Abstract
Background: The common variants in the fat mass and obesity–associated (FTO) gene have been associated with obesity and insulin resistance. Recently, studies also linked FTO variants with macronutrient intakes.
Objective: We aimed to investigate whether diet interventions varying in macronutrients modified the effects of FTO genotypes on changes in insulin resistance.
Methods: We genotyped FTO variants rs1558902 and rs9939609 and measured insulin resistance in fasting plasma samples at baseline and at 6-mo and 2-y visits in 743 overweight or obese adults (aged 30–70 y, 60% women) from a randomized weight-loss dietary interventional trial, the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. We assessed interactions between FTO variants and intakes of dietary fat and protein in relation to change in body weight and insulin resistance using generalized estimating equation models.
Results: We found significant interactions between rs1558902 and dietary fat on changes in homeostasis model assessment of insulin resistance (HOMA-IR) and insulin (P = 0.003 and 0.004, respectively). Each risk allele (A) of rs1558902 showed a trend to be related to a 0.05-unit less reduction in both log(insulin) and log(HOMA-IR) among the participants assigned to low-fat diets (both P = 0.06), but this was not significantly related to reduction in those assigned to high-fat diets (both P > 0.1) during the 2-y period of intervention. Our data showed that the association between rs9939609 and changes in insulin resistance was not modified by diet macronutrient intakes.
Conclusions: Our results show that carriers of the risk alleles of rs1558902 benefit differently in improving insulin sensitivity by consuming high-fat weight-loss diets rather than low-fat diets. Still, given our data, we acknowledge it is difficult to determine whether fat or carbohydrate contributed to the observed associations. This trial was registered at clinicaltrials.gov as NCT00072995.
Keywords: FTO, dietary intervention, gene-diet interaction, diabetes, insulin resistance
Introduction
The prevalence of diabetes in the United States has become of epidemic proportions, reaching ∼8.3% of the population (1), and type 2 diabetes accounts for up to 95% of diabetes cases (2). Insulin resistance, which is closely related to obesity, plays a determinant role in the development of type 2 diabetes (3) and presents 10–20 y before the onset of the disease (4, 5). Interestingly, the common variants in the fat mass and obesity–associated (FTO)9 gene were also related to insulin resistance and risk of type 2 diabetes (6–8).
Recently, we found that genetic variation in FTO was associated with habitual-consumption of macronutrients (9). Similar associations were also found in the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium (10). In addition, a previous diet intervention study found that dietary fat intake might modify one FTO variant in relation to changes in insulin resistance (11). However, this study only assessed the effects of short-term (10-wk) dietary intervention, and it remains unclear whether the FTO-macronutrient interactions persist in long-term interventions.
In the present study, we assessed interactions between 2 common variations in FTO and intakes of dietary fat and protein in relation to change in body weight and insulin resistance among participants in a 2-y randomized dietary intervention weight-loss trial, the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial.
Methods
Study population.
The POUNDS LOST trial is a randomized dietary intervention trial to compare the effects of energy-reduced diets with different compositions of fat, protein, and carbohydrate on weight change over a 2-y period. The study design and methods were previously described in detail (12). In summary, 811 overweight or obese subjects, aged 30–70 y, were randomly assigned to 1 of 4 energy-limited diets. The target percentages of energy derived from fat, protein, and carbohydrate in the 4 diets were 20%, 15%, and 65%; 20%, 25%, and 55%; 40%, 15%, and 45%; and 40%, 25%, and 35%, respectively. Two diets were low in fat (20%) and 2 were high in fat (40%) and 2 were average in protein (15%) and 2 were high in protein (25%) according to a 2-by-2 factorial design. Each participant’s caloric prescription represented a deficit of 750 kcal/d from baseline, as calculated from the person’s resting energy expenditure and activity level. The main exclusion criteria were as follows: participants who had diabetes treated with medication or unstable cardiovascular disease, those who used medications to influence body weight, and those who expressed insufficient motivation to complete the study. The study was approved by the human subjects committee at the Harvard School of Public Health and Brigham and Women’s Hospital, Boston, MA, and the Pennington Biomedical Research Center of the Louisiana State University System, Baton Rouge, LA, and by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All participants provided written informed consent.
