Abstract
Background: Numerous studies have investigated the benefits of fish, fish oil, and ω-3 (n–3) polyunsaturated fatty acids against cardiovascular diseases. However, concern surrounding contamination with persistent organic pollutants (POPs) prompts caution in the recommendation to consume fish and fish oil.
Objective: The present study compared the effects of fish oil contaminated with polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCs) on serum lipid profiles, inflammation, and oxidative stress.
Methods: Twenty eight-day-old male Sprague-Dawley rats (n = 30) consumed diets of unmodified fish oil (FO) consisting of 15% fat by weight, persistent organic pollutant–contaminated fish oil (POP FO) (PCBs at 2.40 μg/g; OCs at 3.80 μg/g FO), or corn oil (control; CO) for 9 wk. Lipid profiles and C-reactive protein concentrations were assessed. Hepatic gene expression related to lipid metabolism was determined by real time quantitative polymerase chain reaction analysis.
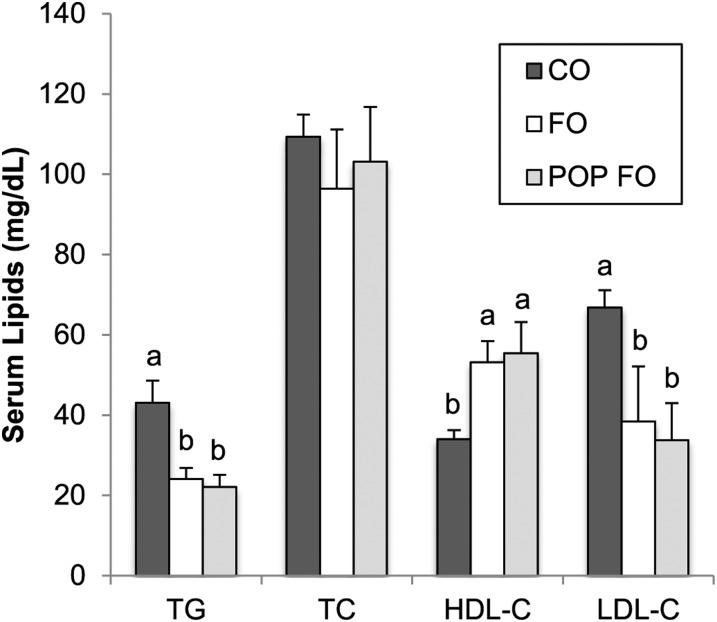
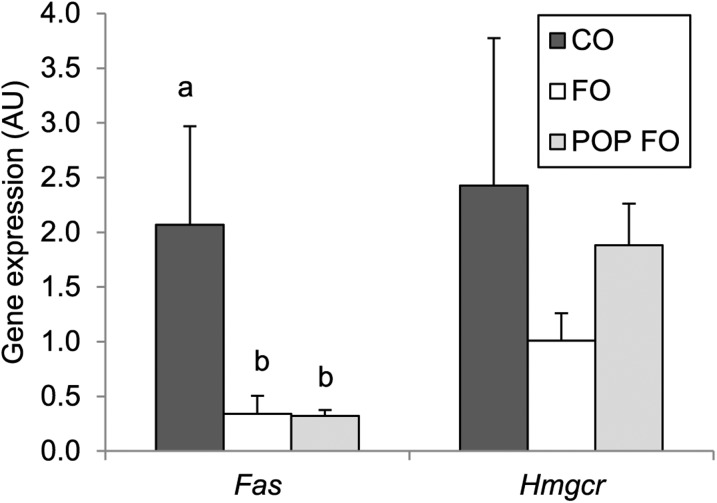
Results: After 9 wk of feeding, accumulation of PCBs and OCs in the fat tissue of the POP FO group compared with the other 2 groups was confirmed (P < 0.01). Both fish oil groups showed greater HDL cholesterol (FO 53 ± 5.3 and POP FO 55 ± 7.7 vs. CO 34 ± 2.3 mg/dL), but lower triglycerides (24 ± 2.8 and 22 ± 3.0 vs. 43 ± 5.6 mg/dL), LDL cholesterol (38 ± 14 and 34 ± 9.2 vs. 67 ± 4.4 mg/dL), and C-reactive protein (113 ± 20 and 120 ± 26 vs. 189 ± 22 μg/dL) compared with the CO group (P < 0.05). Gene expression of fatty acid synthase in both fish oil groups was also less than in the CO group (P < 0.05). However, the POP FO group showed greater lipid peroxidation (5.1 ± 0.7 vs. 2.9 ± 0.9 and 2.6 ± 0.6 μM) and less antioxidant capacity (0.08 ± 0.06 vs. 0.5 ± 0.1 and 0.4 ± 0.1 mM) than the CO and FO groups (P < 0.05).
Conclusions: These findings indicate that, despite exhibiting benefits on serum lipid concentrations and inflammation, contamination with PCBs and OCs showed significant negative effects on oxidative stress and antioxidant capacity in rats. Future studies should investigate the effects of different contaminant doses and the possibility of a dose-dependent response, a lengthened feeding time, and interactions between contaminant mixtures and oils of varying composition to advise on dietary consumption of fish and fish oil.
