Abstract
Introduction:
Short-course chemotherapy containing rifampicin and isoniazid in combination has proved to be highly effective in the treatment of tuberculosis, but one of its adverse effects is hepatotoxicity. Various risk factors have been found to be associated with drug-induced liver injury (DILI) in general population. The study aimed to determine the prevalence of drug-induced hepatitis and the risk factors associated with the DILI among the patients of pulmonary tuberculosis in Indian patients.
Setting and Design:
Prospective nested case control study.
Materials and Methods:
Out of the cohort of 3900 tuberculosis patients who were initiated on anti-tubercular therapy, 150 patients who developed drug-induced liver injury due to short-course chemotherapy under RNTCP were included in the analysis. Thirty cases were being followed up in our hospital and other 120 were referred to us for the management of drug-induced hepatitis from the primary health centers. The diagnostic criteria's for DILI were made according to the American Thoracic Society criteria. Analyses of various risk factors were done for the development of DILI.
Results:
The prevalence of DILI in the present study was 3.8%. It was observed that DILI patients were older and their serum albumin levels were lower, and they had multiple co-morbid conditions. Regular alcohol intake, more extensive disease associated with radiological and female gender were observed to be independent risk factors for the development of DILI.
Conclusions:
Of the various risk factors analyzed, advanced age, hypoalbuminemia, regular alcohol intake and advanced nature of the disease were independent risk factors for the development of DILI. The risk of development of hepatitis is increased in the presence of one or more of these risk factors.
Keywords: Adverse drug reaction, chemotherapy, drug-induced hepatitis, drug-induced liver injury, hepatic failure, tuberculosis
Introduction
Although the global incidence of tuberculosis (TB) peaked around 2003 and now appears to be in slow decline, the worldwide incidence of new cases is estimated to be 9 million every year and the disease is responsible for 1.5 million deaths each year. The most effective TB treatment currently available is the 6-month short-course regimen utilizing isoniazid (INH), rifampicin (RMP) and pyrazinamide (PZA) in the intensive phase, followed by RMP and INH in the continuation phase. This regimen is capable of curing almost all patients with TB due to drug-sensitive organisms, assuming patient compliance. Unfortunately, however, anti-tuberculosis medications have various adverse effects, one of which is drug-induced liver injury (DILI). DILI is usually related to changes in treatment regimens, the use of less effective second-line treatments and prolonged hospitalization.[1] The reported incidence of hepatotoxicity in controlled trials of anti-TB chemotherapy that included INH, RMP and PZA ranged from 0.6% to 3%.[2,3,4]
However, if one of the three drugs has to be finally terminated due to serious side-effects, the patient no longer receives the best treatment available and might be at a higher risk of treatment failure and drug resistance. Hence, it is very important to draw attentions of all health workers toward side effects of anti-TB drugs which can be harmful to the patients. Hepatotoxicity is one of the important side-effects of anti-TB drugs especially during the initial intensive period, and monitoring is crucial during this period, but may be costly. Awareness of the risk groups may decrease the cost as well as the incidence of serious drug-related adverse effects. In this study, we aimed to determine the prevalence of DILI and the risk factors associated with the development of DILI over a period of 5 years.
Materials and Methods
This was a prospective nested case-control study done from 2009 to 2013 in a tertiary level hospital at KLES Dr. Prabhakar Kore Hospital and MRC at Belgaum, Karnataka, India. All these patients received either a regimen consisting of INH, RMP, ethmbutol and PZA or addition of streptomycin, in addition to other four drugs, in patients with underlying drug history intake. Thus, a total of 3900 patients who were registered under these regimens were included in the study. All these patients were initiated on therapy and were referred to primary health center (PHC) for continuation of the treatment under RNTCP; only about 600 patients continued the treatment from our hospital. A total of 150 patients who developed hepatitis due to the short-course anti-tubercular therapy were included in the final analysis. Thirty cases that had DILI were being followed up in our OPD, while the other 120 patients had developed DILI at their place of taking treatment and were referred to us for drug-induced hepatitis.
