Abstract
Background and Aims:
Advanced monitoring targeting haemodynamic and oxygenation variables can improve outcomes of surgery in high-risk patients. We aimed to assess the impact of goal directed therapy (GDT) targeting cardiac index (CI) and oxygen extraction ratio (O2ER) on outcomes of high-risk patients undergoing abdominal surgery.
Methods:
In a prospective randomised trial, forty patients (American Society of Anaesthesiologists II and III) undergoing major abdominal surgeries were randomised into two groups. In-Group A mean arterial pressure ≥ 65 mmHg, central venous pressure ≥ 8–10 mmHg, urine output ≥ 0.5 mL/kg/h and central venous oxygen saturation ≥ 70% were targeted intra-operatively and 12 h postoperatively. In-Group-B (enhanced GDT), in addition to the monitoring in-Group-A, CI ≥ 2.5 L/min/m2 and O2ER ≤ 27% were targeted. The end-points were lactate levels and base deficit during and after surgery. The secondary end points were length of Intensive Care Unit (ICU) and hospital stay and postoperative complications. Wilcoxon Mann Whitney and Chi-square tests were used for statistical assessment.
Results:
Lactate levels postoperatively at 4 and 8 h were lower in-Group-B (P < 0.05). The mean base deficit at 3, 4, 5 and 6 h intra-operatively and postoperatively after 4, 8 and 12 h were lower in-Group-B (P < 0.05). There were no significant differences in ICU stay (2.10 ± 1.52 vs. 2.90 ± 2.51 days) or hospital stay (10.85 + 4.39 vs. 13.35 + 6.77 days) between Group A and B.
Conclusions:
Implementation of enhanced GDT targeting CI and OER was associated with improved tissue oxygenation.
Keywords: Base deficit, goal directed therapy, high-risk abdominal surgery, lactate
INTRODUCTION
Despite advances in anaesthetic techniques and monitoring, the outcomes in certain high-risk groups remain very high.[1] Goal directed therapy (GDT) was introduced with the aim of improving the outcomes in this group. Surgery imposes a stress response that increases oxygen demands. Studies have shown that an inability to increase the supply to the tissues is associated with poorer outcomes after surgery.[2]
Improving the cardiac output (CO) and oxygen delivery (DO2) to meet the tissue demands under stress has been the principle underlying GDT. The targets have been achieved by various monitoring techniques such as the pulmonary artery catheter,[3] oesophageal Doppler[4] and pulse contour analysis systems (LidCO Plus™, PiCCO™, Flotrac-Vigileo™).[5,6] In our study we used the pulse contour derived CO monitor (FloTrac-Vigileo™) that has found to be a reliable indicator of CO. Assessment of tissue perfusion by various end points namely lactate, base deficit and tissue partial pressure of carbon dioxide have been evaluated and can be considered as a reflection of tissue oxygenation.[7]
Imbalance between the oxygen demand and supply manifests in increased levels of lactate and increase in base deficit. An objective measure is the oxygen extraction ratio (O2ER) that is derived from the mathematical equation, O2ER = (SaO2−SvO2)/SaO2 × 100. A high O2ER is the common precedent for postoperative organ dysfunction in major abdominal surgeries.[8] We aimed to improve the DO2 to the tissues by targeting an O2ER ≤ 27% in the treatment group.
METHODS
Based on the key article by Rivers et al.[9], with a mean difference of 3.1 for base deficit among the two groups (2 ± 6.6 vs. 5.1 ± 6.7) with 95% confidence limit, the sample size calculated was 36 in each group. However, due to constraints in time and availability of the CO monitor, our study was restricted to 20 patients in each group. After approval from the Institutional Ethics Committee and obtaining informed consent, 40 patients (American Society of Anaesthesiologists physical status II and III) undergoing high-risk abdominal surgery were randomly allocated to one of the two groups by numbers generated on the random number table; in Group A, we targeted mean arterial pressure (MAP) ≥65 mmHg, central venous pressure (CVP) ≥8–10 mmHg, urine output (UO) ≥0.5 mL/kg/h and central venous oxygen saturation (ScvO2) ≥70% during the surgery and the first 12 h postoperatively [Figure 1].
Figure 1.
