Abstract
Hypertension is widely encountered in family medicine. Despite its prevalence, many patients have uncontrolled or difficult-to-control blood pressure. Resistant hypertension is defined as hypertension that is poorly responsive to treatment and requires the use of multiple medications to achieve acceptable blood pressure ranges. It may be a consequence of secondary hypertension or have no identifiable cause. Resistant hypertension is important to recognise because it places patients at risk of end-organ damage. Primary care physicians should be aware of the therapeutic approach for hypertension when traditional therapy fails. This article aims to familiarise readers with the evaluation and management of resistant hypertension by outlining the most recent evidence-based treatment options.
Keywords: Blood pressure, cardiovascular disease, renal denervation, resistant hypertension
Introduction
Hypertension is defined as persons 18 years of age and over with a systolic pressure reading of 140 mmHg or more or a diastolic reading of 90 mmHg or more, or those taking antihypertensive medications.[1,2] Between 90-95% of cases are essential hypertension in which there is no identifiable cause.[3] The remaining cases of hypertension are secondary to a distinguishable process and would likely resolve if the underlying condition was treated.
A considerable number of patients fail to reach target blood pressure ranges despite lifestyle advice and standard medical therapy. Resistant hypertension is defined as blood pressure that remains above 140/90 mmHg despite use of three antihypertensive medications of different classes at the best tolerated doses, one of which must be a diuretic.[4,5]
Epidemiology
Hypertension is widely encountered in primary care and is the most common condition managed at primary care level.[1] It accounts for 8.6% of all visits to a primary care physician.[2] Hypertension affects 32% of adults in western society, two-thirds of whom are poorly controlled.[6,7] Its prevalence is likely to continue rising.[8]
It is estimated that approximately 10% of hypertensive patients in westernised countries have resistant hypertension.[9,10] Doctors in primary care can expect to encounter resistant hypertension one in every 20 hypertensive patients, compared with higher rates in specialist clinics.[10]
Aetiology and pathogenesis
In most cases the pathogenesis of resistant hypertension is uncertain. Only in a minority of instances is the problem due to secondary hypertension. In the absence of a secondary cause the condition is most likely multifactorial. Proposed mechanisms include genetic factors, aberrant sympathetic nervous system activation and altered renal sodium and water handling due to changes in the renin-angiotensin-aldosterone system.
Clinical features and diagnosis
Although hypertension is usually asymptomatic detection and treatment remains important. Uncontrolled blood pressure is a considerable cardiovascular risk factor that makes target-organ damage more likely.[11] Possible end-organ consequences of untreated hypertension include heart failure, stroke, ischemic heart disease and renal failure.
Resistant hypertension presents in patients who have persistently elevated blood pressure which responds minimally to therapy. Approximately six months of treatment with three conventional antihypertensive agents should be allowed for at least some blood pressure correction.[9] If at six months treatment is proving unsuccessful the possibility of resistant hypertension may be contemplated.
The true prevalence of resistant hypertension is difficult to quantify because many patients actually suffer ‘pseudo-resistant’ hypertension. Pseudo-resistant hypertension refers to poorly controlled disease which appears resistant but is actually attributable to other factors. The commonest reasons for apparent treatment resistance are medication non-compliance and insufficient drug therapy. Other implicated factors include failure to adhere to lifestyle advice, poor measurement technique, white-coat hypertension and the use of medications that interfere with blood pressure [Table 1].
Table 1.
Drugs that interfere with blood pressure control[10]

A key step in diagnosing resistant hypertension is confirming that blood pressure elevation is truly resistant. Pseudo-resistance must be ruled out with a thorough history and medication review, followed by measurement of blood pressure with proper technique. Provided these confounders are absent and standard drug therapy has failed, resistant hypertension is diagnosed.
Every patient with resistant hypertension requires at least some screening for secondary causes [Table 2]. Obstructive sleep apnoea, chronic kidney disease and primary hyperaldosteronism are the most frequent comorbidities.[12] Although secondary hypertension is more likely in resistant hypertension than in patients whose blood pressure is controlled, most still do not have an identifiable cause.[13]
Table 2.
Causes of secondary hypertension[10]

Evaluation for secondary causes should include comprehensive history and physical examination searching for any clues pointing towards to an underlying diagnosis. For example the patient may have symptoms of obstructive sleep apnoea, or alternatively episodic palpitations with headaches and diaphoresis might suggest pheochromocytoma, while an abdominal bruit could indicate renal artery stenosis. Oftentimes however, the clinical assessment will be unremarkable.