Diagnosis of drug-induced liver injury
The diagnostic criteria for drug-induced hepatitis (DILI) are as follows.[5]: (1) A rise of five times the upper limit of normal levels (50 IU/l) of serum aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT); (2) a rise in the level of serum total bilirubin >1.5 mg/dl; (3) any increase in AST and/or ALT above pre-treatment levels together with anorexia, nausea, vomiting, and jaundice; (4) absence of serologic evidence of infection with hepatitis virus A, B, or C. Viral hepatitis markers (HBsAg, IgM anti-HAV, IgM anti-HBc, and anti-HCV second-generation antibodies) were analyzed using ELISA immunoassay kits. The presence of any one of the first three criteria along with the absence of viral hepatitis was considered to be having DILI. Patients with associated chronic illnesses such as cirrhosis of the liver, chronic hepatitis, acute viral hepatitis, gastro-intestinal, renal or cardiac diseases were excluded.
Drug regimens
The drug regimens used are as follows:
Category 1 (2R3H3E3Z3/4R3H3): RMP, INH, ethambutol (EMB) and PZA given three times weekly for 2 months followed by RMP plus INH three times weekly for 4 months.
Category 2 (2S3R3H3E3Z3/1R3H3E3Z3/5R3H3): Streptomycin, RMP, INH, EMB and PZA given three times weekly for 2 months followed by four drugs for another one month of intensive phase and then RMP and INH given three times weekly daily for 5 months.
Drug dosages
The drug dosages were calculated in relation to the weight of the patients as follows:
Streptomycin: 0.75 gm IM (<50 years) and 0.50 gm (>50 years)
Rifampicin: Body weight <450 mg/day; >50 kg – 600 mg
Isoniazid: 600 mg (10–15mg/kg)
Ethambutol: 1200 mg (30 mg/kg)
Pyrazinamide: 1500 mg (30–35mg/kg).
Study design
Data on patient demographics, co-morbidity, use of concomitant medications, alcohol consumption, body weight, baseline transaminases/bilirubin and treatment regimen were recorded for all the patients. All the baseline investigations were performed including HIV status, hepatitis B status and hepatitis C status. In addition to the patients’ data and treatment data, the following information on risk factors were analyzed: Alcohol abuse (>40 g·day-1); i.v. drug abuse; history of hepatitis; hepatic damage at admission (liver enzymes at admission ≥2 times normal values); history of diabetes mellitus; HIV infection and concomitant therapy with other hepatotoxic drugs [Table 1]. The incidence of DILI was determined, and the patient and treatment characteristics of those who developed DILI were compared with the rest of the cohort (non-DH patients). The clinical course and treatment outcome of the patients with DILI were also studied. All patients had baseline serum transaminase and bilirubin levels measured prior to starting treatment and were routinely advised to report immediately when they experience symptoms of hepatitis such as nausea, vomiting or abdominal pain. Monitoring of serum transaminase/bilirubin levels was carried out in high-risk patients (e.g., history of liver disease or alcohol abuse), or if symptoms or signs suggestive of hepatitis occurred. Chest radiography was performed in all the patients with DILI to know the extent of the disease radiologically. It was our operating policy that if a patient developed hepatitis according to the above-mentioned criteria, TB treatment would be temporarily stopped, even in the absence of symptoms. All drugs were stopped and liver function tests were conducted twice a week. Once liver functions were returned to normal, the drug regime was restarted with all drugs at the same time and in full doses. If hepatotoxicity recurred, the drugs were reintroduced in stages as follows: First EMB at the maximum dosage of 1200 mg and INH at 100 mg. The INH dosage was increased by 100 mg/day to the maximum dosage of 300 mg on the third day. RIF was re-introduced from the fourth day starting at 150 mg and increasing by 150 mg on alternate days until the maximum dose of 600 mg was achieved. Once RIF had been re-introduced to its maximum dosages, PZA was started at 500 mg and the dosage increased by 500 mg on alternate days until the maximum dosage of 1500 mg was achieved.
Table 1.