Keywords: fish oil, persistent organic pollutants, inflammation, oxidative stress, CVD
Introduction
Cardiovascular disease (CVD)6 is the leading cause of death worldwide, with a mortality rate of 1 per every 6 people in the United States (1, 2). Given such statistics, a considerable amount of research has been dedicated to the study of various interventions for the prevention of CVD. Fatty fish and fish oils have become a prominent area of interest in this regard because of the presence of n–3 FAs, namely EPA (20:5 n–3) and DHA (22:6 n–3) (3). These n–3 FAs do not affect CVD through a single route; rather, they influence the mosaic of phenotypes related to the disease, such as serum lipid concentrations, systemic inflammation, and oxidative stress (4–10). In a study of individuals with moderate hypertriglyceridemia, Skulas-Ray et al. (9) demonstrated that doses of EPA and DHA decreased serum TGs by 27% compared with placebo (9), indicating that consumption of fish and fish oil may have a similar TG-lowering effect. Additionally, studies in healthy adults as well as patients with stable coronary heart disease reported inverse associations between plasma and erythrocyte membrane n–3 FA concentrations and biomarkers of inflammation, namely C-reactive protein (CRP) and IL-6 (5, 6). Further investigations conducted in female Wistar rats as well as in human populations indicate positive relations between n–3 FA consumption and antioxidant enzymes, specifically superoxide dismutase (SOD) and glutathione peroxidase (GPx) (7, 8).
Despite these benefits, however, fish and fish oil may contain anthropogenic contaminants that accumulated in the fish before going to market. The US FDA warns consumers to be wary when consuming certain types of fish and shellfish because of the risk of dangerous mercury content, with elevated risks for pregnant women, nursing mothers, and young children (11). Other dangers involved in fish and marine-derived supplement consumption are attributed to the biomagnification of persistent organic pollutants (POPs), which results in the increase of contaminant concentrations at higher trophic positions within the food web (12–14). The consumption of fatty fish and fish oil supplements thus becomes an important point of entry of POPs into the human food chain, exposing populations to toxic compounds such as polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCs). PCBs were originally used as lubricants and cooling liquids for transformers, but their production was ceased in 1980 (15). Despite termination of their manufacture, PCBs still persist in the environment today and pose a threat as a toxic component in fish or contaminated fish oils. Clinical manifestations of PCB toxicity include endocrine, neurobehavioral, and developmental disruption (14). Organochlorines, another class of chlorine-containing organic pollutants such as dichloro-diphenyltrichloroethane (DDT), may also lead to endocrine disruption and pose a substantial carcinogenic threat (16). Previous studies have documented substantially increased breast cancer risk associated with PCB and OC concentrations in serum and breast adipose tissue (17, 18).
Given these concerns, there exist debates weighing the potential risks from POP intake against the benefits of n–3 FA consumption (19–23). A few studies examined the risks and benefits of consuming fish and fish oil containing POPs with the use of animal models (24, 25). However, no such study has investigated the effects of fish oil contamination with POPs on CVD risk factors. Therefore, we sought to investigate the effects of POP exposure through fish oil consumption on certain biological functions, including serum lipid modulation, oxidative stress, and inflammation. We hypothesized that the fish oil contaminated with POPs would negate the benefits by increasing oxidative stress and inflammation and decreasing antioxidant concentrations.
Methods
Rats and diets.
Training and procedures for animal use were conducted and approved by the San Diego State University animal subjects committee. Thirty male 28-d-old Sprague-Dawley rats (Harlan) were housed individually in wire-bottom cages on a 12-h light-dark cycle in a research room at San Diego State University. Both temperature and humidity were controlled at ∼20–24°C and 40–45%, respectively.
Rats were divided into 3 groups of 10 and consumed diets consisting of 15% by weight of fat from corn oil (control; CO), 11.5% by weight of fat from unmodified fish oil (FO), and 11.5% by weight of fat from persistent organic pollutant–contaminated fish oil (POP FO) (Supplemental Table 1). Among 4 tested fish oil dietary supplements, Omega-3 (Nordic Natural), had the lowest concentration of PCBs and OCs, and was selected as the unmodified fish oil (Supplemental Table 2). According to the label, its sources were anchovies and sardines; 1600 mg n–3 FAs was included in 5 g fish oil, and the extract was purified by molecular distillation to remove PCBs, heavy metals, and pesticides. A previous study also showed that a molecularly distilled arctic cod liver oil from the same company contained the lowest concentrations of PCBs and OCs compared with other fish oils (26).
For the POP FO diet, the FO was added with individual solid standards of the 7 most common PCB congeners (PCB28, PCB52, PCB101, PCB118, PCB138, PCB153, and PCB180), DDT [p,p’- dichlorodiphenyldichloroethylene (DDE), p,p’-dichlorodiphenyldichloroethane, and p,p’-DDT], and chlordane (γ-chlordane, trans-nonachlor, and cis-nonachlor). Final concentrations of PCB, DDT, and chlordane were 2.40 μg/g, 1.89 μg/g, and 1.91 μg/g, respectively. Concentrations were within the range of PCBs and OCs in fish and fish oil reported in literature (27–30). A literature report of the contaminant composition in cod liver (28) was used to set the concentrations of the individual contaminants shown in Supplemental Table 3.