Measurements.
Body weights were measured in the morning before breakfast at baseline and at the 6-mo and 2-y visits. Height was measured at baseline. BMI was calculated as weight/height2 (kg/m2). Race was self-reported and grouped as white, black, and other. Fasting blood samples were collected at baseline and at the 6-mo and 2-y visits. Analyses of glucose and insulin were performed at the Clinical Laboratory at Pennington. Glucose and insulin were measured by using an immunoassay with chemiluminescent detection on the Immulite analyzer (Diagnostic Products). Insulin resistance was estimated by HOMA-IR and calculated from fasting glucose and insulin concentrations (13) as shown in Equation 1.
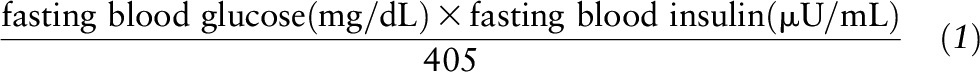 |
β Cell function was estimated by homeostasis model assessment of β cell function (HOMA-β) (13) and calculated as shown in Equation 2.
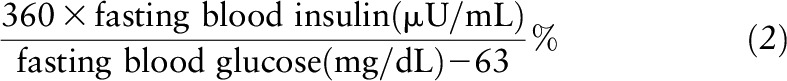 |
Genotyping.
DNA was extracted from the buffy coat fraction of centrifuged blood by using the QIAmp Blood Kit (Qiagen). The FTO variant rs1558902 was genotyped successfully in 743 and the variant rs9939609 in 738 of 811 total participants by using the OpenArray SNP Genotyping System (BioTrove). The genotype success rate was 99%. Replicate quality control samples (10%) were included and genotyped with >99% concordance. The genotype frequencies for both FTO variants in the 2 major races (white and black) were both in Hardy-Weinberg equilibrium (P > 0.05).
Statistical analysis.
Baseline data are presented as means ± SDs for continuous variables and as numbers and percentages for categorical variables. Baseline characteristics were compared by using chi-square test for categorical variables and by a general linear model with the assumption of an additive genetic model for continuous variables. Insulin concentrations, HOMA-IR, and HOMA-β were log-transformed to improve their imperfect normality. The primary outcomes were changes (i.e., the values at follow-up time minus the baseline values) in fasting insulin, glucose, HOMA-IR, and HOMA-β over the time the participant remained in the trial. The effects of genotype and diet intervention on outcomes at 6 mo and 2 y were correlated and analyzed together by using the generalized estimating equation (GEE) method. Covariate adjustment included age, sex, race, the baseline value for the respective outcome, and concurrent weight loss in model 1 and additionally included baseline BMI in model 2. Additive genetic models were used in the analyses. Gene-diet intervention interactions were tested by including the genotype-by-diet interaction multiplicative terms in the GEE models. As a secondary analysis, linear mixed models were used to test the genotype effect on the trajectory of changes in fasting insulin, glucose, HOMA-IR, and HOMA-β by including a genotype-by-time interaction term. In sensitivity analyses, we analyzed the associations between the white participants only.
All reported P values were 2-sided, and 0.05 was considered significant. All data were analyzed with SAS version 9.4 (SAS Institute). This trial was registered at clinicaltrials.gov as NCT00072995.
Results
Characteristics of the study population.
Baseline characteristics of participants according to the FTO rs1558902 and rs9939609 genotypes are presented in Table 1. FTO rs1558902 and rs9939609 were in strong linkage disequilibrium (r2 = 0.83). The minor allele frequency for rs1558902 (A allele) was 0.402 and for rs9939609 (A allele) was 0.447 in the present study population. For variant rs1558902, the genotype frequencies were significantly related to age, sex, and race but not the diet groups. The genotype frequencies for variant rs9939609 were not related to these variables. Insulin, glucose, insulin resistance, and β cell function at baseline were not related to the FTO variants.