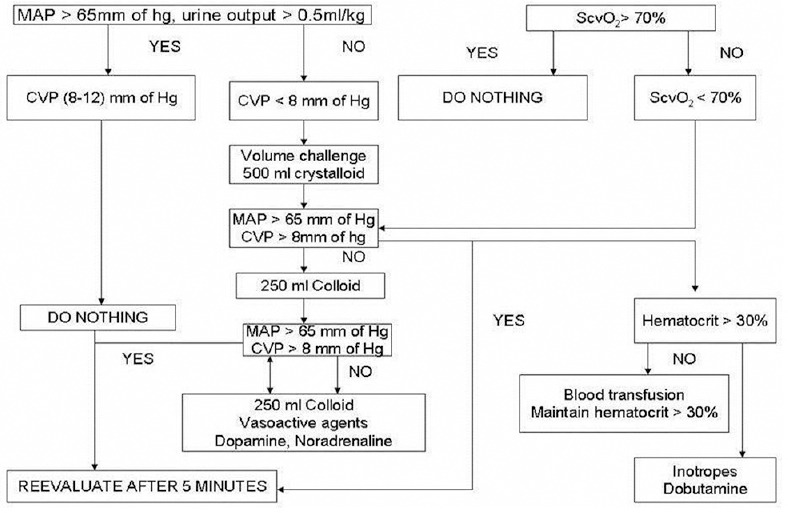
Group A protocol
Group-B was the enhanced GDT group in whom in addition to the targets as mentioned above, advanced CO monitoring was obtained by an arterial pressure analysis with Edwards Lifesciences FloTrac/Vigileo™ targeting a goal (cardiac index [CI] ≥2.5 L/min and O2ER ≤ 27%). This was instituted during surgery and for the first 12 h postoperatively [Figure 2].
Figure 2.
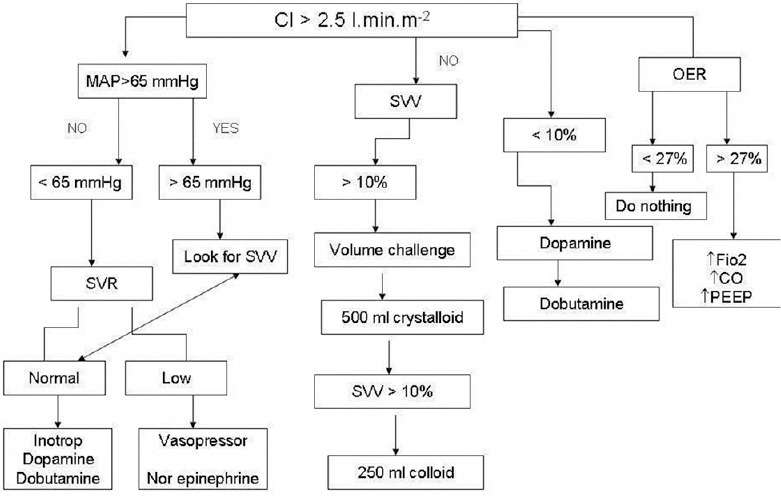
Enhanced GDT (Group B) protocol
The surgeries in study patients included abdominal aortic aneurysm, bowel obstructions, mesenteric ischemia, bowel malignancy involving resection and anastomosis. Those with renal dysfunction, liver dysfunction, poor cardiac reserve with ejection fraction ≤ 35%, those undergoing hepatic resections or any condition that contraindicated fluid resuscitation were excluded.
All patients were evaluated preoperatively and premedicated according to protocols unless bowel obstruction was present. All patients had an epidural and an arterial line inserted under local anaesthesia prior to the induction of anaesthesia. Anaesthesia was induced with fentanyl 2 mcg/kg, midazolam 0.02–0.05 mg/kg and propofol titrated to loss of verbal response. The patients were intubated with vecuronium 0.1 mg/kg or with suxamethonium 1.5 mg/kg. Central venous cannulation was performed using the right jugular access or the right subclavian with a 7.5 Fr catheter. Anaesthesia was maintained with 1% isoflurane in 40% oxygen and air with intermittent top ups of muscle relaxant. In the study Group-B, the FloTrac/Vigileo™ was connected and CI, O2ER were monitored.
Readings of heart rate (HR) blood pressure (BP), UO, ScvO2, CI and O2ER were noted every hour during surgery and 4th hourly in the first 12 h postoperatively. The base deficit and serum lactate levels were considered to reflect tissue perfusion per operatively and the readings were obtained hourly from the blood gas analysis.