Beyond history and examination, all patients with resistant hypertension should be investigated at minimum with a serum creatinine test, estimated glomerular filtration rate and urine dipstick.[10] This is due to the high prevalence of chronic kidney disease in this population. Any derangement in these baseline investigations warrants a renal ultrasound scan.[10] It is also advisable to order serum sodium, potassium and glucose because the test is simple and often helpful. Because of the confounding nature of the white-coat effect, 24-hour ambulatory blood pressure monitoring should also be undertaken.[11,14,15] Pressure readings from ambulatory monitoring correlate more closely with morbidity and mortality than measurements obtained in clinic.[14]
Further screening investigations for secondary causes are not compulsory. Additional tests [Table 3] should be chosen depending on the clinical circumstances of the patient as revealed by history and examination.
Table 3.

Finally, all patients with ongoing uncontrolled hypertension should be assessed routinely for any signs of end-organ compromise. This may include measures such as yearly fundoscopy, electrocardiogram and urine dipstick.
Management
Primary care physicians are familiar with the management of essential hypertension. Treatment should comprise both lifestyle modification and pharmacologic therapy. The blood pressure goal in uncomplicated patients is 140/90 mmHg which could be relaxed to 150/90 in patients greater than 60 years of age.[17] A more appropriate treatment target in patients with end-organ damage is 130/80 mmHg.[5,15]
Non-pharmacological treatment
Non-pharmacologic measures should be introduced in patients with hypertension of any severity. Recommendations include smoking cessation, reduction in alcohol intake, dietary sodium restriction, healthy eating plans, increased physical activity and weight loss. Lifestyle interventions complement the efficacy of drug therapy and, alone, are often satisfactory in uncomplicated essential hypertension.
Pharmacological treatment
There is little randomised trial data to guide choice of drug regimen for patients with resistant hypertension and recommendations are largely empirical. In general, the best strategy is to formulate a combination therapy that targets different physiological mechanisms and accounts for patient comorbidities.
The preferred initial drug choices are the same as for essential hypertension.[18] The guidelines of the National Institute for Health and Clinical Excellence (NICE) recommend initial treatment with an angiotensin-converting enzyme inhibitor (ACE inhibitor) in patients younger than 55 years of age, or a dihydropyridine calcium channel blocker (CCB) in patients older than 55 or black patients of any age.[19] These options can then be trialled in combination and titrated as necessary before adding a thiazide as the third medication.
Guidelines endorse commencement of antihypertensive treatment with only one drug because adequate monotherapy controls hypertension in 30% of cases.[20] If monotherapy is insufficient the regimen could be modified depending on therapeutic effect by altering dose or adding an additional class of drug. Triple therapy must be optimised before selecting further add-on therapy because optimal dosing and drug selection can see blood pressure normalise in many patients.
Whatever regimen is chosen it is important that it be tailored to the individual patient. Comorbidities must be considered when selecting an antihypertensive agent. For instance, in patients over 55 years of age with evidence of heart failure a thiazide diuretic might be a more suitable first-line option than a CCB. Alternatively, if patients are intolerant to an ACEI because of cough it is acceptable to replace the ACEI with an angiotensin receptor blocker (ARB). This should achieve a comparable blood pressure lowering effect.
Most patients with hypertension are prescribed hydrochlorothiazide as the diuretic of choice. However, several recent clinical trials have demonstrated superior blood pressure reduction with chlorthalidone, especially in patients with resistant hypertension.[20,21,22,23,24] Chlorthalidone is a thiazide-like diuretic with a longer half-life and greater potency than hydrochlorothiazide. A good initial step in controlling refractory hypertension is therefore to switch patients from hydrochlorothiazide to chlorthalidone. The starting dose of chlorthalidone is 12.5 mg daily, taken in the morning, titrated if necessary to a maximum of 50 mg daily.[25,26]
Indapamide is an alternative thiazide-like agent which may be more suitable than chlorthalidone in some patients. Chlorthalidone has a longer half-life and duration of action than indapamide and is associated with a higher risk of renal impairment and hypokalaemia. Thus in predisposed patients such as the elderly or those with renal insufficiency indapamide might be a superior substitute.[27] An appropriate starting dose is a 1.5 mg controlled-release tablet each morning.[19] Patients with severe renal impairment (GFR below 30 mls/min) should have their thiazide replaced by a loop diuretic under specialist guidance.[12]
In some cases the drug regimen may be altered by having patients take one antihypertensive agent at night-time instead of conventional morning-only dosing. Blood pressure generally lowers physiologically during sleep and nocturnal hypertension, or so-called ‘non-dipping’, has been associated with poorer cardiovascular prognosis.[28,29] There is recent trial data on resistant hypertension which suggests improved blood pressure reduction with fewer cardiovascular events if one antihypertensive is ingested nocturnally.[30] Importantly, there is no significant difference in adverse outcomes with this approach.[31] Therefore, shifting one agent to bedtime dosing is not an unreasonable decision in patents with resistant hypertension. However, if electing to trial nocturnal therapy it is generally wise to avoid a diuretic as the night-time medication.