Patient's characteristics at baseline (n=150 who developed DILI)
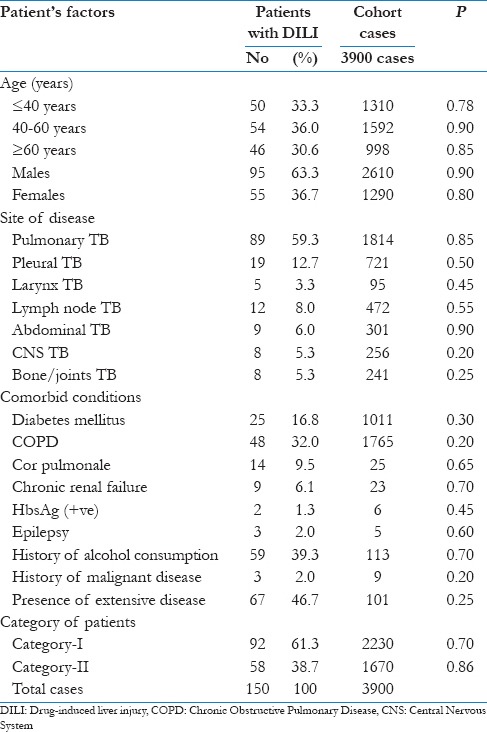
The risk factors for the development of DILI were analyzed in details: Age, gender, past history of anti-TB treatment, extensive nature of radiological disease, co-morbid disorders and drug resistance for the development and recurrence of hepatotoxicity. If the sputum status remained positive even after 2 months of chemotherapy, sputum was sent for AFB culture and sensitivity testing for confirmation of drug resistance. The INH acetylation status was not analyzed in this study as we do not have the facility for the same. Ethical clearance was taken from the Institutional Ethical committee.
Data analysis
Statistical analysis was done using computer software (SPSS version 13.0, SPSS Inc., Chicago, IL, USA). Data were analyzed by Chi-square (χ2) test and logistic regression analysis. Data were expressed as “mean (standard deviation; SD),” minimum-maximum and percent (%) where appropriate. A P value < 0.05 was considered statistically significant. Continuous variables (ALT and AST) that failed in the assumption of normality and homogeneity of variance were compared across the groups using the Mann–Whitney test. Binary logistic regression was used to calculate the adjusted odds ratio for the significant risk factors of DILI. Logistic regression univariate analysis was preformed to analyze the risk factors associated with DILI. Multivariate logistic regression analysis was done to remove confounding variables to assess the role of independent risk factors for development of DILI.
Results
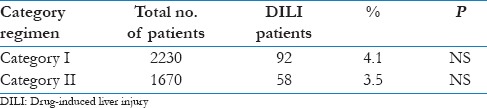
The risk of development of DILI in the present study was 3.8% (150 patients out of total cohort of 3900 patients). The detail baseline characteristics of the patients are shown in Table 1. The mean age was 47 ± 7.2 years. Majority of the patients were above the age of 40 years (66.6%). The average duration of development of DILI was 20 days after starting anti-tubercular therapy and lasted for average of 14 days. Hepatotoxicity was observed to develop for once in 81.9% (n = 95) of patients while it recurred for more than once in 18.1% (n = 21) patients. Majority of the patients had pulmonary TB (46.5%), followed by pleural TB (18.5%). Most of the patients had associated co-morbid conditions, with the most common being COPD (30.0%), followed by diabetes mellitus (16.8%). Fifty-nine patients (39.3%) had history of alcohol consumption. RNTCP Category I patients contributed to 57.2% of the patients, while another 42.8% were under Category II regimen. It was observed that the prevalence of DH was almost the same in both the categories [Table 2].
Table 2.
Drug-induced liver injury (DILI) among different categories
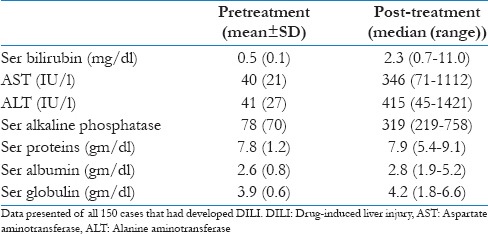
The rise in ALT and AST was almost five times the upper limit of normal (ULN), while the bilirubin was raised to >2.0 mg/dl, with 11 patients (7.3%) had raised bilirubin up to 10 mg/dl [Table 3]. There were 23 cases (15.3%) where ALT and AST were raised to three times ULN along with symptoms of hepatitis and we had to stop the therapy.
Table 3.