In Group-A, CVP was targeted with boluses of crystalloids or colloids, MAP with fluids or Inotropes/ vasopressor (noradrenaline, dopamine) and ScvO2 measured hourly was maintained with fluids, transfusions targeting a haemoglobin ≥10 g/dL and by infusions of dobutamine according to the protocol outlined [Figure 1].
In the enhanced GDT group, the CI was maintained within the desired range throughout surgery by administering boluses of crystalloid, 4% hydroxyethyl starch and blood transfusions when indicated. The use of Inotropes/ vasopressor dopamine, dobutamine or noradrenaline, was in accordance to the protocol [Figure 2]. The O2ER was calculated hourly using the formula O2ER = (SaO2−ScvO2)/SaO2 × 100. SpO2 was obtained from pulse-oximetry and ScvO2 from central venous blood gas analysis measured hourly.
At the end of surgery, patients considered stable were extubated while patients with haemodynamic instability or hypothermia were electively ventilated. Both groups of patients were shifted to Intensive Care Unit (ICU), and readings were obtained at 4th hourly intervals for 12 h postoperatively.
The primary end points were levels of lactate and base deficit intra-operatively and for 12 h postoperatively. Secondary outcomes were length of ICU stay, length of hospital stay, organ dysfunction, need for ventilation and postoperative complications.
Mean and standard deviations were calculated for all quantitative variables using IBM SPSS 15.0 statistical software, P < 0.05 was considered significant. Wilcoxon Mann Whitney test was used to compare the values of base deficit, lactate levels, ICU stay, hospital stay, intra-operative fluid usage and demographic variables. The Chi-square test was used to find out the association between postoperative ventilation and gender.
RESULTS
The demographic variables and surgical profile in both groups were similar. Table 1 denotes the distribution of various types of surgeries the patients underwent in both the groups. The duration of surgery was comparable between groups A and B (4.62 ± 1.47h and 4.46 ± 1.38h) respectively. Figure 3 shows thelactate levels during and after surgery in both groups. The baseline values of lactate in both the groups were similar (P = 0.786). Although a trend towards higher lactate levels were seen in-Group-A at 2, 3 and 4 h, this was not statistically significant. The highest value of lactate was found to be 6.3 mmol/L in-Group-A patients as compared to Group-B patients in whom the highest lactate level was only 4.8 mmol/L [Figure 3].
Table 1.
Distribution of number of patients undergoing different surgeries

Figure 3.
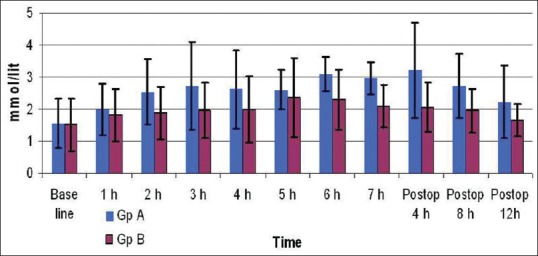
Intraop and postop lactate
There was a trend towards decreasing lactate in both groups postoperatively. However, lower levels were seen at 4 and 8 h and a trend towards lower levels was seen in Group-B even at 12 h after surgery [Figure 3].
Figure 4 shows the mean values of base deficit measured hourly intra-operatively and postoperatively. The mean values of base deficit intra-operatively were less in Group-B compared to Group-A at 3, 4, 5 and 6 h of surgery. The highest base deficit observed in Group-A was − 8.8 mmol/L in as compared to that in Group-B in whom the highest value was − 6.3 mmol/L. The values in Group-B after surgery were significantly lesser than Group-B at all-time points [Figure 4].
Figure 4.
