Abstract
Renal Ewing sarcoma (ES) is a rare malignant tumor characterized by fusion of the EWSR1 gene with a member of the ETS family of oncogenes, arising at a specific chromosomal translocation. Diagnosis of ES can be problematic, especially from cytological or small bioptical specimens because the differential diagnoses comprising a diverse group of small round blue cell tumors (SRBCTs). We report a case of primary renal ES in a young male, which had a t(11;22) (q24;q12) chromosome translocation encoding a type2 EWSR1/FLI1 fusion transcript. The tumor cells showed diffuse cytoplasmic immunoreactivity for CD99 and diffuse nuclear immunoreactivity for NKX2.2, an important oncogenic transcriptional target of EWSR1/FLI1, not only in the histological, but also in the cytological specimens. From the results of this case, we speculate that NKX2.2, in combination with CD99, may be a useful immunocytochemical marker to distinguish renal ES from other SRBCTs of kidney.
Keywords: CD99, Ewing sarcoma, immunocytochemistry, kidney neoplasm, NKX2.2
Introduction
Renal Ewing sarcoma (ES) is a rare small round blue cell tumor (SRBCT), which predominantly affects adolescents and young adults.[1] Cytogenetically, renal ESs commonly harbor characteristic chromosomal translocations, more than 90% of which are reciprocal translocation t (11;22) (q24;q12), resulting in an EWSR1/FLI1 fusion transcript.[1,2] We here report a case of renal ES with a t (11;22) (q24;q12) chromosome translocation encoding an EWSR1/FLI1 fusion transcript. The tumor cells in both histological and cytological specimens showed diffuse and strong nuclear immunoreactivity for NKX2.2, an oncogenic transcriptional target of EWSR1/FLI1, which may be a useful immunocytochemical marker for renal ES harboring EWSR1/FLI1 fusion transcript.
Case Report
A 35-year-old male presented with a 2-week history of abdominal pain and hematuria. Computed tomography revealed a large mass (15.5 cm × 13.5 cm × 10 cm) replacing the right kidney and expanding into the right inferior vena cava, as well as a small metastatic nodule in the right lung. A needle biopsy of the renal mass showed mixture of sheets and Homer-Wright rosettes composed of small round cells [Figure 1a]. PAS staining demonstrated the presence of variable amounts of diastase-digestible glycogen. Immunohistochemistry revealed that the tumor cells showed diffuse membranous and cytoplasmic expression of CD99 [Figure 1b], and diffuse nuclear NKX2.2 expression with strong intensity [Figure 1c]. We interpreted the tumor as renal ES and the patient underwent curative resection of both of the primary and the metastatic lesion. Papanicolaou staining of touch imprint smears of the lesion showed a well dispersed uniform population of small round cells [Figure 1d]. The tumor cells had vesicular and clear cytoplasm of which rim was distinct. The nuclei were round to oval, with irregular borders, coarsely granular chromatin and inconspicuous nucleoli. Mitotic figures and apoptotic figures were frequently found. Rosette formation was rarely observed. In immunocytochemistry, the tumor cells showed diffuse cytoplasmic expression of CD99 [Figure 1e]; diffuse nuclear expression of NKX2.2 with strong intensity [Figure 1f]. Fluorescence in situ hybridization (FISH) was performed using probes made from BAC clone; RP11-945M21 for EWSR1 and RP11-75P14 for FLI1. Probe DNA was labeled by nick translation kit using: SpectrumOrange (Abott) for EWSR1 and SpectrumGreen (Abott) for FLI1. The touch imprint specimen revealed the presence of t (11;22) (q24;q12) translocation [Figure 2a]. Reverse transcription polymerase chain reaction (RT-PCR) using the removed tumor specimen could amplify the fusion transcript [Figure 2b], following sequence analysis revealed type 2 EWSR1/FLI1 fusion that involved EWSR1 exon 7 fused to FLI1 exon 5 [Figure 2c]. Nevertheless of six cycles of intensive chemotherapy, including cyclophosphamide, vincristine, doxorubicin, the tumor relapsed, and the patient died of the disease 27 months after the operation.
Figure 1.