Pre-treatment and post-treatment liver function tests in 150 DILI patients
Risk factors for development of DILI
Elderly patients (>60 years) were observed to be at higher risk of developing DILI. It was observed that DILI was lower among younger age group (<20 years) (10.3%), while it was observed to be present in 39.5% of patients >60 years of age group. Hepatotoxicity was identified in 9.2% of patients with limited disease while 35.3% of patients had radiological extensive disease. The development of hepatotoxicity was significantly more common in patients with extensive disease (P = 0.003; Table 3). Co-morbid disorders were evident in 102 cases, with some patients having multiple co-morbid conditions. The development of hepatotoxicity was significantly more common in patients with associated co-morbid conditions [Table 1]. Hepatotoxicity was identified in 19.1% of the patients with previous history of TB. Alcohol consumption was common especially among the younger age group, and these patients developed DILI in 42.2% of the cases. It was also found to be an independent risk factor for the development of DILI. About 12 patients had previous history of hepatitis, this may be viral hepatitis with jaundice, and these patients were at higher risk of development of DILI following anti-TB therapy. A lower serum albumin level was also found to be associated with DILI. The mean serum albumin level in patients with DILI was 2.0 gm/dl.
A total of 21 patients (14%) developed recurrence of DILI. It was observed that the factors that contributed for the development of recurrent DILI were: Previous hepatitis episode, previous ant-TB treatment, age >60 years, extensive disease, hypoalbuminemia and alcohol consumption. Past history of anti-TB treatment was the only risk factor determined to be significantly associated with recurrence (P = 0.027).
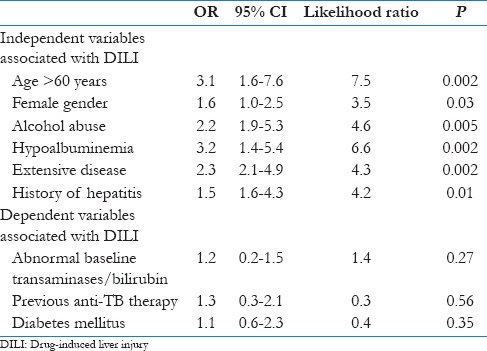
On univariate analysis, the factors that were significantly associated with DILI were prior history of hepatitis, age >60 years, female sex, alcohol consumption, previous ant-TB therapy, hypoalbuminemia, extensive nature of disease and diabetes mellitus [Table 4]. On multivariate analysis, the significant risk factors that were associated with DILI were female sex, prior history of hepatitis, alcohol consumption, hypoalbuminemia, age >60 years and extensive nature of disease radiological [Table 5].
Table 4.
Univariate analysis of risk factors for DILI (n=150)
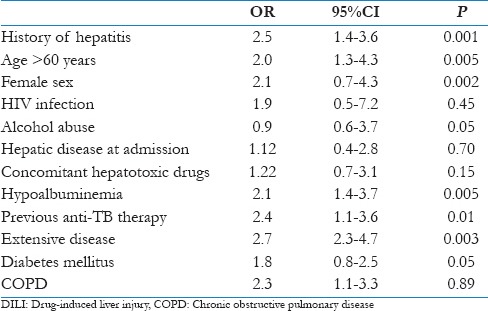
Table 5.
Multivariate analysis of risk factors for DILI (n=150)
Management of hepatotoxicity
Anti-TB treatment was continued at full dosage after the normalization of liver enzyme levels in 82.7% (n = 96) of patients with hepatotoxicity. In recurrent hepatotoxicity, a step-by-step anti-TB treatment was re-started and majority of the patients could tolerate all the drugs successfully. Only in 12 patients we had to change the regimen by avoiding RMP and PZA. All these patients received daily regimens as the tolerance level was much better was the daily regimens as compared with the intermittent regimen. Thus, it was possible to administer the treatment regimen in majority of the patients according to the guidelines.