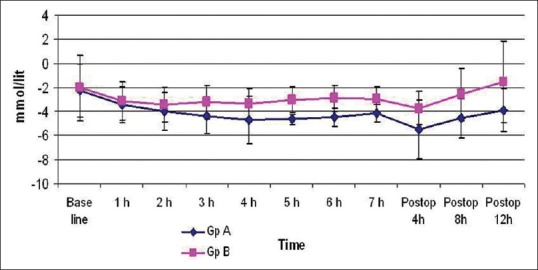
Intraop and postop base deficit
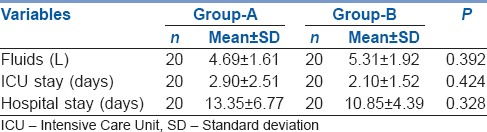
In Group A and B, the length of ICU stay (2.90 ± 2.51 vs. 2.10 ± 1.52 days) or hospital stay (13.35 ± 6.77 vs. 10.85 ± 4.39 days) was not significantly different [Table 2]. The fluid requirements were higher in Group-B (5.31 ± 1.92 vs. 4.69 ± 1.61 L) but they were not significant. The use of inotropes although marginally higher in Group-B was not statistically different from Group-A [Table 2].
Table 2.
Comparison of fluid requirements, ICU and hospital stays
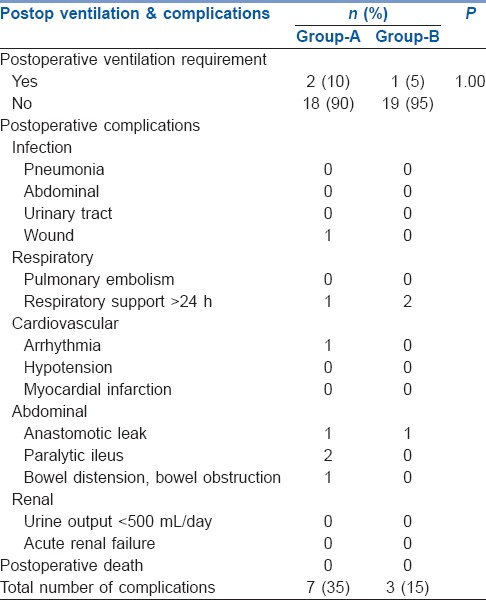
Two patients in Group-A and 1 patient in-Group-B needed ventilation postoperatively, which was comparable (P = 1.00, Table 3). Seven patients in-Group-A and three patients in Group-B developed postoperative complications [Table 3].
Table 3.
Comparison of postoperative ventilation and distribution of postoperative complications among groups
DISCUSSION
The emphasis on haemodynamic goals began with the study by Rivers et al.[9] in patients with sepsis in the first 6 h wherein the strategy of ScvO2 ≥ 70% in addition to standard care management reduced the mortality from 46.5% to 30.5%. The principles in management have been extended to the management of high-risk surgical patients also. In the above study maintaining a ScvO2 above 70% also resulted in reduced lactate and base deficits and improved outcomes. We compared the impact of targeting CI and tissue oxygenation measured by O2ER as an enhancement of GDT guided by ScvO2 in high-risk patients. We evaluated the effects of targeting CI ≥ 2.5 L/min/m2 and O2ER on the tissue perfusion intra- and post-operatively by measurements of serum lactate and base deficit. We found that the base deficit was significantly less intra- and post-operatively in the enhanced GDT group. In addition, a trend towards lower lactate intra-operatively and significantly lower values postoperatively at 4 h and 8 h were noted in the enhanced GDT group even with the limited numbers of patients enrolled in our study.
Lactate levels have been considered as a reflection of tissue hypoxia and trends in lactate have been used to predict recovery.[10] The crystalloids (Ringer's lactate, normal saline) and colloids used in both groups were similar. As per prevailing protocols, both the crystalloids were used equally and were comparable between both the groups. We had excluded patients with hepatic dysfunction as they would have an inability to metabolise lactate and hypothesised that the lactate in Ringer's lactate would be metabolised to bicarbonate. Blood transfusions were administered as per the protocol or when losses exceeded 10% of estimated blood volume. The need for transfusion was limited, only 3 patients in Group-A and 2 in Group-B needed blood replacement. The pre- and intra-operative lactate levels were similar in both groups and the mean value of lactate was found to be lower in Group-B (1.67 ± 0.49) mmol/L than in Group-A (2.24 ± 1.14) mmol/L. We hypothesised that the fluid and haemodynamic resuscitation in the enhanced GDT could have contributed to the improved tissue perfusion represented by lactate levels.