Spironolactone is recommended as the fourth antihypertensive drug.[12,32] The Scandinavian Cardiac Outcomes Trial (ASCOT) showed mean blood pressure reduction of 22/10 mmHg at one year follow-up in patients with resistant hypertension randomised to receive spironolactone as the fourth medication.[27] A general rule is to commence patients on 25 mg per day, which may require about 2 weeks for full effect. This dose can be increased gradually, preferably over several months, to a maximum of 100 mg daily if necessary. Adverse effects of spironolactone usually appear at higher doses where patients may complain of gynecomastia and breast tenderness, menstrual irregularities and sexual dysfunction. Amiloride, a potassium-sparing diuretic, is a reasonable alternative in those intolerant of spironolactone. Its dose ranges from 2.5-10 mg daily. A caveat for both spironolactone and amiloride is the need for careful routine monitoring of serum potassium. If the blood potassium level exceeds 4.5 mmol/L intensification of thiazide therapy should be considered.[33]
The dual renal-angiotensin-aldosterone system blockade offered by spironolactone with ACEIs is not matched by combining ACEIs with ARBs. The landmark Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET) demonstrated no additional benefit between using ACEIs or ARBs alone and in combination.[34] Treatment regimens therefore should not include both an ACEI and ARB simultaneously.
Difficult patients will require referral to a hypertension specialist. Indications for referral include patients with uncontrolled blood pressure despite receiving maximum tolerated doses of four medications, patients suffering end-organ damage as a consequence of their high blood pressure, or patients with a suspected secondary cause [Table 4]. All patients unresponsive to quadruple therapy should be revaluated for a secondary cause.
Table 4.
Indications for specialist referral in hypertensive patients[9]

Most patients with resistant hypertension do achieve blood pressure control through pharmacotherapy provided they are properly evaluated and treated.[35] Patients who remain hypertensive despite four agents can have additional medications trialled sequentially with direction from a specialist [Figure 1]. Drug selection must be stepwise with careful appraisal of clinical circumstances at each stage. Patient comorbidities often dictate medication choices and it is judicious to begin each medication at the lowest dose practicable. Occasionally these less-frequently used agents will require earlier introduction depending on clinical circumstances.
Figure 1.
Stepwise pharmacologic management of resistant hypertension
Vasodilating beta blockers, such as carvedilol and labetalol, are a preferable next option as fifth-line drug therapy.[12] They are not recommended as first-line therapy for patients with uncomplicated hypertension because they offer less cardiovascular protection than other agents. For example, patients treated with beta blockers in the Controlled ONset Verapamil INvestigation of Cardiovascular Endpoints (CONVINCE) trial had higher rates of stroke than with any of CCBs, ACEIs or thiazides.[36] Carvedilol should be initiated at 12.5 mg daily for at least two days, before increasing by 12.5 mg every two weeks to a maximum dose of 50 mg per day. Labetalol is commenced initially at 100 mg twice daily and titrated to a maximum of 2.4 g daily. Labetalol must not be increased faster than by 200 mg increments per day. In some patients such as those with ischemic heart disease or poor glycaemic control, traditional beta blockers like atenolol or metoprolol may instead be indicated. Their antihypertensive benefit is presumably smaller although head-to-head comparison data is lacking.[12]
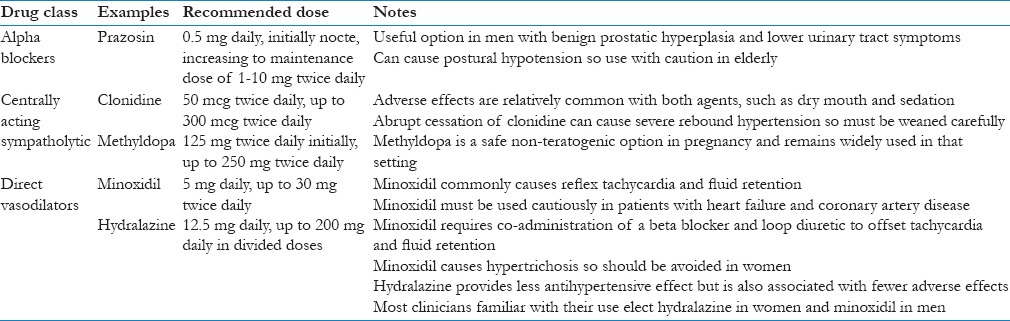
Other options include alpha blockers, clonidine, methyldopa and direct vasodilators such as hydralazine or minoxidil. These seldom form part of the routine management of hypertension and should be prescribed with expert advice. Although such agents are very efficacious in controlling blood pressure their use must be tempered by side effect profiles and the comorbidities of the patient [Table 5]. These medications have not been adequately studied in resistant hypertension and the choice of agent depends largely on patient situation and individual prescriber preference. Most clinicians tend to opt for centrally-acting agents before the direct vasodilator drugs because experience with their use is wider.