TABLE 1.
Characteristics of study participants according to FTO rs1558902 and rs9939609 genotypes1
| rs1558902 |
rs9939609 |
|||||||
| Baseline characteristics | TT (n = 282) | AT (n = 325) | AA (n = 136) | P | TT (n = 228) | AT (n = 360) | AA (n = 150) | P |
| Age, y | 49.8 ± 9.4 | 51.8 ± 9.3 | 51.5 ± 8.4 | 0.04 | 50.0 ± 9.9 | 51.5 ± 9.1 | 51.4 ± 8.5 | 0.10 |
| Male | 93 (33.0) | 136 (41.9) | 59 (43.4) | 0.04 | 86 (37.7) | 145 (40.3) | 54 (36.0) | 0.62 |
| Race | <0.01 | 0.05 | ||||||
| White | 180 (63.8) | 285 (87.7) | 130 (95.6) | 182 (79.7) | 284 (78.9) | 124 (82.7) | ||
| Black | 84 (29.8) | 25 (7.7) | 3 (2.2) | 28 (12.3) | 61 (16.9) | 23 (15.3) | ||
| Other | 18 (6.4) | 15 (4.6) | 3 (2.2) | 18 (7.9) | 15 (4.2) | 3 (2.0) | ||
| Dietary fat composition | 0.48 | 0.41 | ||||||
| High-fat diets (40% of energy) | 136 (48.2) | 160 (49.2) | 74 (54.4) | 112 (49.1) | 174 (48.3) | 82 (54.7) | ||
| Low-fat diets (20% of energy) | 146 (51.8) | 165 (50.8) | 62 (45.6) | 116 (50.9) | 186 (51.7) | 68 (45.3) | ||
| Dietary protein composition | 0.69 | 0.59 | ||||||
| High-protein diets (25% of energy) | 145 (51.4) | 157 (48.3) | 65 (47.8) | 119 (52.2) | 175 (48.6) | 71 (47.3) | ||
| Average-protein diets (15% of energy) | 137 (48.6) | 168 (51.7) | 71 (52.2) | 109 (47.8) | 185 (51.4) | 79 (52.7) | ||
| BMI, kg/m2 | 33.0 ± 3.7 | 32.3 ± 4 | 33.1 ± 3.7 | 0.61 | 32.7 ± 3.8 | 32.5 ± 3.9 | 33.1 ± 3.8 | 0.51 |
| Fasting glucose, mg/dL | 91.1 ± 11.4 | 92.6 ± 12.6 | 91.9 ± 10.5 | 0.31 | 91.3 ± 11.8 | 92.3 ± 12.2 | 91.8 ± 11 | 0.58 |
| Fasting insulin,2 μU/ml | 10.4 [9.0] | 10.6 [8.0] | 10.6 [8.1] | 0.80 | 10.2 [8.3] | 10.5 [8.4] | 10.7 [8.5] | 0.63 |
| HOMA-IR2 | 2.3 [2.1] | 2.4 [2.0] | 2.5 [2.2] | 0.63 | 2.3 [2.0] | 2.4 [2.1] | 2.5 [2.3] | 0.56 |
| HOMA-β2 | 1.4 [1.1] | 1.3 [1.0] | 1.3 [1.0] | 0.45 | 1.4 [1.0] | 1.3 [1.0] | 1.3 [1.0] | 0.87 |
| Changes in outcomes at 2 y | ||||||||
| ΔFasting glucose, mg/dL | 2.4 [9.4] | 2.0 [9.2] | 2.4 [8.9] | 0.94 | 1.6 [8.8] | 2.3 [8.9] | 2.9 [10.5] | 0.25 |
| ΔFasting insulin, μU/mL | −1.0 [8.2] | −1.4 [5.7] | −1.5 [4.9] | 0.70 | −1.1 [8.6] | −1.4 [5.6] | −1.1 [5.2] | 0.76 |
| ΔHOMA-IR | −0.1 [2.1] | −0.2 [1.6] | −0.3 [1.4] | 0.75 | −0.2 [2.2] | −0.2 [1.6] | −0.1 [1.6] | 0.63 |
| ΔHOMA-β | −0.3 [1.1] | −0.3 [0.8] | −0.3 [0.6] | 0.65 | −0.3 [1.3] | −0.3 [0.8] | −0.3 [0.6] | 0.88 |
Values are means ± SDs, n (%), or medians [IQRs]. P values were calculated by chi-square test for categorical variables and by a general linear model for continuous variables with the assumption of an additive genetic model. FTO, fat mass and obesity–associated; HOMA-β, homeostasis model assessment of β cell function; ∆, change in respective outcomes (i.e., the values at 2 y minus baseline values); for ΔFasting insulin, ΔHOMA-IR, and ΔHOMA-β, this means the log-transformed values at 2 y minus the log-transformed baseline values.
