Abstract
Aim:
To determine the correlation between the results of thyroid fine-needle aspirations interpreted using the Bethesda system and final histopathological reports for patients at an oncology hospital (OH) and non-oncology hospitals (NOHs).
Materials and Methods:
A retrospective, cross-sectional, descriptive study was performed to compare the cytology and histopathology results for patients with thyroid nodules in three Colombian hospitals. The final correlation of diagnoses between the two methods is reported. In Colombia, the health system provides the existence of general care hospitals and hospitals specializing in care of patients with cancer.
Results:
A total of 196 reports were reviewed, of which 53% were from OH and 47% were from NOHs. A greater proportion of category V (37.5%) was diagnosed at the OH, whereas NOHs diagnosed a greater proportion of category II (42.3%). The global correlation between diagnoses made using cytology and histopathology was 93.3% for categories V and VI (based on the final malignant diagnosis) and 86.9% for benign category II. Significant differences between institution types were observed when category IV and V and malignant histopathology were compared (56.3% OH vs. 23.5% NOH; P = 0.05 for category IV, 97.4% OH vs. 82.3% NOH; P = 0.03 for category V), while no significant difference between institution types was observed when category II and final benign diagnosis were compared (P = 0.6).
Conclusions:
The Bethesda system for thyroid cytology correlates adequately with final histopathological diagnosis in Colombia. Significant differences were identified in the diagnostic correlation for malignant lesions between the OH and NOHs in categories IV and V caused by selection bias of the population.
Keywords: Bethesda system, biopsy, fine needle, multicenter study, pathology, terminology, thyroid cytopathology, thyroid gland, thyroid neoplasms
Introduction
The Bethesda system for reporting thyroid cytopathology (TBSRTC)[1] was introduced in 2007 in an attempt to standardize international terminology and to categorize morphological criteria in fine-needle aspirations (FNAs) from patients with thyroid nodules. FNA is currently the preferred screening test for guiding the diagnosis and treatment of thyroid nodules.[2]
TBSRTC establishes six diagnostic categories for FNA results and assigns a malignancy risk and recommendations for patient management for each category.[1] Global studies of the incorporation of TBSRTC in diagnostic algorithms for patients with thyroid nodules have concluded that TBSRTC reduces unnecessary thyroidectomies while also ensuring the quality of thyroid malignancy detection.[3]
In Colombia, the health system provides the existence of general care hospitals and hospitals specializing in the care of patients with cancer. In both types of hospitals, there are trained cytologists and pathologists for the diagnosis of the most common diseases, and it is supposed that an oncologic pathologist outperforms the general pathologist in the cytological diagnosis of thyroid samples.
Few studies have been published on the implementation of the TBSRTC in Colombian health institutions and its diagnostic correlation with final histopathological results.[4] The objective of this study was to determine the correlation between diagnoses made based on FNA results interpreted using TBSRTC and the final histopathological diagnosis for patients at oncology hospitals (OHs) and non-oncology hospitals (NOHs).
Materials and Methods
Pathology laboratory archives from three different institutions were reviewed. A cross-sectional study was designed using FNA results of patients with thyroid nodules classified based on the TBSRTC for whom histopathological reports on the resected surgical lesion were available (n = 196) and who were treated between 2010 and 2012.
The study included 104 (53%) patients at an OH, in Bogotá, and 92 (47%) patients at NOHs in Bucaramanga and Bogotá, Colombia.
The histopathological reports on partial or total thyroidectomies of patients who participated in the study were diagnosed based on the World Health Organization (WHO) 2004 classification for tumors of the thyroid gland.[5]
Statistical analysis
Frequency measures are reported for the categorical variables and central tendency and dispersion measures are reported for continuous variables. In addition, frequency tables were constructed for each category in TBSRTC for comparison with the final histopathological report. Values of P were calculated using Fisher's exact test.