Water and food were available to the rats at all times. Body weight was measured weekly, and 48 h food and water intake was also measured throughout the study. After 9 wk of feeding, rats were killed with the use of a CO2 overdose. Blood was collected and liver, spleen, and epididymal fat pads were harvested and weighed.
Measurement of PCBs and OCs in epididymal fat tissue.
Epididymal fat tissue was weighed and homogenized individually with the use of a mortar and pestle with equal part baked sodium sulfate until dry. Sample preparation procedures followed Rochman et al. (31). All final samples were analyzed with the use of an Agilent 6890 series GC connected to an Agilent 5973 MS, with ultrapure grade helium (99.995%; Airgas West) as the carrier gas and a Restek Rxi-5 Sil MS column (30 m × 0.25 mm i.d. × 0.25 μm thickness) integrated with a 5 m guard column. Selected ion monitoring was used to detect PCBs (PCB28, PCB52, PCB101, PCB118, PCB138, PCB153, and PCB180), OCs (p,p’-DDT, p.p’-DDE, p,p’- dichlorodiphenyldichloroethane, γ-chlordane, and cis- and trans-nonachlor), and the internal and recovery standards. Instrumental variables used in this study were developed previously in our laboratory (32). The 4 fish oils were analyzed by the same approach, except for exclusion of the first extraction step.
Serum lipids.
Serum total cholesterol (no. 1010–225), HDL cholesterol (no. 0599–020), and TGs (no. 2100–225) were assessed with the use of kits from Stanbio. LDL cholesterol was calculated by subtracting HDL cholesterol from total cholesterol, and then subtracting the quantity of TGs divided by 5 from the previously attained number.
Quantification of CRP.
Serum concentrations of CRP were determined with the use of the Rat C-Reactive Protein ELISA Kit (BD Biosciences, no. 557825). Standards and samples were added to the 96-well plate coated with a rabbit antirat CRP antibody. Substrate solution (3,3′,5,5′-tetramethylbenzidine) was added, producing a blue color with an absorbance directly proportional to the amount of CRP present.
Determination of thiobarbituric acid reactive substances.
Concentrations of malondialdehyde, a naturally occurring product of lipid peroxidation, were measured with the use of Cayman’s TBARs Assay Kit (Cayman, no. 10009055).
Total antioxidant capacity.
Total antioxidant capacity was determined with the use of an antioxidant assay kit (Sigma, no. CS0790). Serum concentrations of the radical cation of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) were determined by measurement with a spectrophotometer at 405 nm.
Antioxidant enzyme activity.
Activities of SOD (no. 706002), catalase (no. 707002), glutathione S-transferase (no. 703302), and GPx (no. 703102) were determined with the use of the Cayman Chemical kits and the manufacturer’s protocols.
Hepatic enzyme activity.
Activities of aspartate aminotransferase (no. 2920–430), alanine aminotransferase (no. 2930–430), alkaline phosphatase (no. 2900–430), and lactate dehydrogenase (no. 2940–430) were determined with the use of kits from Stanbio.
Hepatic gene expression.
Gene expression of fatty acid synthase (Fas) and 3-hydroxy-3-methyl-glutaryl-CoA reductase (Hmgcr) was measured with the use of PCR. cDNA was produced by SuperScript III Reverse Transcriptase (Life Technologies) with oligo(dT)12–18 primers. Gene expression was quantified by real time PCR (ViiA7, Life Technologies) with the use of TaqMan probes and normalization with r18S expression (33).
Statistical Analysis.
All data were analyzed with the use of SPSS software (IBM). One-factor ANOVAs were used to test differences between groups on lipid profile, oxidative stress, antioxidant status, inflammation, and gene expression. Student-Newman-Keuls (SNK) multiple comparison tests analyzed differences between individual means. Levene tests were performed to account for unequal variance. The level of significance was set at P < 0.05. Values are presented as means ± SEs.
Results
PCBs and 6 OCs were analyzed in the adipose tissue from each rat and are summarized in Table 1. The concentrations of all contaminants except PCB28 and PCB52 were significantly higher in rats fed the POP FO diet than in the rats fed the FO and CO diets (P < 0.01). There was no difference in contaminant accumulation between the FO and CO groups. This result confirmed that added PCBs and OCs were taken up by rats fed the POP FO, and that the FO was nearly free of the contaminants, as also verified by quantitative analysis of the unmodified oil (Supplemental Table 2).
TABLE 1.