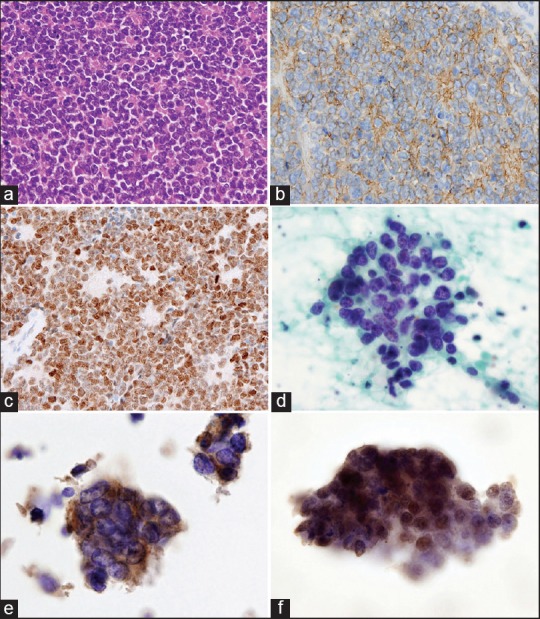
Renal Ewing sarcoma with EWSR1/FLI1 fusion. (a) Lobar architecture with Homer-Wright rosettes (H and E). (b) Immunohistochemistry with anti-CD99 (Leica, NCL-CD99). (c) Immunohistochemistry with anti-NKX2.2 (Sigma, A71669). (d) Touch imprint smear. Tumor cells have round to oval nuclei with coarsely granular chromatin (Pap). (e) The tumor cells show cytoplasmic expression of CD99. (f) The tumor cells show strong and diffuse nuclear expression of NKX2.2
Figure 2.
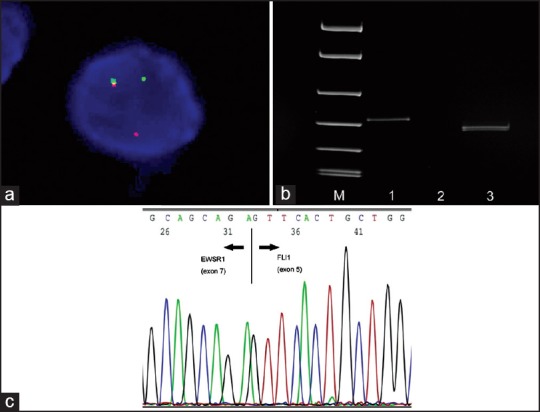
(a) Fluorescence in situ hybridization using dual-color probe for EWSR1 region (orange) and FLI1 region (green). A tumor cell of touch imprint smear showing one fused, one red and one green signal pattern. (b) Polyacrylamide gel electrophoresis of Reverse transcription polymerase chain reaction (RT-PCR) products. The present case showing a 166 bp band of type2 EWSR1/FLI1 fusion (lane 1). (Lane M: DNA marker, lane 2: Negative control, lane 3: Fusion transcripts of a skeletal Ewing sarcoma, a 130 bp band of type 1 EWSR1/FLI1 fusion), (c) Sequence analysis of the RT-PCR product of the present case. EWSR1 exon 7 fused to FLI1 exon 5
Discussion
Renal ESs commonly show an adverse prognosis as the present case. Cases of local recurrence and metastasis have been shown to comprise more than 50% of the clinical presentations.[1] In a recent review of 25 cases, the mean patient survival was approximately 10 months.[2] Therefore, accurate and reliable preoperative procedures, such as fine-needle aspiration cytology (FNAC), are necessary to facilitate timely treatment. However, it can be difficult to distinguish renal ES from other SRBCTs desmoplastic small cell round tumor, synovial sarcoma, and malignant lymphoma, by FNAC because these tumors, such as Wilms tumor, neuroblastoma, small cell neuroendocrine tumor, often show overlapping cytomorphologic features.[2,3] Therefore, the cytodiagnosis requires confirmation by other ancillary approaches, including immunocytochemistry and/or genetic analyses, such as FISH and/or RT-PCR, as our present case.[3] However, these genetic analyses are not always suitable for screening because they are costly and laborious, and the amount of applicable RNA from cytological specimen is very little. Therefore, conventional immunocytochemistry with reliable markers will help to confirm the preoperative cytodiagnosis.[4]
CD99 is frequently applied as an immunohistochemical marker for the diagnosis of ES.[1,2,3,4] However, CD99 expression has been found in a wide variety of renal non-ES SRBCTs, such as Wilms tumor, small cell neuroendocrine tumor, and synovial sarcoma.[5] Therefore, CD99 cannot be applied as an independent diagnostic marker for ES, even though its expression, as well as that of NKX2.2, is regulated by EWSR1/FLI1 in ES.[6] Meanwhile, FLI1 also might be a sensitive immunohistochemical marker for ES; however, it could not be used as the sole diagnostic marker, because FLI1 expression also has been reported in various non-ES SRBCTs, such as lymphoblastic lymphomas, Burkitt lymphomas, and synovial sarcomas, and its specificity for the ES is lower than that of CD99.[7]
Recently, NKX2.2 has been identified as a target of EWSR1/FLI1 and upregulated in ESs.[6] NKX2.2 functions as transcriptional repressors through the recruitment of corepressors, such as histone deacetylase, and play important roles in the oncogenesis of ES.[6] A recent immunohistochemical study of peripheral SRBCTs has shown that NKX2.2 is a valuable immunohistochemical marker for peripheral ES, with a sensitivity of 93% and a specificity of 89%, and that the reactivity was commonly diffuse with moderate or strong intensity.