Results
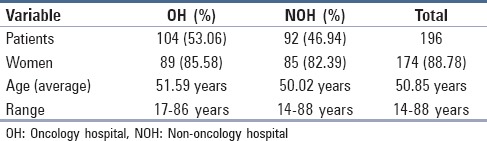
A total of 196 patients were included in the study, 174 of whom were women (88.78%), with a mean age of 50.8 years (range: 14-88 years). Details of the other characteristics of the population are presented in Table 1.
Table 1.
Characteristics of the study population
The following FNA results by TBSRTC category were obtained for the total population:
Non-diagnostic or unsatisfactory (ND/UNS), 4.1%;
Benign, 23.5%;
Atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS), 2%;
Follicular neoplasm or suspicious for a follicular neoplasm, 16.8%;
Suspicious for malignancy (SM), 37.2%; and
Malignant, 16.3%. When grouped by OH and NOHs, more categories V, SM and VI, malignant (68.3%) and less category II, benign (6.7%) were diagnosed at the OH, whereas more benign FNA results (42.4%) were obtained at the NOHs, followed by category V, SM (37%) and category IV, follicular neoplasm or suspicious for a follicular neoplasm (18.5%; Table 2).
Table 2.
FNA results and histopathology based on type of hospital
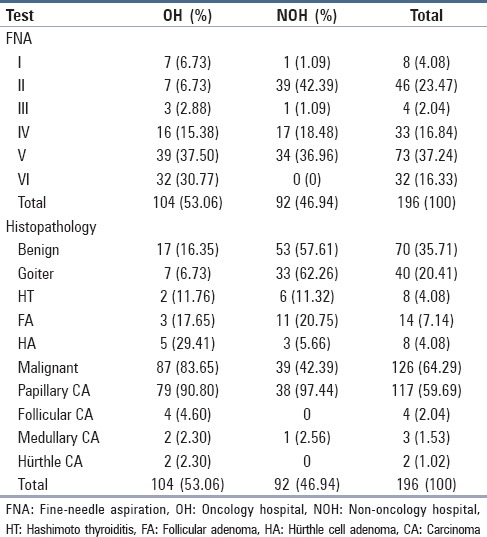
About the histopathological diagnoses, 64.3% were malignant and 35.7% were benign. The most frequent benign diagnosis was goiter (20.4%) and the most frequent malignant diagnosis was papillary carcinoma (59.7%). When the results were compared based on type of hospital, the OH made a final malignant histopathological diagnosis in 83.6% of cases, whereas NOHs made a final malignant diagnosis in 42.4% of the cases [Table 2].
When the results of FNA and histopathology were compared, FNA categories V and VI were associated with a histopathological malignancy frequency of 90.4% and 100%, respectively (this last category was not used for any FNA at the NOHs), while category II, benign, was associated with a benign histopathological diagnosis in 86.9% of all cases. With respect to category IV, follicular neoplasm or suspicious for a follicular neoplasm, 39.4% of cases were associated with a final malignant histopathological result [Table 3].
Table 3.
FNA frequency and histopathological final diagnosis based on institution
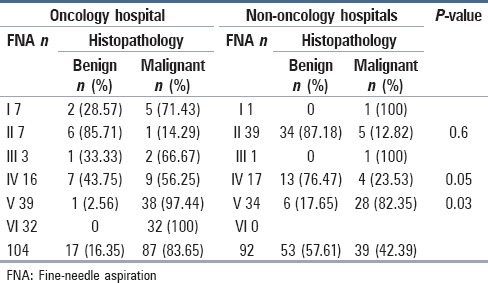
A comparison of FNA with histopathology results based on institution type revealed that FNA category V was associated with a higher frequency of final malignant histopathological results at the OH (OH 97.4% vs. NOHs 82.3%; P = 0.03). For benign category II FNA, no significant difference between type of institution was observed when correlations between final benign and malignant histopathological results were compared (OH: 85.7% benign and 14.3% malignant vs. NOHs: 87.2% benign and 12.8% malignant; P = 0.6). For a FNA follicular neoplasm or suspicious for a follicular neoplasm category IV, a greater proportion of benign results were obtained at the NOHs (76.5% NOHs vs. 43.8% OH), and a greater proportion of malignant histopathological results were obtained at the OH (56.3% OH vs. 23.5% NOHs; P = 0.05) [Table 3].