Adipose tissue contaminant concentrations in male rats fed the CO, FO, or POP FO diet for 9 wk1
| Diet, ng/g |
|||
| CO | FO | POP FO | |
| PCB 28 | <0.005 | <0.005 | <0.005 |
| PCB 52 | <0.01 | 3.12 ± 6.58 | 3.63 ± 5.66 |
| PCB 101 | 0.812 ± 0.923b | 0.338 ± 0.736b | 37.6 ± 13.7a |
| PCB 118 | 1.09 ± 2.15b | 1.95 ± 2.11b | 231 ± 76.4a |
| PCB 138 | 2.07 ± 0.719b | 2.58 ± 1.10b | 561 ± 194a |
| PCB 153 | 3.57 ± 1.10b | 2.80 ± 1.78b | 317 ± 93.7a |
| PCB 180 | 1.58 ± 0.467b | 1.63 ± 0.757b | 112 ± 38.2a |
| p,p’-DDE | 0.096 ± 0.304b | 0.405 ± 0.878b | 413 ± 148a |
| p,p’-DDD | <0.05b | 6.80 ± 5.22b | 125 ± 48.1a |
| p,p’-DDT | <0.05b | <0.05b | 465 ± 139a |
| γ -Chlordane | <0.03b | <0.03b | 0.203 ± 0.24a |
| trans-Nonachlor | <0.03b | <0.03b | 6.05 ± 2.92a |
| cis-Nonachlor | <0.03b | <0.03b | 28.2 ± 12.0a |
Values are means ± SDs, n = 10 per group. Labeled means in a row without a common letter differ significantly, P < 0.01. CO, control; DDD, dichlorodiphenyldichloroethane; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; FO, unmodified fish oil; PCB, polychlorinated biphenyl; POP FO, persistent organic pollutant–contaminated fish oil.
Initial and final body weights of rats in each experimental group are presented in Supplemental Table 4. No significant differences were seen in initial body weight, final body weight, or body weight gain among the groups over the 9 wk. Additionally, there were no significant differences in 48 h food or water intake, or food efficiency. No significant differences in organ weights were seen among groups (data not shown).
Rats fed diets supplemented with fish oil, regardless of contamination level, had significantly higher concentrations of serum HDL cholesterol (P = 0.016), as well as significantly lower concentrations of serum TGs (P = 0.002) and LDL cholesterol (P = 0.038) than did the CO group (Figure 1). Serum concentrations of CRP were significantly lower in both the FO and POP FO groups (113 ± 20.2 μg/dL and 120 ± 26.0 μg/dL, respectively) than in the CO group (189 ± 21.7 μg/dL) (P = 0.045). Hepatic gene expression of Fas was downregulated in both fish oil groups (P = 0.050) (Figure 2) compared with the CO group. No significant differences were found in Hmgcr transcription among groups.
FIGURE 1.
Serum lipid concentrations in male rats fed the CO, FO, or POP FO diet for 9 wk. Values are means ± SEs, n = 10 per group. Labeled means without a common letter differ significantly, P < 0.05. CO, control; FO, unmodified fish oil; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; POP FO, persistent organic pollutant–contaminated fish oil; TC, total cholesterol.
FIGURE 2.
Hepatic gene expression of Fas and Hmgcr in male rats fed the CO, FO, or POP FO diet for 9 wk. Values are means ± SEs, n = 10 per group. Labeled means without a common letter differ significantly, P < 0.05. AU, arbitrary unit; CO, control; Fas, fatty acid synthase; FO, unmodified fish oil; Hmgcr, 3-hydroxy-3-methyl-glutaryl-CoA reductase; POP FO, persistent organic pollutant–contaminated fish oil.
The effects of each experimental diet on liver function enzymes are presented in Table 2. Rats consuming diets supplemented with POP FO had significantly higher concentrations of alanine aminotransferase (P = 0.029) than did rats consuming the FO or CO diets. The FO group had significantly lower concentrations of lactate dehydrogenase (P = 0.040) than did the POP FO and CO groups. No significant differences in aspartate aminotransferase or alkaline phosphatase were observed between the groups.
TABLE 2.
Serum liver function enzyme activities in male rats fed the CO, FO, or POP FO diet for 9 wk1
| Diet, U/L |
|||
| CO | FO | POP FO | |
| AST | 97.7 ± 6.62 | 97.8 ± 14.6 | 97.8 ± 14.6 |
| ALT | 31.3 ± 4.03b | 35.4 ± 6.30b | 64.1 ± 13.5a |
| AP | 91.6 ± 9.41 | 71.6 ± 5.63 | 78.9 ± 4.01 |
| LDH | 304 ± 24.5a | 243 ± 12.2b | 310 ± 19.3a |
Values are means ± SEs, n = 10 per group. Labeled means in a row without a common letter differ significantly, P < 0.05. ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; CO, control; FO, unmodified fish oil; LDH, lactate dehydrogenase; POP FO, persistent organic pollutant–contaminated fish oil.
The effects of experimental diets on oxidative stress, antioxidant capacity, and antioxidant enzyme concentrations are presented in Table 3. The POP FO group exhibited higher serum concentrations of TBARs (P = 0.045) and lower total antioxidant capacity (P = 0.016) than did the FO and CO groups. Regarding antioxidant enzyme activity, the FO group had significantly higher concentrations of SOD (P = 0.001) and glutathione S-transferase (P = 0.027) than did the POP FO and CO groups, whereas no differences in catalase and GPx were observed between groups.
TABLE 3.