Table 5.
Interventional treatment
There is a need for alternative treatment strategies in refractory patients who remain hypertensive despite optimum medical therapy. Two potentially effective interventional therapies are currently being assessed for this purpose. These are renal denervation and carotid sinus stimulation.
Renal denervation (RD) is a minimally invasive percutaneous approach that involves placement of an electrode in the renal arteries followed by ablation of sympathetic nerves. Sympathetic innervation stimulates renin release from the juxtoglomerular apparatus and afferent vasoconstriction of renal vessels, both of which lead to increased tubular reabsorption of sodium and elevated blood pressure. Renal denervation is therefore based on the principle that deactivation of renal sympathetic nerves will curb hypertension.
Until recently, available data indicated that this procedure provides moderate blood pressure reduction in patients with resistant hypertension. This was demonstrated in several unblinded studies, including SYMPLICITY-HTN-2. That trial, conducted in centres across Australia, New Zealand and Europe, demonstrated a mean pressure reduction of 32/11 mmHg at six months post-RD, and was not associated with any major complications.[41]
However, a blinded randomised controlled trial released in April 2014, SYMPLICITY-HTN-3, failed to demonstrate any blood pressure benefit for RD versus a sham control procedure in 535 patients with resistant hypertension.[42] This paper represents the most definitive evidence available to date, in contradiction to past studies, and therefore the utility of RD remains unestablished.
The other emerging intervention is carotid sinus stimulation. This is founded on the theory that continuous electrical stimulation of carotid baroreceptors, via an implantable device, should inhibit sympathetic output. Carotid baroreflex activation is a new procedure with limited experience. Although several feasibility studies have demonstrated appreciable pressure reduction the safety of this technique is not yet certain.
The Rheos Pivotal Trial is the only major double-blind randomised study published on carotid sinus stimulation. It reported substantial blood pressure reduction but was also associated with serious procedure-related adverse effects in 25.2% of participants.[43] Complications were primarily related to nerve injury during device implantation though there was also a 2.3% incidence of hypertension-induced stroke. This event rate exceeds procedural safety allowances and is unsatisfactory.
Experience with carotid sinus stimulation is currently limited. Knowledge of device safety and long-term efficacy remains insufficient and it is not a routinely available management option at this time.
Conclusion
Hypertension is a challenging clinical problem with a significant proportion of patients failing to achieve blood pressure control despite extensive medical therapy. Resistant hypertension is defined as blood pressure that remains above 140/90 mmHg despite optimal use of three antihypertensive medications of different classes, including a diuretic. Such patients are more likely to have a secondary cause and to suffer end-organ damage. Most individuals with resistant hypertension will achieve normotension with conscientious treatment decisions. Patients must be approached in a stepwise manner beginning with traditional antihypertensive therapy followed gradually by additional agents to reach a quadruple or five-drug compound regimen if necessary. In those who remain hypertensive despite thorough medical management, there are interventional options currently under development which are promising but require further research.
Acknowledgement
We are grateful to Dr Suku Thambar for his valuable feedback on earlier drafts of this paper.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Australian Institute of Health and Welfare. High Blood Pressure [internet] 2013. [updated 2013 Sep 18; cited 2014 Jan 14]. Available from: http://www.aihw.gov.au/high-blood-pressure .
