurgie. 1999;45:312–5. [PubMed] [Google Scholar]
- 21.Patnaik A, Mishra SS, Mishra S, Deo RC. Intradiploic ossified giant cavernous hemangioma of skull with a dural tail sign mimicking primary calvarial meningioma. Neurol India. 2012;60:250–2. doi: 10.4103/0028-3886.96431. [DOI] [PubMed] [Google Scholar]
- 22.Peterson DL, Murk SE, Story JL. Multifocal cavernous hemangioma of the skull: Report of a case and review of the literature. Neurosurgery. 1992;30:778–82. [PubMed] [Google Scholar]
- 23.Politi M, Romeike BF, Papanagiotou P, Nabhan A, Struffert T, Feiden W, et al. Intraosseous hemangioma of the skull with dural tail sign: Radiologic features with pathologic correlation. AJNR Am J Neuroradiol. 2005;26:2049–52. [PMC free article] [PubMed] [Google Scholar]
- 24.Relf SJ, Bartley GB, Unni KK. Primary orbital intraosseous hemangioma. Ophthalmology. 1991;98:541–7. [PubMed] [Google Scholar]
- 25.Sharma RR, Pawar SJ, Lad SD, Netalkar AS, Musa MM. Frontal intraosseous cryptic hemangioma presenting with supraorbital neuralgia. Clin Neurol Neurosurg. 1999;101:215–9. doi: 10.1016/s0303-8467(99)00047-5. [DOI] [PubMed] [Google Scholar]
- 26.Sinnreich Z, Kremer S, Sade J, Bernheim J. Cavernous hemangioma of the frontal bone. ORL J Otorhinolaryngol Relat Spec. 1990;52:269–72. doi: 10.1159/000276149. [DOI] [PubMed] [Google Scholar]
- 27.Suss RA, Kumar AJ, Dorfman HD, Miller NR, Rosenbaum AE. Capillary hemangioma of the sphenoid bone. Skeletal Radiol. 1984;11:102–7. doi: 10.1007/BF00348797. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki Y, Ikeda H, Matsumoto K. Neuroradiological features of intraosseous cavernous hemangioma-case report. Neurol Med Chir (Tokyo) 2001;41:279–82. doi: 10.2176/nmc.41.279. [DOI] [PubMed] [Google Scholar]
- 29.Tashiro T, Inoue Y, Nemoto Y, Shakudo M, Mochizuki K, Katsuyama J, et al. Cavernous hemangioma of the clivus: Case report and review of the literature. AJNR Am J Neuroradiol. 1991;12:1193–4. [PMC free article] [PubMed] [Google Scholar]
- 30.Vanhoenacker FM, De Praeter G, Kools D, Voormolen M, Parizel PM. Unusual lesion of the clivus: Diagnosis and discussion. Skeletal Radiol. 2011;40:243. doi: 10.1007/s00256-010-0981-6. [DOI] [PubMed] [Google Scholar]
- 31.Wyke BD. Primary hemangioma of the skull; a rare cranial tumor; review of the literature and report of a case, with special reference to the roentgenographic appearances. Am J Roentgenol Radium Ther. 1949;61:302–16. [PubMed] [Google Scholar]
- 32.Xu P, Lan S, Liang Y, Xiao Q. Multiple cavernous hemangiomas of the skull with dural tail sign: A case report and literature review. BMC Neurol. 2013;25:155. doi: 10.1186/1471-2377-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]