Serum oxidative stress, total antioxidant capacity, and antioxidant enzyme activities in male rats fed the CO, FO, or POP FO diet for 9wk1
| CO | FO | POP FO | |
| TBARs, μM | 2.91 ± 0.92b | 2.61 ± 0.57b | 5.05 ± 0.68a |
| Total antioxidant capacity, mM | 0.48 ± 0.10a | 0.40 ± 0.12a | 0.08 ± 0.06b |
| Antioxidant enzyme activities | |||
| SOD, U/mL | 28.7 ± 0.76b | 44.3 ± 1.57a | 28.7 ± 0.76b |
| CAT, nmol/(min · mL) | 343 ± 51.2 | 438 ± 38.5 | 359 ± 31.5 |
| GST, nmol/(min · mL) | 5.00 ± 0.74b | 8.20 ± 0.69a | 5.39 ± 1.02b |
| GPx, nmol/(min · mL) | 1.64 ± 0.19 | 1.76 ± 0.35 | 1.69 ± 0.34 |
Data are means ± SEs, n = 10 per group. Labeled means in a row without a common letter differ significantly, P < 0.05. CAT, catalase; CO, control; FO, unmodified fish oil; GST, glutathione S-transferase; GPx, glutathione peroxidase; POP FO, persistent organic pollutant–contaminated fish oil; SOD, superoxide dismutase.
Discussion
The present study sought to investigate the effects of PCB and OC contamination in fish oil on biological functions, including serum lipid modulation, inflammation, and oxidative stress. The level of contamination employed in the current work did not appear to have adverse effects on the lipid lowering activity of the n–3 FAs, as evidenced by the significantly lowered concentrations of TG and gene expression of Fas in both contaminated fish oil and FO exposure groups. We also observed improved lipoprotein profiles in both fish oil exposure groups, with both exhibiting a greater concentration of HDL cholesterol and lower concentration of LDL cholesterol. Furthermore, given the significantly lowered concentrations of CRP in both fish oil exposure groups, the anti-inflammatory effects of fish oil were not compromised by the presence of PCBs and OCs. CRP is one of several markers for inflammation, and further research is needed to determine if the observed toxicity is mediated by other markers (e.g., IL-6, TNF-α, serum amyloid A, or fibrinogen). Contamination with PCBs and OCs did appear to have negative effects on total antioxidant capacity and lipid peroxidation. These results raise questions about the differential mechanisms by which n–3 FAs modulate lipid concentrations, systemic inflammation, and oxidative stress.
The cardio-protective effects of fatty fish and fish oil have been well documented (34). Supplementation with n–3 FAs has led to decreased serum TG concentrations in a dose-dependent fashion (9). Potential mechanisms by which this is accomplished involve EPA and DHA acting as ligands for nuclear transcription factors such as PPARG and PPARG-α and sterol regulatory element-binding protein 1 (Srebp-1), leading to modulation of the transcription of enzymes involved in β-oxidation and cholesterol, FA, and TG synthesis (10). Secondary to this mechanism is the inhibition of hepatic VLDL–TG synthesis and secretion, thus lowering serum TG and VLDL concentrations (34). These mechanisms are in line with the observations from the current work, in which TG, LDL cholesterol, and gene expression of Fas were all significantly lowered because of FO supplementation. However, these beneficial effects were also seen in rats consuming POP FO. These results are consistent with a study conducted by Turunen et al. (35) in which a Finnish population with high fatty fish consumption accompanied by high environmental pollutant exposure still exhibited decreased serum TG concentrations (35). However, a separate study conducted in Sprague-Dawley rats determined that POPs present in crude salmon oil upregulated genetic expression of Srebp-1 and Fas, which may exert fat synthesis, counteracting the protective effects originally conferred by n–3 FAs (25).
Systemic inflammation has also been recognized as an independent risk factor for CVD (5, 6, 33). Studies in healthy adults, as well as in patients with stable coronary heart disease, reported inverse associations between n–3 FA concentrations in plasma and erythrocyte membrane and biomarkers of inflammation, namely CRP and IL-6 (5, 6). Furthermore, Turunen et al. (35) observed a tendency for serum CRP to decrease with increasing fish consumption; however, this decrease was accompanied by increased exposure to pollutant concentrations (35). These results are also consistent with observations from the current work, but inconsistent with previous studies describing a proinflammatory effect from PCBs (36). This discrepancy may be due to differential effects related to dietary components that have the potential to attenuate or exacerbate PCB toxicity, specifically n–3 vs. n–6 FAs. For example, whereas n–3 FAs have led to a lower level of inflammatory biomarkers (5, 6), a study in transgenic mice documented n–6 FAs potentiating the toxicity of PCBs as evidenced by increased proinflammatory cytokine concentrations in mice fed diets rich in n–6 FAs and PCBs, compared with those given PCBs alone (37). Future research should seek to further investigate the different lipophilic interactions PCBs experience with different dietary fats and the physiologic relevance of these interactions.