Discussion
The presence of DILI in the present study was observed to be 3.8%, and it was observed to be same among two different RNTCP categories. The frequency of DILI, which is the most important side effects of TB treatment, varies in different countries, ranging from 1% to 10%. In developing countries it is reported to vary from 8% to 10%, while in Western countries it varies from 1% to 3.3%.[6] Meaningful comparison of the incidences of reported hepatotoxicity across different treatment centers is often not possible, as hepatitis has not been consistently defined in the literature. Definitions have ranged from asymptomatic elevation of transaminases of 2 × ULN, to symptomatic, jaundiced individuals with AST >150 U/L.[7] The relatively higher incidence of hepatotoxicity in the developing countries has been attributed to various factors such as older age, higher alcohol intake, malnutrition, intestinal parasitism, past history of jaundice, chronic liver disease, indiscriminate use of drugs, and viral hepatitis.[8] Ambreen et al.,[9] have observed that female gender, low body mass index, extra-pulmonary and positive HIV were at higher risk for DILI and it was also observed that higher level of malondialdehyde enzyme in these patients may be due to oxidative stress-resulting from anti-TB drugs. In HIV-positive TB patients the risk of DILI is much higher, and in one study it was observed that the presence of disseminated TB and malnutrition (BMI < 18.5 kg/m2) were independent predictors in the development of DILI in these TB/HIV co-infected patients.[10]
INH and RFP are two key drugs used in the treatment of TB and both often cause DILI via different mechanisms. Direct INH toxicity or toxicity from an INH metabolite is most likely responsible for hepatocyte death, which results in elevation of the level of transaminase. The histopathologic appearance of DILI caused by INH toxicity closely resembles that of viral hepatitis.[11] RFP is metabolized in the liver and excreted through the bile ducts. RFP is known to interact with the metabolism of other drugs (e.g. warfarin), especially via enterohepatic circulation.[12] The various reported risk factors for DILI include older age, female sex, poor nutritional status, high alcohol intake, pre-existing liver disease, hepatitis B and C infections, extensive disease, hypoalbuminemia and acetylator status. In all disease groups, close follow-up is required during the treatment with periodical clinical laboratory tests.[11] In one meta-analysis, the presence of RMP in a multidrug treatment regimen was reported to increase the incidence of significant hepatotoxicity among adults from 1.6% to 2.55%.[12] The PZA has also been demonstrated to contribute to increased incidence or severity of hepatotoxicity.[13] Kato et al.,[14] have observed decreased activities of daily living and chronic cardiac disease to be associated with higher prevalence of DILI with bilirubin >2 mg/dl in patients with anti-tubercular therapy. Elderly patients are at a higher risk of development of DILI than younger generation. Babalik et al.[15] have observed that patients with age >40 years were at higher risk for the development of DILI, while another study from India[16] has also observed higher prevalence among >60 years of age group. The higher incidence of DILI in older age may be due to increased prevalence of co-morbid conditions as well as use of related additional drugs in this age group. Female gender has also been found to have higher prevalence of DILI.[16]
On multivariate analysis, the risk factors that were independently associated with DILI were alcohol abuse, extensive nature of disease, and hypoalbuminemia. Malnourished children have been observed to have threefold increased incidence of DILI in one study,[17] while in another study[4] it was found that patients with hypoalbuminemia had a two-fold higher risk of developing DILI. The possibility that hypoalbuminemia was caused partly by the development of hepatitis itself cannot be ruled out. High alcohol intake and advanced TB were also associated with development of DILI.[18,19] Moderately/far advanced pulmonary TB has been found to be an independent predictor of DILI in many studies.[5,15] The extensive nature of disease is a significant risk factor for the development of hepatitis. In patients with advanced disease, multiple factors may play a role in developing DILI. This includes underlying nutritional status, hypoalbuminemia, alcohol abuse and long-standing nature of disease which will lead to undernourishment of an individual.[15]
The addition of PZA to the regimen increases the risk of DILI.[20] Another factor that may be responsible for DILI is the acetylation status of the patients. But it has been observed that both fast and slow acetylators are prone to develop DILI with short-course chemotherapy.[21,22] Pande et al.,[16] have observed DILI to be more frequent among slow acetylators. We could not assess the acetylation status of an individual and but one should keep acetylation factor in mind. All patients who developed viral hepatitis during anti-TB treatment were excluded in this study, although the possibility that a few of them had viral hepatitis that was not detected by the serological tests used cannot be excluded. Serological markers were evaluated only for hepatitis A, B, and C virus. Kumar et al.,[23] have stated that high incidence of DILI in developing countries was, to a significant extent, attributable to these viral infections.