- 2.Canberra: National Heart Foundation of Australia; 2012. National Heart Foundation of Australia. High blood pressure statistics. [Google Scholar]
- 3.Murtagh J. North Ryde: McGraw-Hill Australia Pty Ltd; 2011. Murtagh's General Practice. [Google Scholar]
- 4.James P, Oparil S, Carter B, Cushman W, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-based guidelines for the management of high blood pressure in adults; Report from the panel members appointed to the eighth joint national committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 5.Pimenta E, Calhoun D. Treatment of resistant hypertension. J Hypertension. 2010;28:2194–5. doi: 10.1097/HJH.0b013e32833eafa3. [DOI] [PubMed] [Google Scholar]
- 6.Briganti E, McNeil J, Shaw J, Zimmet P, Chadban S, Atkins R, et al. Untreated hypertension among Australian adults: The 1999-2000 Australian diabetes, obesity and lifestyle study. Med J Aust. 2003;179:135–9. doi: 10.5694/j.1326-5377.2003.tb05114.x. [DOI] [PubMed] [Google Scholar]
- 7.Canberra: ABS; 2012. Australian Bureau of Statistics. Australian Health Survey: Health service usage and health related actions. ABS publication 4364.0.55.002. [Google Scholar]
- 8.Tu K, Zhongliang C, Lipscome L. Prevalence and incidence of hypertension from 1995 to 2005: A population-based study. Can Med Assoc J. 2008;178(11):1429–35. doi: 10.1503/cmaj.071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Persell S. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076–80. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 10.Johnson D. How to treat: Resistant hypertension. Aust Doc. 2007;20:27–34. [Google Scholar]
- 11.Moser M, Setaro J. Resistant or Difficult-to-Control Hypertension. N Engl J Med. 2006;355:4. doi: 10.1056/NEJMcp041698. [DOI] [PubMed] [Google Scholar]
- 12.Viera A. Resistant hypertension. J Am Board Fam Med. 2012;25:487–95. doi: 10.3122/jabfm.2012.04.110275. [DOI] [PubMed] [Google Scholar]
- 13.Setaro J, Black H. Refractory hypertension. N Engl J Med. 1992;327:543–7. doi: 10.1056/NEJM199208203270808. [DOI] [PubMed] [Google Scholar]
- 14.National Heart Foundation and High Blood Pressure Research Council of Australia Ambulatory Blood Pressure Monitoring Consensus Committee. Ambulatory blood pressure monitoring. Aust Fam Phys. 2011;40:877–80. [PubMed] [Google Scholar]
- 15.Canberra: National Heart Foundation of Australia; 2010. National Heart Foundation of Australia. Guide to management of hypertension 2008. [Google Scholar]
- 16.Rimoldi S, Scherrer U, Messerli F. Secondary arterial hypertension: When, who, and how to screen? Eur Heart J. 2014;35:1245–54. doi: 10.1093/eurheartj/eht534. [DOI] [PubMed] [Google Scholar]
- 17.James P, Oparil S, Carter B, Cushman W, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guidelines for the management of high blood pressure in adults; report from the panel members appointed to the eighth joint national committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun D, Jones D, Textor S, Goff D, Murphy T, Toto R, et al. Resistant hypertension: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–19. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 19.London: National Institute for Health and Clinical Excellence; 2011. National Institute for Health and Clinical Excellence. Hypertension: Clinical management of primary hypertension in adults. [Google Scholar]
- 20.Frank J. Managing hypertension using combination therapy. Am Fam Phys. 2008;77:1279–86. [PubMed] [Google Scholar]
- 21.Kholsa N, Chua D, Elliot W, Bakris G. Are chlorthalidone and hydrochlorothiazide equivalent blood-pressure-lowering medications? J Clin Hypertens. 2005;7:354. doi: 10.1111/j.1524-6175.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan N. The choice of thiazide diuretics: Why chlorthalidone may replace hydrochlorothiazide. Hypertension. 2009;54:951. doi: 10.1161/HYPERTENSIONAHA.109.135061. [DOI] [PubMed] [Google Scholar]
- 23.Kumar N, Calhoun D, Dudenbostel T. Management of patients with resistant hypertension: Current treatment options. Integr Blood Press Control. 2013;6:139–51. doi: 10.2147/IBPC.S33984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter B, Ernst M, Cohen J. Hydrochlorothiazide versus chlorthalidone: Evidence supporting their interchangeability. Hypertension. 2004;43:4. doi: 10.1161/01.HYP.0000103632.19915.0E. [DOI] [PubMed] [Google Scholar]
- 25.Chlorthalidone. 50th ed. Sydney: MIMS Australia; 2013. Monthly Index of Medical Specialties; p. 57. [Google Scholar]
- 26.eTG complete. 2015. [Last updated on 2012 Oct 01; cited on 2014 Feb 15]. Available from: www.online.tg.org.au .