Discussion
Thyroid carcinoma is the most frequent endocrine neoplasm; it presents at all ages and predominantly affects women.[6] Based on the Globocan 2012 results,[7] the estimated incidence per 100,000 persons per year of thyroid cancer varies from country to country: The incidence is 13.2 in the United States, 8.4 in France and 5.1 in Colombia, clearly indicating regional differences in the incidence of thyroid cancer and the need to establish diagnostic correlations between FNA and histopathology for each country. According to the National Institute of Cancer, Colombia, thyroid carcinoma is the fifth most frequent malignant neoplasm in Colombia.[8]
FNA is essential when evaluating patients with a unique thyroid nodule. When used, analyzed and interpreted correctly, FNA reduces the number of unnecessary thyroidectomies in patients with benign nodules and is highly sensitive for diagnosing patients with malignant thyroid tumors.[3,9,10]
TBSRTC is a unified system that complements cytological findings from thyroid FNA and represents a consensus among multiple experts in different areas of medicine.[11] In Colombia, implementation of TBSRTC at a single institution that specializes in the treatment of cancer patients revealed an 89% correlation with the final histopathological report for multiple malignant lesions;[4] however, the sample size and the population selection bias limits the external validity of these results. No data are available on the diagnostic correlation between TBSRTC and the final histopathology report in NOHs in Colombia and whether there are differences in the implementation of TBSRTC and diagnostic accuracy among pathologists and cytologists with more training in the diagnosis of cancer at OH and those trained in diagnosing general diseases at NOHs.
In this study, histopathology and cytopathology results were compared for the same thyroid lesions in patients at three different health institutions: One OH and two NOHs. All institutions participating in the study used TBSRTC for the interpretation of FNA; however, at the NOHs, cytopathologists did not use category malignant in their FNA reports regarding the characteristics of the study population in these hospitals, which have a relatively low frequency of carcinomas, producing distrust of the use of the category. We do not take into account the percentage of incidental papillary carcinoma in the resections for analysis.
The distribution of FNA categories and the distribution of pathologic diagnoses were analyzed. Differences in the two stratified populations were observed. Histopathological and cytopathological diagnoses of benign lesions were frequent in the NOHs, whereas diagnoses of malignant neoplasms were more frequent in the OH.
Comparison of the distribution of the samples in each TBSRTC category with previous reports[9,12,13,14] reveals similarities in the proportion of FNA category III AUS/FLUS (2% vs. 3-3.2%). These results reflect a strict adherence to the established criteria for this category and contradict the general belief that this category represents a catchall for those cases that cannot be classified as any other category.
However, the same comparison for category IV, follicular neoplasm or suspicious for a follicular neoplasm, reveals that the proportion varies from 5.5% and 11.6% in the literature, while the value in the present study was greater, 16.8% (OH 15.4% vs. NOHs 18.5%). Of these cases, 39.4% were associated with a final malignant histopathology report, the majority of which were the follicular variant of papillary thyroid carcinoma, and a statistically significant difference was noted between the OH and NOHs for the final malignant histology diagnosis in this category (56.3% OH vs. 23.5% NOHs; P = 0.05). Differences in these categories are likely due to a cognitive bias of pathologists, i.e., the percentage of benign tumors is greater in NOHs when using this category and the percentage of malignancies is higher in OH. In a meta-analysis that specifically evaluated category IV before and after the implementation of TBSRTC, an increase in the utilization of this category (6.1% to 7.4%, P = 0.0002) and an increase in the final frequency of histopathological malignancy (22% to 28%, P = 0.03) was observed, indicating the difficulty of differentiating neoplasms with follicular morphology from benign samples and confirming the necessity of histopathological examination for a final classification. These data justify surgical resection of TBSRTC category IV lesions.[6,15,16,17]
Our data differ from those in the literature for the relative frequencies of categories II, benign (23.5 vs. 64.6-73.8%), V, SM (37.2% vs. 1.3-2.6%) and VI, malignant (16.3% vs. 5.2-7.5%). The distribution of cases in this study indicates a greater proportion of malignancies and a reduced proportion of benign results. The malignancy rate was 64.29% in the whole series. As the incidence of thyroid carcinoma is 5.1 per 100,000 persons per year in Colombia, this high rate of malignancy may be attributable to the population selection bias.