According to recommendations, if the diagnosis is DILI, anti-TB drugs should be stopped and the drugs must be withheld until the normalization of the liver function tests.[24,25] American Thoracic Society (ATS) recommends initiation of the new treatment regime following hepatotoxicity provided that ALT levels are below the two-fold of upper normal limits. In this study, treatment was re-initiated only after normalization of liver enzymes. WHO recommended re-introduction of all the drugs at once when drug-induced hepatitis is resolved with discontinuation of the latest drug added in case of symptom recurrence or abnormality in liver function tests.[24] In the present study, we started the full drug dosages after the normalization of the enzyme values in all the cases and 21 of 150 cases (14%) had recurrent hepatotoxicity. In recurrent hepatotoxicity a step-by-step anti-TB treatment was re-started and majority of the patients could tolerate all the drugs successfully. In 12 patients we had to change the regimen by avoiding RMP and PZA. All these patients received daily regimens as the tolerance level was much better was the daily regimens as compared with the intermittent regimen. Another study[12] had observed the prevalence of 21.7% risk of DILI during reintroduction of the drugs. The risk factor associated with recurrent hepatotoxicity was past anti-TB history, which has been observed in other study also.[26] Reintroduction of antitubercular therapy must be balanced with the knowledge of adaptation, and close monitoring for early clinical symptoms cannot be underestimated.[27] Simultaneous rechallenge with combination drugs or sequential treatment has similar incidence of DILI and warrants careful use.
Management of active TB includes the early initiation and proper completion of the anti-TB therapy, and also effective management of side effects related to anti-TB drugs. The study showed that DILI is a frequent side effect of anti-TB therapy under DOTS therapy. DILI could considerably impact the anti-TB treatment, potentially leading to unsuccessful treatment outcomes and the prolongation of intensive treatment phase. Early diagnosis and identification of the risk factors for DILI is important to prevent hepatitis-induced mortality.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Parthasarathy R, Sarma GR, Janardhanam B, Ramachandran P, Santha T, Sivasubramanian S, et al. Hepatic toxicity in south Indian patients during treatment of tuberculosis with short-course regimens containing isoniazid, rifampicin and pyrazinamide. Tubercle. 1986;67:99–108. doi: 10.1016/0041-3879(86)90003-6. [DOI] [PubMed] [Google Scholar]
- 2.Singapore Tuberculosis Service/British Medical Research Council. Assessment of a daily combined preparation of isoniazid, rifampicin, and pyrazinamide in a controlled trial of three 6- month regimens for smear-positive pulmonary tuberculosis. Am Rev Respir Dis. 1991;143:707–12. doi: 10.1164/ajrccm/143.4_Pt_1.707. [DOI] [PubMed] [Google Scholar]
- 3.Myesr JP. New recommendations for the treatment of tuberculosis. Curr Opin Infect Dis. 2005;18:133–40. doi: 10.1097/01.qco.0000160902.48942.31. [DOI] [PubMed] [Google Scholar]
- 4.Singapore Tuberculosis Service/British Medical Research Council. Clinical trial of six month and four month regimens of chemotherapy in the treatment of pulmonary tuberculosis: The results up to 30 months. Tubercle. 1981;62:95–102. doi: 10.1016/0041-3879(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 5.Sharma SK, Balamurugan A, Saha PK, Pandey RM, Mehra NK. Evaluation of clinical and immunogenetic risk factors for the development of hepatotoxicity during antituberculosis treatment. Am J Respir Crit Care Med. 2002;166:916–9. doi: 10.1164/rccm.2108091. [DOI] [PubMed] [Google Scholar]
- 6.Tost JR, Vidal R, Caylà J, Díaz-Cabanela D, Jiménez A, Broquetas JM. Severe hepatotoxicity due to anti-tuberculosis drugs in Spain. Int J Tuberc Lung Dis. 2005;9:534–40. [PubMed] [Google Scholar]
- 7.Thompson NP, Caplin ME, Hamilton MI, Gillepsie SH, Clarke SW, Burrough AK, et al. Anti-tuberculosis medication and the liver: Dangers and recommendations in management. Eur Respir J. 1995:1384–8. doi: 10.1183/09031936.95.08081384. [DOI] [PubMed] [Google Scholar]