- 27.Madkour H, Gadallah M, Riveline B, Plante GE, Massry SG. Indapamide is superior to thiazide in the preservation of renal function in patients with renal insufficiency and systemic hypertension. Am J Cardiol. 1996;77:23–25. doi: 10.1016/s0002-9149(97)89236-3. [DOI] [PubMed] [Google Scholar]
- 28.Friedman O, Logan A. Can nocturnal hypertension predict cardiovascular risk? Integr Blood Press Control. 2009;2:25–37. doi: 10.2147/ibpc.s4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto L, Gamboa A, Shibao C, Black B, Diedrich A, Raj S, et al. Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension. 2009;52:363–9. doi: 10.1161/HYPERTENSIONAHA.108.124552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayala DE, Hermida RC, Mojón A, Fernández JR. Cardiovascular risk of resistant hypertension: Dependence on treatment-time regimen of blood pressure-lowering medications. Chronobiol Int. 2013;30:340–52. doi: 10.3109/07420528.2012.701455. [DOI] [PubMed] [Google Scholar]
- 31.Carter B, Chrischilles E, Rosenthal G, Gryzlak B, Eisenstein E, Vander Weg M. Efficacy and safety of nighttime dosing of antihypertensives: Review of the literature and design of a pragmatic clinical trial. J Clin Hypertens. 2014;16(2):115–21. doi: 10.1111/jch.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman N, Dobson J, Wilson S, Dahlhoef B, Sever P, Wedel H, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839. doi: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 33.Myat A, Redwood S, Qureshi A, Spertus J, Williams B. Resistant Hypertension. BMJ. 2012;345:7473–70. doi: 10.1136/bmj.e7473. [DOI] [PubMed] [Google Scholar]
- 34.Mann J, Schmeider R, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicenter, randomised, double-blind, controlled trial. Lancet. 2008;372:547. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 35.Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076–80. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 36.Black H, Elliot W, Grandits G, Grambasch P, Lucente T, White W, et al. Principal results of the Controlled Onset INvestigation of Cardiovascular Endpoints (CONVINCE) Trial. JAMA. 2003;289:2073. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 37.Brown M, Cruickshank J, Dominiczak A, MacGregor G, Poulter N, Russell G, et al. Better blood pressure control: How to combine drugs. J Hum Hypertens. 2003;17:81–6. doi: 10.1038/sj.jhh.1001511. [DOI] [PubMed] [Google Scholar]
- 38.Carter B, Ernst M, Cohen J. Hydrochlorothiazide versus chlorthalidone: Evidence supporting their interchangeability. Hypertension. 2004;43:4. doi: 10.1161/01.HYP.0000103632.19915.0E. [DOI] [PubMed] [Google Scholar]
- 39.Gottlieb T, Katz F, Chidsey C. Combined therapy with vasodilator drugs and beta-adrenergic blockade in hypertension: A comparative study of minoxidil and hydralazine. Circulation. 1972;45:571–82. doi: 10.1161/01.cir.45.3.571. [DOI] [PubMed] [Google Scholar]
- 40.Adelaide: Pharmaceutical Society of Australia; 2010. Australian Medicines Handbook. [Google Scholar]
- 41.Esler M, Krum H, Sobotka P, Schlaich M, Schmeider R, Boehm M, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The SymplicityHTN-2 Trial): A randomised controlled trial. Lancet. 2010;376:1903–09. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 42.Bhatt D, Kandzari D, O’Neil W, D’Agostino R, Flack J, Katzen B, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 43.Bisognano J, Bakris G, Nadim M, Sanchez L, Kroon A, Schafer J, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension; results from the double blind, randomised, placebo-controlled Rheos Pivotal Trial. J Am Coll Cardiol. 2011;58:765–73. doi: 10.1016/j.jacc.2011.06.008. [DOI] [PubMed] [Google Scholar]