The proportion of category I, ND/UNS cases in this study is lower (4.1 vs. 10.4-11.1%) than that reported in similar studies[10,13,14,18]; however, the proportion of cases treated in the OH was greater than in the NOHs (6.7% vs. 1.1%).
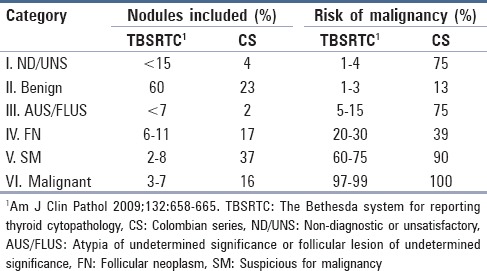
TBSRTC assigns a risk of malignancy to each category in an ascending manner. In our series, this tendency is preserved, but the frequency of malignancy was greater for all categories when compared with TBSRTC,[1] particularly for category III, AUS/FLUS (75% vs. 15% of TBSRTC; Table 4). Notably, only four of 196 FNAs were included in this category and, of these cases, three patients were judged to have carcinoma as the final histopathological report. The results of recently published studies demonstrate that the malignancy frequency for nodules classified as category III AUS/FLUS is greater than that estimated by TBSRTC,[19,20] and a review of the guidelines and clinical protocols for TBSRTC is advised.
Table 4.
Comparison between the Bethesda system overall categories and the Colombian series: Frequency of nodules included in each category and absolute risk of malignancy
Conclusion
In conclusion, for the interpretation of aspiration cytology of thyroid lesions in Colombia, TBSRTC is a useful tool that enhances the diagnostic accuracy of FNA, exhibits an adequate diagnostic correlation with the final histopathological examination and enables a comparison of results between different institutions. Significant differences in the diagnostic correlation of FNA between the OH and the NOHs in the study were observed, specifically for the percentage of malignancy in categories IV and V. It can be caused by selection bias of the population that consults each type of hospital and also by a cognitive bias of the pathologists who favor malignancy or benignity based on their own workload.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Cibas ES, Ali SZ. NCIThyroidFNA State of the Science Conference. The Bethesda System for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658–65. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3.Richmond BK, O’Brien BA, Mangano W, Thompson S, Kemper S. The impact of implementation of the Bethesda System for reporting thyroid cytopathology on the surgical treatment of thyroid nodules. Am Surg. 2012;78:706–10. [PubMed] [Google Scholar]
- 4.Romero-Rojas A, Melo-Uribe M. Implementation of the Bethesda system for cytology reporting of thyroid fine needle aspirates with histological follow-up: Experience in a cancer treatment center. Rev Colomb Cancerol. 2014;18:3–7. [Google Scholar]
- 5.DeLellis R, Lloyd R, Heitz P, Eng C. Lyon: IARC Press; 2004. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs; p. 50. [Google Scholar]
- 6.Bose S, Walts AE. Thyroid fine needle aspirate: A post-Bethesda update. Adv Anat Pathol. 2012;19:160–9. doi: 10.1097/PAP.0b013e3182534610. [DOI] [PubMed] [Google Scholar]
- 7.Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. [Last accessed on 2014 July 17]. Available from: http://www.globocan.iarc.fr .