The base deficit or the acidosis reflects a metabolic acidosis resulting from inadequate tissue oxygenation. Evaluation of the trends in base deficit is a prognostic guide for resuscitation.[10] The baseline base deficit levels were not statistically significant (P = 0.705) and both groups were comparable. The mean values of base deficit were found to be statistically significant after 3, 4, 5 and 6 h of surgery. The highest base deficit observed in Group-A was − 8.8 mmol/L as compared to that in Group-B where the highest value was − 6.3 mmol/L. This could be because the fluid and haemodynamic targeted Group-B probably had a better tissue perfusion and better acid base profile than the Group-A. However, the intervention to keep ScvO2 ≥ 70% in Group-A could have resulted in improved tissue perfusion in comparison with standard care.
There has been considerable interest in ways of identifying high-risk patients for surgery and identifying strategies to reduce their disproportionately high mortality.[2,11] High-risk may be related to patient as well as surgical factors and has been identified to include surgery for aortic aneurysm, bowel resection with anastomosis, cardio-respiratory illness, massive blood loss and renal dysfunction.[1]
Careful monitoring of fluid administration by individualised GDT has been shown to reduce organ failure and hospital stay.[4,5,12] In our study we found that although there was a reduction in the length of stay in the hospital (13.35 vs. 10.85 days) and ICU (2.90 vs. 2.10 days) in the enhanced GDT group, these values were not significant. The enhanced GDT group appeared to have fewer complications (7 vs. 3) but the numbers were too small for significance.
Complex surgeries are associated with an increased stress response and an increased oxygen demand. Length of ICU and hospital stay is increased in high-risk patients in whom preoperative risks are high and unavoidable. Strategies to detect and treat potential triggers like tissue perfusion are important in the high-risk population.[13] Patients unable to meet the oxygen demands had a higher incidence of complications and death. As the tissue demands increase, the extraction of oxygen increases until it reaches a critical value of DO2 beyond which tissue hypoxia occurs. The impact of oxygenation and positive influence on outcomes has been shown in several studies.[14,15,16]
Donati and colleagues had shown that maintaining tissue DO2 reduced organ failure and length of hospital stay.[8] They found significantly lower length of hospital stay and number of organ failures in patients randomised to receive GDT starting intra-operatively and in whom the OER was maintained at ≤27%. They concluded that perioperative augmentation of DO2 through GDT was associated with improved outcome.
Pearse and his colleagues evaluated the effects of postoperative GDT targeting DO2 in 60 patients undergoing high-risk surgery.[5] In this study, patients were randomised to receive GDT targeting an oxygen delivery index of ≥ 600 mL/min. They concluded that the GDT group patients had shorter duration of hospitalization and lesser complication rates than the control group.
We also evaluated the effect of tissue oxygenation as reflected by lactate levels and base excess on outcomes with and without targeting O2ER between similar groups of patients. We obtained the value of O2ER from a simple mathematical difference between the arterial and central venous saturations instead of the mixed venous saturations, which is a close estimate to the O2ER. This did not involve any extra invasive lines for the patients and provided an appropriate representation in the balance between DO2 and consumption (SaO2–ScvO2/SaO2 × 100). In most patients, this was easily achieved during anaesthesia. In those patients with a value >27%, the target was obtained by either improving CI or by increasing the FiO2 delivered. As an oxygenation target, we found that attention to this value could have been associated with better tissue perfusions and outcomes in the GDT group. However, assessments of O2ER were performed only hourly and could have been improvised with continuous ScvO2 measurements using specialised central venous catheters. The measurements of O2ER were dependent upon ScvO2, which was targeted in-Group-A as well and could have limited the positive impact of GDT in the study group.
Goal directed therapy with larger volumes of fluid resuscitation has been controversial in terms of postoperative recovery.[17] Studies targeting GDT while improving outcomes have always been associated with larger volumes of fluid replacement.[4,5,6,9] In our study, we targeted fluid administration in Group-A to achieve a ScvO2 > 70% and enhanced haemodynamic targets in Group-B. Although this did not comply with the restrictive strategy, we did not observe postoperative complications relating to bowel oedema or delayed bowel recovery in either of the groups.
The CO monitor used, the FloTrac/Vigileo™ works on the analysis of the arterial pressure tracing and has found to be a reliable indicator of CO.[18] This concept of pulse contour analysis is based upon the relationship between the BP, stroke volume, arterial compliance and systemic vascular resistance (SVR).[19] The stroke volume and the CO can be calculated from the arterial pressure waveform if the compliance and SVR are known. The FloTrac system is operator independent, needs no external calibration and requires only a peripheral arterial catheter. We used the FloTrac/Vigileo™ for ensuring the target CI while need for fluids or vasopressors was assessed by other haemodynamic parameters as outlined in the figures [Figures 1 and 2].