Essential FAs present within marine-based foods and oils cannot be synthesized de novo in mammals and, therefore, must be obtained from the diet (6, 34, 38). Thus, fish products become a point of entry for POPs into the human food chain. These include PCBs and OCs, which have been shown to influence endocrine, neurobehavioral, and developmental homeostasis, as well as affect aspects of metabolism (15–18, 36, 39). Dioxin-like PCBs exert their toxicity by binding to nuclear receptors, including the aryl hydrocarbon receptor, thus increasing reactive oxygen species, which in turn can lead to upregulation of nuclear factor (erythroid-derived 2)-like 2, and, consequently, production of proinflammatory proteins (36). Although these cellular pathways do not explain the inflammatory responses seen in the current work, they do explain the lower total antioxidant capacity and greater oxidative stress observed in rats consuming the fish oil contaminated with POPs. Such an outcome can be explained by the fact that dioxin-like PCBs were not added to the fish oil diet in the current study. Other studies suggest that the type of FAs that contaminants interact with plays a key role in determining the contaminants’ toxicity. DHA has been shown to enhance the protective antioxidant response to PCB toxicity (36), whereas linoleic acid (cis,cis-9,12-octadecadienoic acid, 18:2n–6) has been observed to potentiate the negative effects of coplanar PCBs, as evidenced by increased oxidative stress in an in vitro model (40).
In the current study, serum total antioxidant capacity was determined as a function of fish oil contaminated with POPs. Use of the total antioxidant capacity measurement in vivo has been questioned (41, 42). Therefore, further analysis of nonprotein small molecule antioxidants such as ascorbate and tocopherol remains to be determined.
OCs such as DDT and its major metabolite, p,p’-DDE, can trigger oxidative stress and the production of reactive oxygen species (16, 43). A study focused on Sertoli cells of Sprague-Dawley rats demonstrated a dose-dependent oxidative stress response, with higher concentrations of p,p’-DDE leading to increased ROS and malondialdehyde concentrations, as well as decreased SOD activity (43). These results are consistent with the current work and suggest future studies to investigate any molecular interplay between PCB and DDT that may enhance their induction of oxidative stress.
In summary, the level of POP contamination administered in the current study did not affect the lipid or CRP lowering effects of FO. However, POPs in fish oil did have negative effects on total antioxidant capacity and lipid peroxidation. The present study provides evidence for differential physiologic effects from POP toxicity. Contamination with POPs in fish is not limited to certain regions or species. It is, rather, global and ubiquitous (44–46). The current results prompt caution in their consumption and encourage further investigation of health effects from exposure to POPs via consumption of fatty fish and fish oil. For instance, research into the effects of different doses of contaminants and feeding durations is essential for the evaluation of long-term exposure and assessment of a critical window of exposure. In particular, the high accumulation of PCBs and OCs in the fatty tissue of the contaminated fish oil group raises concerns surrounding a generational effect through maternal transfer. The current study only dealt with 7 generally abundant PCBs and selected OCs. However, true exposure includes a large number of additional POPs, including other PCB congeners, dioxin-like PCBs, and polybrominated diphenyl ethers (14, 42, 47–49). The uptake of this chemical mixture via fatty fish and fish oil consumption must also be evaluated.
Recent years have seen an increase in the sale of fish oil n–3 FA dietary supplements. To maximize health benefits and minimize potential negative effects from supplement consumption, further research as suggested above is critically important. This will inform clinical nutrition strategies and advise on criteria for the quality of fish oil supplements.
Supplementary Material
Acknowledgments
We thank Kayo Watanabe, Chris Millow, and Dr. Chelsea Rochman for assistance with chemical analysis and Dr. Nathan Dodder for editing the manuscript. MYH and EH designed the research and analyzed the data; MYH, JL, PM, RS, and EH conducted the research; MYH, JL, and EH wrote the paper; and MYH had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CO, control; CRP, C-reactive protein; CVD, cardiovascular disease; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; Fas, fatty acid synthase; FO, unmodified fish oil; GPx, glutathione peroxidase; Hmgcr, 3-hydroxy-3-methyl-glutaryl-CoA reductase; OC, organochlorine pesticide; PCB, polychlorinated biphenyl; POP, persistent organic pollutant; POP FO, persistent organic pollutant–contaminated fish oil; SOD, superoxide dismutase; Srebp-1, sterol regulatory element-binding protein 1.
References
- 1.Centers for Disease Control and Prevention [Internet]. 2011. Leading causes of death. Final data for 2011. [cited 2014 Oct 5] Available from: http://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm.
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. . Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:e28–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilk JB, Tsai MY, Hanson NQ, Gaziano JM, Djoussé L. Plasma and dietary omega-3 fatty acids, fish intake, and heart failure risk in the Physicians’ Health Study. Am J Clin Nutr 2012;96:882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson EJ, Thayne KA, Harris M, Shaikh SR, Darden TM, Lark DS, Williams JM, Chitwood WR, Kypson AP, Rodriguez E. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARγ activation? Antioxid Redox Signal 2014;21:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n–3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis 2009;205:538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalogeropoulos N, Panagiotakos DB, Pitsavos C, Chrysohoou C, Rousinou G, Toutouza M, Stefanadis C. Unsaturated fatty acids are inversely associated and n–6/n–3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin Chim Acta 2010;411:584–91. [DOI] [PubMed] [Google Scholar]
- 7.Lluís L, Taltavull N, Muñoz-Cortés M, Sánchez-Martos V, Romeu M, Giralt M, Molinar-Toribio E, Torres JL, Pérez-Jiménez J, Pazos M, et al. . Protective effect of the omega-3 polyunsaturated fatty acids: eicosapentaenoic acid/docosahexaenoic acid 1:1 ratio on cardiovascular disease risk markers in rats. Lipids Health Dis 2013;12:140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romieu I, Garcia-Esteban R, Sunyer J, Rios C, Alcaraz-Zubeldia M, Velasco SR, Holguin F. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM2.5. Environ Health Perspect 2008;116:1237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr 2011;93:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillander V, Bjørndal B, Burri L, Bohov P, Skorve J, Berge RK, Alexson SHE. Fish oil and krill oil supplementations differentially regulated lipid catabolic and synthetic pathways in mice. Nutr Metab 2014;11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration2004. What You Need to Know About Mercury in Fish and Shellfish [Internet]. [cited 2014 Oct 5] Available from: http://www.fda.gov/food/resourcesforyou/consumers/ucm 110591.htm.