P values were calculated for the log-transformed values.
FTO genotypes and change in insulin resistance.
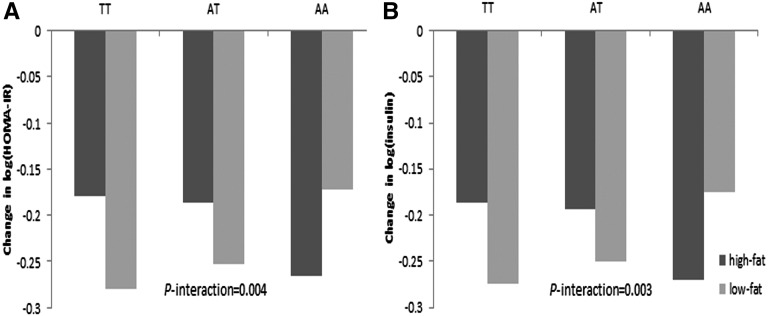
Table 2 shows the associations of FTO genotypes with changes in fasting insulin, glucose, insulin resistance calculated by HOMA-IR and β cell function calculated by HOMA-β according to intake of dietary fat by using repeated measures of changes in these markers at 6 mo and 2 y, with adjustment for age, sex, race, the baseline value for the respective outcome, and concurrent weight loss in model 1 and with additional adjustment for baseline BMI in model 2. In these 2 models, the directions of genetic effects were the same, whereas the magnitudes were similar, and the significance of gene-diet interaction on changes in insulin and HOMA-IR remained after adjusting for baseline BMI. In model 2, each risk allele (A) of rs1558902 showed a trend to be associated with a smaller reduction in both log(insulin) and log(HOMA-IR) among participants assigned to the low-fat diets (both P = 0.06), but they were not significantly related with reduction among those assigned to high-fat diets (both P > 0.1) during the 2-y period of intervention (P-interaction = 0.003 and 0.004, respectively; Table 2, Figure 1). The associations between rs9939609 and changes in glucose, insulin, HOMA-IR, or HOMA-β were not significantly modified by dietary fat intakes. Dietary protein did not significantly modify the genetic effects on these outcomes. The results in white participants only were similar to those observed in the whole study population, although the significance of the interaction between dietary fat and rs1558902 was attenuated (Supplemental Table 1). The β-coefficients of the genetic association of rs1558902 with changes in insulin and HOMA-IR were both 0.04 in the low-fat group and were both −0.03 in the high-fat group (both P-interaction = 0.07).
TABLE 2.