CONCLUSIONS
Enhanced GDT targeted at CI and O2ER resulted in lesser values of lactate and base deficit and correlated with better tissue oxygenation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Lees N, Hamilton M, Rhodes A. Clinical review: Goal-directed therapy in high risk surgical patients. Crit Care. 2009;13:231. doi: 10.1186/cc8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearse RM, Harrison DA, James P, Watson D, Hinds C, Rhodes A, et al. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10:R81. doi: 10.1186/cc4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14. doi: 10.1056/NEJMoa021108. [DOI] [PubMed] [Google Scholar]
- 4.Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95:634–42. doi: 10.1093/bja/aei223. [DOI] [PubMed] [Google Scholar]
- 5.Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445] Crit Care. 2005;9:R687–93. doi: 10.1186/cc3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer J, Boldt J, Mengistu AM, Röhm KD, Suttner S. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: A randomized, controlled trial. Crit Care. 2010;14:R18. doi: 10.1186/cc8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez G, Wulf-Gutierrez ME, Reines HD. Monitoring oxygen transport and tissue oxygenation. Curr Opin Anaesthesiol. 2004;17:107–17. doi: 10.1097/00001503-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Donati A, Loggi S, Preiser JC, Orsetti G, Münch C, Gabbanelli V, et al. Goal-directed intraoperative therapy reduces morbidity and length of hospital stay in high-risk surgical patients. Chest. 2007;132:1817–24. doi: 10.1378/chest.07-0621. [DOI] [PubMed] [Google Scholar]
- 9.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 10.Englehart MS, Schreiber MA. Measurement of acid-base resuscitation endpoints: Lactate, base deficit, bicarbonate or what? Curr Opin Crit Care. 2006;12:569–74. doi: 10.1097/MCC.0b013e328010ba4f. [DOI] [PubMed] [Google Scholar]
- 11.Older P, Hall A. Clinical review: How to identify high-risk surgical patients. Crit Care. 2004;8:369–72. doi: 10.1186/cc2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97:820–6. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Lobo SM, Salgado PF, Castillo VG, Borim AA, Polachini CA, Palchetti JC, et al. Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med. 2000;28:3396–404. doi: 10.1097/00003246-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Jouffroy R, Lamhaut L, Guyard A, Phillipe P, Deluze T, Jaffry M, et al. Base excess and lactate as prognostic indicators for patients treated by extra corporeal life support after out hospital cardiac arrest due to acute coronary syndrome. Resuscitation. 2014;85:1764–8. doi: 10.1016/j.resuscitation.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Smith I, Kumar P, Molloy S, Rhodes A, Newman PJ, Grounds RM, et al. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med. 2001;27:74–83. doi: 10.1007/s001340051352. [DOI] [PubMed] [Google Scholar]
- 16.Abdelmalak BB, Cata JP, Bonilla A, You J, Kopyeva T, Vogel JD, et al. Intraoperative tissue oxygenation and postoperative outcomes after major non-cardiac surgery: An observational study. Br J Anaesth. 2013;110:241–9. doi: 10.1093/bja/aes378. [DOI] [PubMed] [Google Scholar]
- 17.Lobo MS, Ronchi SL, Oliviera NG, Brandao PG, Froes A, Cunrath GS, et al. Restrictive strategy of intra operative fluid maintenance during optimization of oxygen delivery decreases major complications after high-risk surgery. Crit Care. 2011;15:R226. doi: 10.1186/cc10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer J, Boldt J, Poland R, Peterson A, Manecke GR., Jr Continuous arterial pressure waveform-based cardiac output using the FloTrac/Vigileo: A review and meta-analysis. J Cardiothorac Vasc Anesth. 2009;23:401–6. doi: 10.1053/j.jvca.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Marik PE. Noninvasive cardiac output monitors: A state-of the-art review. J Cardiothorac Vasc Anesth. 2013;27:121–34. doi: 10.1053/j.jvca.2012.03.022. [DOI] [PubMed] [Google Scholar]