- 12.Hennig B, Ormsbee L, McClain CJ, Watkins BA, Blumberg B, Bachas LG, Sanderson W, Thompson C, Suk WA. Nutrition can modulate the toxicity of environmental pollutants: implications in risk assessment and human health. Environ Health Perspect 2012;120:771–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs DR Jr, Ruzzin J, Lee DH. Environmental pollutants: downgrading the fish food stock affects chronic disease risk. J Intern Med 2014;276:240–2. [DOI] [PubMed] [Google Scholar]
- 14.Martí M, Ortiz X, Gasser M, Martí R, Montaña MJ, Díaz-Ferrero J. Persistent organic pollutants (PCDD/Fs, dioxin-like PCBs, marker PCBs, and PBDEs) in health supplements on the Spanish market. Chemosphere 2010;78:1256–62. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz X, Carabellido L, Martí M, Martí R, Tomás X, Díaz-Ferrero J. Elimination of persistent organic pollutants from fish oil with solid adsorbents. Chemosphere 2011;82:1301–7. [DOI] [PubMed] [Google Scholar]
- 16.Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 2013;307:74–88. [DOI] [PubMed] [Google Scholar]
- 17.Aronson KJ, Miller AB, Woolcott CG, Sterns EE, McCready DR, Lickley LA, Fish EB, Hiraki GY, Holloway C, Ross T, et al. . Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2000;9:55–63. [PubMed] [Google Scholar]
- 18.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect 2007;115:1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assmuth T. Policy and science implications of the framing and qualities of uncertainty in risks: toxic and beneficial fish from the Baltic Sea. Ambio 2011;40:158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewailly E, Ayotte P, Lucas M, Blanchet C. Risk and benefits from consuming salmon and trout: a Canadian perspective. Food Chem Toxicol 2007;45:1343–8. [DOI] [PubMed] [Google Scholar]
- 21.Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Risk-based consumption advice for farmed Atlantic and wild Pacific salmon contaminated with dioxins anddioxin-like compounds. Environ Health Perspect 2005;113:552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruzzin J, Jacobs DR. The secret story of fish: decreasing nutritional value due to pollution? Br J Nutr 2012;108:397–9. [DOI] [PubMed] [Google Scholar]
- 23.Turyk ME, Bhavsar SP, Bowerman W, Boysen E, Clark M, Diamond M, Mergler D, Pantazopoulos P, Schantz S, Carpenter DO. Risks and benefits of consumption of Great Lakes fish. Environ Health Perspect 2012;120:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim MM, Fjære E, Lock EJ, Naville D, Amlund H, Meugnier E, Le Magueresse Battistoni B, Frøyland L, Madsen L, Jessen N, et al. . Chronic consumption of farmed salmon containing persistent organic pollutants causes insulin resistance andobesity in mice. PLoS ONE 2011;6:e25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock E, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, et al. . Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect 2010;118:465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoh E, Lehotay SJ, Pangallo KC, Mastovska K, Ngo HL, Reddy CM, Vetter W. Simultaneous quantification of multiple classes of organohalogen compounds in fish oils with direct sample introduction comprehensive two-dimensional gas chromatography and time-of-flight mass spectrometry. J Agric Food Chem 2009;57:2653–60. [DOI] [PubMed] [Google Scholar]
- 27.Baeyens W, Leermakers M, Eiskens M, Van Larebeke N, De Bont R, Vanderperren H, Fontaine A, Degroodt JM, Goeyens L, Hanot V, et al. . PCBs and PCDD/FS in fish and fish products and their impact on the human body burden in Belgium. Arch Environ Contam Toxicol 2007;52:563–71. [DOI] [PubMed] [Google Scholar]
- 28.Glasius M, Christensen JH, Platz J, Vorkamp K. Halogenated organic contaminants in marine fish and mussels from southern Greenland–pilot study on relations to trophic levels and local sources. J Environ Monit 2005;7:127–31. [DOI] [PubMed] [Google Scholar]
- 29.Green NW, Knutzen J. Organohalogens and metals in marine fish and mussels and some relationships to biological variables at reference localities in Norway. Mar Pollut Bull 2003;46:362–74. [DOI] [PubMed] [Google Scholar]
- 30.Szlinder-Richert J, Barska I, Mazerski J, Usydus Z. PCBs in fish from the southern Baltic Sea: levels, bioaccumulation features, and temporal trends during the period from 1997 to 2006. Mar Pollut Bull 2009;58:85–92. [DOI] [PubMed] [Google Scholar]