Effects of FTO rs1558902 and rs9939609 genotypes on the response of glucose, insulin, and insulin resistance to the dietary fat intervention1
| rs1558902 |
rs9939609 |
|||||||||||||
| Low-fat |
High-fat |
Low-fat |
High-fat |
|||||||||||
| Outcomes | β | SE | P | β | SE | P | P-interaction | β | SE | P | β | SE | P | P-interaction |
| Model 1 | ||||||||||||||
| ΔFasting glucose, mg/dL | 0.29 | 0.44 | 0.50 | −0.17 | 0.49 | 0.50 | 0.28 | 0.08 | 0.46 | 0.86 | 0.47 | 0.56 | 0.40 | 0.44 |
| ΔFasting insulin,2 μU/mL | 0.05 | 0.02 | 0.06 | −0.04 | 0.03 | 0.12 | 0.003 | 0.04 | 0.02 | 0.10 | −0.02 | 0.03 | 0.37 | 0.12 |
| ΔHOMA-IR2 | 0.05 | 0.03 | 0.06 | −0.04 | 0.03 | 0.14 | 0.004 | 0.04 | 0.03 | 0.12 | −0.02 | 0.03 | 0.52 | 0.20 |
| ΔHOMA-β2 | 0.04 | 0.03 | 0.21 | −0.03 | 0.03 | 0.18 | 0.04 | 0.04 | 0.03 | 0.20 | −0.03 | 0.03 | 0.21 | 0.08 |
| Model 2 | ||||||||||||||
| ΔFasting glucose, mg/dL | 0.29 | 0.43 | 0.51 | −0.13 | 0.47 | 0.78 | 0.30 | 0.06 | 0.46 | 0.90 | 0.52 | 0.55 | 0.34 | 0.43 |
| ΔFasting insulin,2 μU/mL | 0.05 | 0.02 | 0.06 | −0.04 | 0.02 | 0.12 | 0.003 | 0.04 | 0.02 | 0.13 | −0.02 | 0.02 | 0.38 | 0.15 |
| ΔHOMA-IR2 | 0.05 | 0.03 | 0.06 | −0.04 | 0.03 | 0.14 | 0.004 | 0.03 | 0.03 | 0.26 | −0.02 | 0.03 | 0.52 | 0.24 |
| ΔHOMA-β2 | 0.03 | 0.03 | 0.22 | −0.03 | 0.03 | 0.19 | 0.05 | 0.03 | 0.03 | 0.24 | −0.03 | 0.02 | 0.22 | 0.10 |
P values for model 1 were adjusted for age, sex, race, follow-up time, baseline values for respective outcomes, and concurrent weight change; P values for model 2 were further adjusted for baseline BMI. FTO, fat mass and obesity–associated; HOMA-β, homeostasis model assessment of β cell function; β, β-coefficient; ∆, change in respective outcomes (i.e., the values at 2 y minus baseline values).
Log-transformed values at 2 y minus log-transformed baseline values.
FIGURE 1.
Change in insulin resistance measured as HOMA-IR (A) and insulin (B) by FTO rs1558902 genotype and dietary fat groups. P values were adjusted for age, sex, race, weight change, follow-up time, baseline values for respective outcomes, and baseline BMI. Values are means. FTO, fat mass and obesity–associated.
Trajectory of changes in insulin resistance by FTO rs1558902 in response to high-/low-fat weight-loss diets.
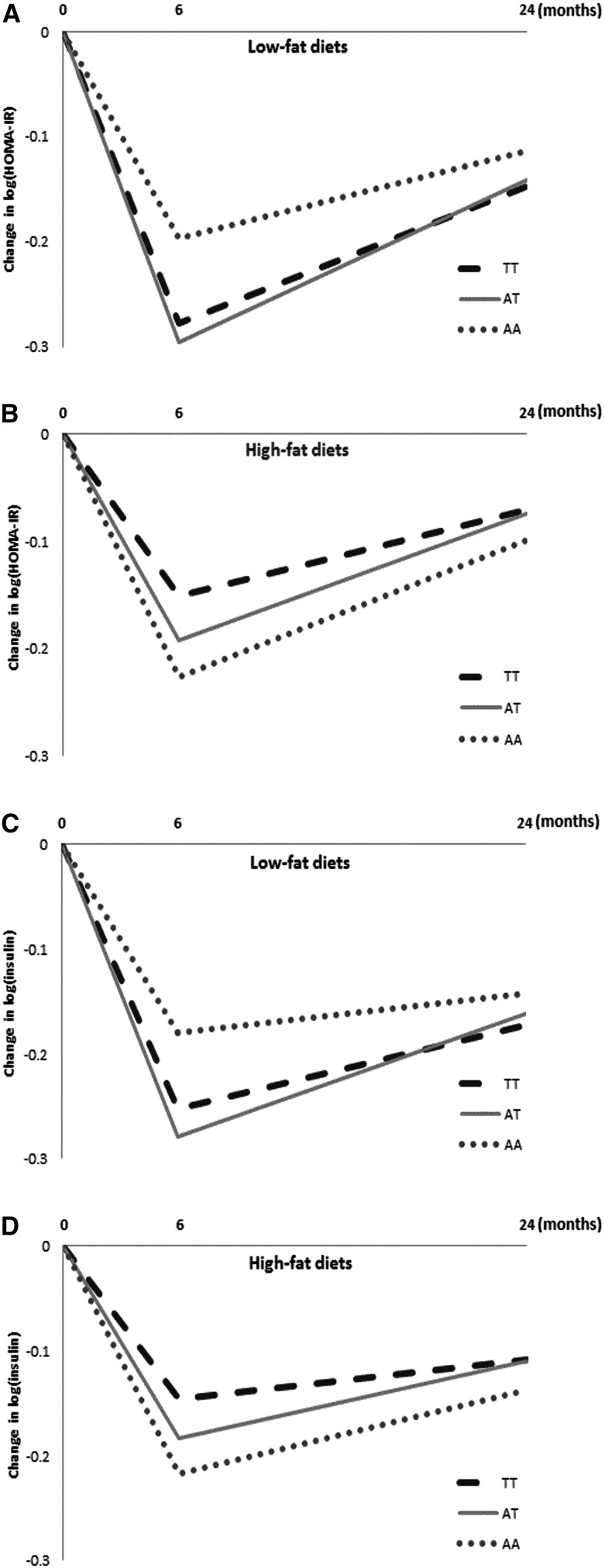