- 8.Garavito G, Llamas A, Cadena E, De Los Reyes A, Hurtado G, Rojas L, et al. Differentiated Thyroid Cancer Multidisciplinary Management at the Colombian National Cancer Institute. Rev Colomb Cancerol. 2010;14:65–77. [Google Scholar]
- 9.Ozluk Y, Pehlivan E, Gulluoglu MG, Poyanli A, Salmaslioglu A, Colak N, et al. The use of the Bethesda terminology in thyroid fine-needle aspiration results in a lower rate of surgery for nonmalignant nodules: A report from a reference center in Turkey. Int J Surg Pathol. 2011;19:761–71. doi: 10.1177/1066896911415667. [DOI] [PubMed] [Google Scholar]
- 10.Crowe A, Linder A, Hameed O, Salih C, Roberson J, Gidley J, et al. The impact of implementation of the bethesda system for reporting thyroid cytopathology on the quality of reporting, ‘risk’ of malignancy, surgical rate, and rate of frozen sections requested for thyroid lesions. Cancer Cytopathol. 2011;119:315–21. doi: 10.1002/cncy.20174. [DOI] [PubMed] [Google Scholar]
- 11.Firat P, Cochand-Priollet B. The Bethesda system for reporting thyroid fine needle aspiration cytology: A study comparing the results of two centers from two different countries. (415-20).Ann Pathol. 2012;32:e29–34. doi: 10.1016/j.annpat.2012.09.204. [DOI] [PubMed] [Google Scholar]
- 12.Theoharis CG, Schofield KM, Hammers L, Udelsman R, Chhieng DC. The Bethesda thyroid fine- needle aspiration classification system: Year 1 at an academic institution. Thyroid. 2009;19:1215–23. doi: 10.1089/thy.2009.0155. [DOI] [PubMed] [Google Scholar]
- 13.Rabaglia J, Kabbani W, Wallace L, Holt S, Watumull L, Pruitt J, et al. Effect of the Bethesda system for reporting thyroid cytopathology on thyroidectomy rates and malignancy risk in cytologically indeterminate lesions. Surgery. 2010;148:1267–73. doi: 10.1016/j.surg.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: A study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–15. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 15.Bongiovanni M, Piana S, Spitale A, Valli R, Carlinfante G, Gardini G. Comparison of the diagnostic accuracy of thyroid fine-needle aspiration in follicular-patterned lesions using a 5-tiered and a 6-tiered diagnostic system: A Double-Blind Study of 140 Cases with Histological Confirmation. Diagn Cytopathol. 2014;42:744–50. doi: 10.1002/dc.23115. [DOI] [PubMed] [Google Scholar]
- 16.Williams BA, Bullock MJ, Trites JR, Taylor SM, Hart RD. Rates of thyroid malignancy by FNA diagnostic category. J Otolaryngol Head Neck Surg. 2013;42:61. doi: 10.1186/1916-0216-42-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu HH, Rose C, Elsheikh TM. The Bethesda system for reporting thyroid cytopathology: An experience of 1,382 cases in a community practice setting with the implication for risk of neoplasm and risk of malignancy. Diagn Cytopathol. 2012;40:399–403. doi: 10.1002/dc.21754. [DOI] [PubMed] [Google Scholar]
- 18.Boonyaarunnate T, Olson MT, Ali SZ. ‘Suspicious for a follicular neoplasm’ before and after the bethesda system for reporting thyroid cytopathology: Impact of standardized terminology. Acta Cytol. 2013;57:455–63. doi: 10.1159/000351664. [DOI] [PubMed] [Google Scholar]
- 19.Ho AS, Sarti EE, Jain KS, Wang H, Nixon IJ, Shaha AR, et al. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS) Thyroid. 2014;24:832–9. doi: 10.1089/thy.2013.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyeon J, Ahn S, Shin JH, Oh YL. The prediction of malignant risk in the category “atypia of undetermined significance/follicular lesion of undetermined significance” of the Bethesda System for Reporting Thyroid Cytopathology using subcategorization and BRAF mutation results. Cancer Cytopathol. 2014;122:368–76. doi: 10.1002/cncy.21396. [DOI] [PubMed] [Google Scholar]