- 31.Rochman CM, Hoh E, Kurobe T, Teh SJ. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci Rep 2013;3:3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van A, Rochman CM, Flores EM, Hill KL, Vargas E, Vargas SA, Hoh E. Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere 2012;86:258–63. [DOI] [PubMed] [Google Scholar]
- 33.Marsh TG, Straub RK, Villalobos F, Hong MY. Soy protein supports cardiovascular health by downregulating hydroxymethylglutaryl-coenzyme A reductase and sterol regulatory element-binding protein-2 and increasing antioxidant enzyme activity in rats with dextran sodium sulfate-induced mild systemic inflammation. Nutr Res 2011;31:922–8. [DOI] [PubMed] [Google Scholar]
- 34.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis 2008;197:12–24. [DOI] [PubMed] [Google Scholar]
- 35.Turunen AW, Jula A, Suominen AL, Männistö S, Marniemi J, Kiviranta H, Tiittanen P, Kranko H, Moilanen L, Nieminen MS, et al. . Fish consumption, omega-3 fatty acids, and environmental contaminants in relation to low-grade inflammation and early atherosclerosis. Environ Res 2013;120:43–54. [DOI] [PubMed] [Google Scholar]
- 36.Petriello MC, Newsome B, Hennig B. Influence of nutrition in PCB-induced vascular inflammation. Environ Sci Pollut Res Int 2014;21:6410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hennig B, Reiterer G, Toborek M, Matveev SV, Daugherty A, Smart E, Robertson LW. Dietary fat interacts with PCBs to induce changes in lipid metabolism in mice deficient in low-density lipoprotein receptor. Environ Health Perspect 2005;113:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLennan PL. Cardiac physiology and clinical efficacy of dietary fish oil clarified through cellular mechanisms of omega-3 polyunsaturated fatty acids. Eur J Appl Physiol 2014;114:1333–56. [DOI] [PubMed] [Google Scholar]
- 39.Bergkvist C, Kippler M, Larsson SC, Berglund M, Glynn A, Wolk A, Akesson A. Dietary exposure to polychlorinated biphenyls is associated with increased risk of stroke in women. J Intern Med 2014;276:248–59. [DOI] [PubMed] [Google Scholar]
- 40.Hennig B, Slim R, Toborek M, Robertson LW. Linoleic acid amplifies polychlorinated biphenyl-mediated dysfunction of endothelial cells. J Biochem Mol Toxicol 1999;13:83–91. [DOI] [PubMed] [Google Scholar]
- 41.Fraga CG, Oteiza PI, Galleano M. In vitro measurements and interpretation of total antioxidant capacity. Biochim Biophys Acta. 2014;1840:931–4. [DOI] [PubMed]
- 42.Sies H. Total antioxidant capacity: appraisal of a concept. J Nutr 2007;137:1493–5. [DOI] [PubMed] [Google Scholar]
- 43.Song Y, Liang X, Hu Y, Wang Y, Yu H, Yang K. p,p’-DDE induces mitochondria-mediated apoptosis of cultured rat Sertoli cells. Toxicology 2008;253:53–61. [DOI] [PubMed] [Google Scholar]
- 44.Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Global assessment of organic contaminants in farmed salmon. Science 2004;303:226–9. [DOI] [PubMed] [Google Scholar]
- 45.Shaw SD, Brenner D, Berger ML, Carpenter DO, Hong CS, Kannan K. PCBs, PCDD/Fs, and Organochlorine Pesticides in Farmed Atlantic Salmon from Maine, Eastern Canada, and Norway, and Wild Salmon from Alaska. Environ Sci Technol 2006;40:5347–54. [DOI] [PubMed] [Google Scholar]
- 46.van Leeuwen SP, van Velzen MJ, Swart CP, van der Veen I, Traag WA, de Boer J. Halogenated contaminants in farmed salmon, trout, tilapia, pangasius, and shrimp. Environ Sci Technol 2009;43:4009–15. [DOI] [PubMed] [Google Scholar]
- 47.Cirillo T, Viscardi V, Fasano E, Farina A, Amodio-Cocchieri R. Polychlorinated biphenyls, organochlorine pesticides, and polycyclic aromatic hydrocarbons in wild, farmed, and frozen marine seafood marketed in Campania, Italy. J Food Prot 2009;72:1677–85. [DOI] [PubMed] [Google Scholar]
- 48.Hayward D, Wong J, Krynitsky AJ. Polybrominated diphenyl ethers and polychlorinated biphenyls in commercially wild caught and farm-raised fish fillets in the United States. Environ Res 2007;103:46–54. [DOI] [PubMed] [Google Scholar]
- 49.Hites RA, Foran JA, Schwager SJ, Knuth BA, Hamilton MC, Carpenter DO. Global assessment of polybrominated diphenyl ethers in farmed and wild salmon. Environ Sci Technol 2004;38:4945–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.