In a secondary analysis, we used linear mixed models to assess the genotype-by-time interactive effect over the 2-y trial in those assigned to the low- or high-fat diet (Figure 2). In both the low- and high-fat diet groups, the genetic associations with changes in fasting insulin and HOMA-IR were more pronounced at the 6-mo visit than at the 2-y visit. However, we did not observe significant genotype-by-time interactions. Similar results were observed when the analyses were restricted to white participants.
FIGURE 2.
Changes from baseline in insulin resistance measured as HOMA-IR (A, B), and insulin (C, D) at the 6-mo and 2-y visits by FTO rs1558902 genotype in both the low-fat and high-fat diet groups. No significant genotype-by-time interactions were detected after adjusting for age, sex, race, weight change, baseline values for respective outcomes, and baseline BMI (all P > 0.05). Values are means. FTO, fat mass and obesity–associated.
Discussion
In the present study, we investigated whether dietary macronutrient composition modified the associations between FTO variants and changes in insulin sensitivity in a large, long-term randomized trial with weight-loss dietary interventions, the POUNDS LOST trial. We identified potential gene (FTO rs1558902)-by-diet (high- vs. low-fat weight-loss diets) interactions on reduction in fasting insulin and HOMA-IR over the 2-y intervention period. Carriers of the risk alleles (A) of rs1558902 might benefit differently by consuming high-fat weight-loss diets but benefit less by consuming low-fat weight-loss diets compared with noncarriers.
Our findings are in line with a previous European short-term dietary intervention trial in which a significant FTO–dietary fat interaction was observed in relation to insulin resistance (11), and both studies consistently showed that the risk allele carriers of FTO variant may benefit differently from high-fat diets in improving insulin resistance than from low-fat diets. In the European study (Sweden, Denmark, United Kingdom, The Netherlands, Czech Republic, France, and Spain), obese carriers of FTO rs9939609 risk alleles showed a greater decrease in insulin resistance with consumption of high-fat diets than did those consuming low-fat diets; however, FTO rs1558902 was not genotyped. The single nucleotide polymorphism rs1558902 showed more significant interaction than rs9939609 with dietary fat on insulin resistance. FTO rs1558902 and rs9939609 were in strong linkage disequilibrium (r2 = 0.83) in our study. The discrepancy between our study and the European study may be partly due to the heterogeneity in genomic structure among various populations, such as the difference in the minor allele frequency of the specific single nucleotide polymorphisms.