[8] Moreover, Sibuya et al. recently evaluated the usefulness of immunohistochemical expression of NKX2.2 for the differential diagnosis between ESs and non-ES SRBCTs, and showed its sensitivity and specificity were 80 and 84%, respectively, and the specificity got higher (98%) when CD99 and NKX2.2 were combined.[4] Although NKX2.2 can be positive in small number of non-ES SRCBTs, including a minor subset of small cell carcinomas, synovial sarcomas, and malignant melanomas,[8] these data suggest that NKX2.2 may be a potential diagnostic marker of ES, as is the case for TLE1 in synovial sarcoma, a transcription factor, which is activated by the SYT/SSX fusion protein, of which efficiency were proofed in recent immunohistochemical studies.[9,10]
In the present study, the ES cells harboring EWSR1/FLI1 fusion showed diffuse nuclear expression of NKX2.2 with strong intensity. Although, the final confirmation of ES does include the demonstration of the translocation by molecular methods, immunostaining of cytological specimens with the combination of anti-CD99 and anti-NKX2.2 may be useful to distinguish renal ES from other SRBCTs of kidney.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Eble J, Epstein J, Sesterhenn I, Sauter G. 3rd ed. Lyon: IARC Press; 2002. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; pp. 83–4. [Google Scholar]
- 2.Jimenez RE, Folpe AL, Lapham RL, Ro JY, O’Shea PA, Weiss SW, et al. Primary Ewing's sarcoma/primitive neuroectodermal tumor of the kidney: A clinicopathologic and immunohistochemical analysis of 11 cases. Am J Surg Pathol. 2002;26:320–7. doi: 10.1097/00000478-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R, Gautam U, Srinivasan R, Lal A, Sharma U, Nijhawan R, et al. Primary Ewing's sarcoma/primitive neuroectodermal tumor of the kidney: Report of a case diagnosed by fine needle aspiration cytology and confirmed by immunocytochemistry and RT-PCR along with review of literature. Diagn Cytopathol. 2012;40(Suppl 2):E156–61. doi: 10.1002/dc.21717. [DOI] [PubMed] [Google Scholar]
- 4.Shibuya R, Matsuyama A, Nakamoto M, Shiba E, Kasai T, Hisaoka M. The combination of CD99 and NKX2.2, a transcriptional target of EWSR1-FLI1, is highly specific for the diagnosis of Ewing sarcoma. Virchows Arch. 2014;465:599–605. doi: 10.1007/s00428-014-1627-1. [DOI] [PubMed] [Google Scholar]
- 5.Riggi N, Cironi L, Provero P, Suvà ML, Kaloulis K, Garcia-Echeverria C, et al. Development of Ewing's sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65:11459–68. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 6.Smith R, Owen LA, Trem DJ, Wong JS, Whangbo JS, Golub TR, et al. Expression profiling of EWS/FLI identifies NKX2.2 as a critical target gene in Ewing's sarcoma. Cancer Cell. 2006;9:405–16. doi: 10.1016/j.ccr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Mhawech-Fauceglia P, Herrmann F, Penetrante R, Beck A, Sait S, Block AM, et al. Diagnostic utility of FLI-1 monoclonal antibody and dual-colour, break-apart probe fluorescence in situ (FISH) analysis in Ewing's sarcoma/primitive neuroectodermal tumour (EWS/PNET). A comparative study with CD99 and FLI-1 polyclonal antibodies. Histopathology. 2006;49:569–75. doi: 10.1111/j.1365-2559.2006.02535.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida A, Sekine S, Tsuta K, Fukayama M, Furuta K, Tsuda H. NKX2. 2 is a useful immunohistochemical marker for Ewing sarcoma. Am J Surg Pathol. 2012;36:993–9. doi: 10.1097/PAS.0b013e31824ee43c. [DOI] [PubMed] [Google Scholar]
- 9.Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, et al. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol. 2007;31:240–6. doi: 10.1097/01.pas.0000213330.71745.39. [DOI] [PubMed] [Google Scholar]
- 10.Jagdis A, Rubin BP, Tubbs RR, Pacheco M, Nielsen TO. Prospective evaluation of TLE1 as a diagnostic immunohistochemical marker in synovial sarcoma. Am J Surg Pathol. 2009;33:1743–51. doi: 10.1097/PAS.0b013e3181b7ed36. [DOI] [PubMed] [Google Scholar]