- 8.Gangadharan PR. Isoniazid, rifampicin and hepatotoxicity. Am Rev Respir Dis. 1986;133:963–5. doi: 10.1164/arrd.1986.133.6.963. [DOI] [PubMed] [Google Scholar]
- 9.Ambreen K, Sharma R, Singh KP, Khan FH, Kumar S. Risk factors for anti-tuberculosis drug-induced hepatotoxicity and its association with oxidative stress in North Indian population. Ann Trop Med Public Health. 2012;5:574–80. [Google Scholar]
- 10.Hassen Ali A, Belachew T, Yami A, Ayen WY. Anti-Tuberculosis Drug Induced Hepatotoxicity among TB/HIV Co-Infected Patients at Jimma University Hospital, Ethiopia: Nested Case-Control Study. PLoS ONE. 2013;8:e64622. doi: 10.1371/journal.pone.0064622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saukkonen JL, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: Hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–52. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 12.Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest. 1991;99:465–71. doi: 10.1378/chest.99.2.465. [DOI] [PubMed] [Google Scholar]
- 13.Chang KC, Leung CC, Yew WW, Lau TY, Tam CM. Tuberculosis and chest service, centre for health hepatotoxicity of pyrazinamide cohort and case-control analyses. Am J Respir Crit Care Med. 2008;177:1391–6. doi: 10.1164/rccm.200802-355OC. [DOI] [PubMed] [Google Scholar]
- 14.Kato H, Horita N, Miyazawa N, Yoshiyama T, Ueda A, Ishigatsubo Y. Risk factors for liver injury with an elevated serum bilirubin concentration caused by antituberculous drugs. Intern Med. 2013;52:2209–14. doi: 10.2169/internalmedicine.52.0545. [DOI] [PubMed] [Google Scholar]
- 15.Babalik A, Arda H, Bakirci N, Agca S, Oruc K, Kiziltas Ş, et al. Management of and risk factors related to hepatotoxicity during tuberculosis treatment. Tuberk Toraks. 2012;60:136–44. [PubMed] [Google Scholar]
- 16.Pande JN, Singh SP, Khilnani GC, Khilnani S, Tandon RK. Risk factors for hepatotoxicity from antituberculosis drugs: A case-control study. Thorax. 1996;51:132–6. doi: 10.1136/thx.51.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grönhagen-Riska C, Hellstrom PE, Froseth B. Predisposing factors in hepatitis induced by isoniazid-rifampicin treatment of tuberculosis. Am Rev Respir Dis. 1978;118:461–6. doi: 10.1164/arrd.1978.118.3.461. [DOI] [PubMed] [Google Scholar]
- 18.Mehta S. Malnutrition and drugs: Clinical implications. Dev Pharmacol Ther. 1990;15:159–65. doi: 10.1159/000457640. [DOI] [PubMed] [Google Scholar]
- 19.Mo P, Zhu Q, Teter C, Yang R, Deng L, Yan Y, et al. Prevalence, drug-induced hepatotoxicity, and mortality among patients multi-infected with HIV, tuberculosis, and hepatitis virus. Int J Infect Dis. 2014;28:95–100. doi: 10.1016/j.ijid.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Riaska N. Hepatitis cases in isoniazid treated groups and in a control group. Bull Int Union Tuberc. 1976;51:203–6. [PubMed] [Google Scholar]
- 21.Teleman MD, Chee CB, Earnest A, Wang YT. Hepatotoxicity of tuberculosis chemotherapy under general programme conditions in Singapore. Int J Tuberc Lung Dis. 2002;6:699–705. [PubMed] [Google Scholar]
- 22.Gurumurthy P, Krisnamurthy MS, Nazareth O, Parthasarthy R, Sarma GR, Somasundaran PR, et al. Lack of relationship between hepatic toxicity and acetylator phenotypes in 3000 south Indian patients during treatment with isoniazid for tuberculosis. Am Rev Respir Dis. 1984;129:58–61. doi: 10.1164/arrd.1984.129.1.58. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Misra PK, Mehrotra R, Govil YC, Rana GS. Hepatotoxicity of rifampicin and isoniazid: Is it all drug induced hepatitis? Am Rev Respir Dis. 1991;143:1350–2. doi: 10.1164/ajrccm/143.6.1350. [DOI] [PubMed] [Google Scholar]
- 24.4th ed 2009. WHO/HTM/TB/2009. 420: Treatment of Tuberculosis: Guidelines for National Programmes. [Google Scholar]
- 25.Jong E, Conradie F, Berhanu R, Black A, John MA, Meintjes G, et al. Consensus statement: Management of drug-induced liver injury in HIV-positive patients treated for TB S Afr. J HIV Med. 2013;14:113–9. [Google Scholar]
- 26.Tahaoğlu K, Ataç G, Sevim T, Tärün T, Yazicioğlu O, Horzum G. The management of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2001;5:65–9. [PubMed] [Google Scholar]
- 27.Devarbhavi H. Anti-tuberculous drug-induced liver injury: Current perspective. Trop Gastroenterol. 2011;32:167–74. [PubMed] [Google Scholar]