Previous studies suggested that the effects of FTO genotype on insulin resistance and diabetes may be not be through adiposity (14, 15). In our study, the interaction between FTO variant and dietary fat on improvement in insulin and HOMA-IR remained significant with adjustment for concurrent weight loss. In this context, our results suggest that the interaction between FTO rs1558902 and dietary fat on the changes in insulin resistance might be independent of weight/adiposity change.
The potential mechanism underlying these findings remains unclear. FTO expression was found to be increased in skeletal muscle from patients with type 2 diabetes compared with nondiabetic individuals, independent of obesity (16). Several previous studies reported that FTO expression could be affected by fasting and feeding status (17–19), suggesting a potential regulatory role of dietary factors. However, evidence directly linking dietary fat and FTO gene function is still lacking, and further experimental data are warranted to clarify the potential mechanisms.
The genetic associations with changes in insulin resistance were more pronounced at the 6-mo visit than at the 2-y visit, which is consistent with the trajectory of changes in weight in the POUNDS LOST trial (12). Similar to other weight-loss trials, the diminished adherence to assigned diets that occurred between 6 mo and 2 y may partially explain these decreased genetic associations after 6 mo. The FTO variant rs1558902 was reported to have the strongest association with obesity in various populations (20, 21), and variant rs9939609 is the first hit of genome-wide association studies on BMI (22). However, the main effect of these FTO variants on weight loss or change in insulin resistance was not significant in the present study. Possible explanations could include the following: that the FTO variants primarily influence body fatness early in life, although the effect persists into adulthood; the participants in our study were homogenous regarding BMI and insulin sensitivity; and that the metabolism and biology mechanism of weight loss are different from those of weight gain (11).
The present study is among the first to investigate interactions between FTO genetic variation and dietary macronutrients on changes in insulin resistance in a long-term randomized dietary intervention trial. The study design allowed for reliable control of the dietary effects. The large sample size and long intervention period provided a decent chance of detecting moderate gene-diet interactions on change in insulin resistance. We combined repeated measures of outcomes in the analysis by using the GEE method to further increase statistical power. The GEE model is fairly robust to the choices of the correlation structure and flexible for missing data compared with other models (23). Nevertheless, we acknowledge that the current study is exploratory and might be underpowered to detect a small gene-diet interaction effect. There are many possible extensions to this work. The replication of the current finding in an independent trial with similar design is needed. Further research into the underlying mechanism of the identified interaction effect on insulin resistance is warranted. The generalization of this study is limited to whites because 80% of our population was white. High fat usually accompanies low carbohydrate in a diet and vice versa. Therefore, given our data, it is difficult to determine which nutrient (fat or carbohydrate) drove the observed associations.
In conclusion, we report an interactive effect between FTO rs1558902 and dietary fat on change in insulin resistance independent of weight loss over a large 2-y dietary intervention trial. Our data suggest that carriers of the risk alleles of rs1558902 might benefit differently in improving insulin sensitivity by consuming high-fat diets than by consuming low-fat diets. However, we also acknowledge that over and above any impact of the dietary intervention on weight loss, there was no significant main effect of the weight-loss diets on changes in insulin sensitivity regardless of FTO genotype. Although further investigation is warranted, our findings provide the potential to bring new insights to personalized dietary intervention for diabetes prevention and therapy by using genome-customized approaches.
Supplementary Material
Acknowledgments
YZ and LQ contributed to the study concept and design, data analysis and interpretation, and drafting and critical revision of the manuscript; TH and XZ critically revised the manuscript; GAB and FMS contributed to the study concept, acquisition of data, design and funding of the initial project, and critical revision of the manuscript; JR contributed to the administration, laboratory measurements, and acquisition of data; and LQ is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: FTO, fat mass and obesity–associated; GEE, generalized estimating equation; HOMA-β, homeostasis model assessment of β cell function; POUNDS LOST, Preventing Overweight Using Novel Dietary Strategies.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta (GA): US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2.Campbell RK. Type 2 diabetes: where we are today: an overview of disease burden, current treatments, and treatment strategies. J Am Pharm Assoc 2009;49 Suppl 1:S3–9. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol 2002;90: 5A:3G–10G. [DOI] [PubMed] [Google Scholar]
- 4.Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H, Freymond D, Nyomba BL, Zurlo F, Swinburn B, Bogardus C. Impaired glucose tolerance as a disorder of insulin action: longitudinal and cross-sectional studies in Pima Indians. N Engl J Med 1988;318:1217–25. [DOI] [PubMed] [Google Scholar]
- 5.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 1990;113:909–15. [DOI] [PubMed] [Google Scholar]
- 6.Freathy RM, Timpson NJ, Lawlor DA, Pouta A, Ben-Shlomo Y, Ruokonen A, Ebrahim S, Shields B, Zeggini E, Weedon MN, et al. Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes 2008;57:1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasan SK, Karpe F, Gu HF, Brismar K, Fall CH, Ingelsson E, Fall T. FTO genetic variants and risk of obesity and type 2 diabetes: a meta-analysis of 28,394 Indians. Obesity (Silver Spring) 2014;22:964–70. [DOI] [PubMed] [Google Scholar]
- 8.Rees SD, Islam M, Hydrie MZ, Chaudhary B, Bellary S, Hashmi S, O'Hare JP, Kumar S, Sanghera DK, Chaturvedi N, et al. An FTO variant is associated with type 2 diabetes in South Asian populations after accounting for body mass index and waist circumference. Diabet Med 2011;28(6):673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JS, Group CNW, Qi Q, Curhan GC, et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum Mol Genet 2013;22:1895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, Houston DK, Kanoni S, Lemaitre RN, Luan J, et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am J Clin Nutr 2013;97:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grau K, Hansen T, Holst C, Astrup A, Saris WH, Arner P, Rossner S, Macdonald I, Polak J, Oppert JM, et al. Macronutrient-specific effect of FTO rs9939609 in response to a 10-week randomized hypo-energetic diet among obese Europeans. Int J Obes (Lond) 2009;33:1227–34. [DOI] [PubMed] [Google Scholar]
- 12.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsson JA, Klovins J, Kapa I, Danielsson P, Svensson V, Ridderstrale M, Gyllensten U, Marcus C, Fredriksson R, Schioth HB. Novel genetic variant in FTO influences insulin levels and insulin resistance in severely obese children and adolescents. Int J Obes (Lond) 2008;32:1730–5. [DOI] [PubMed] [Google Scholar]
- 15.Bressler J, Kao WH, Pankow JS, Boerwinkle E. Risk of type 2 diabetes and obesity is differentially associated with variation in FTO in whites and African-Americans in the ARIC study. PLoS ONE 2010;5:e10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bravard A, Lefai E, Meugnier E, Pesenti S, Disse E, Vouillarmet J, Peretti N, Rabasa-Lhoret R, Laville M, Vidal H, et al. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes 2011;60:258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stratigopoulos G, Padilla SL, LeDuc CA, Watson E, Hattersley AT, McCarthy MI, Zeltser LM, Chung WK, Leibel RL. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol 2008;294:R1185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredriksson R, Hagglund M, Olszewski PK, Stephansson O, Jacobsson JA, Olszewska AM, Levine AS, Lindblom J, Schioth HB. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology 2008;149:2062–71. [DOI] [PubMed] [Google Scholar]
- 19.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007;318:1469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Lango Allen H, Lindgren CM, Luan J, Magi R, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotta K, Nakata Y, Matsuo T, Kamohara S, Kotani K, Komatsu R, Itoh N, Mineo I, Wada J, Masuzaki H, et al. Variations in the FTO gene are associated with severe obesity in the Japanese. J Hum Genet 2008;53:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988;44:1049–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.