Abstract
Degeneration of the intervertebral discs is strongly implicated as a cause of low back pain. Since current treatments for discogenic low back pain show poor long-term efficacy, a number of new, biological strategies are being pursued. For such therapies to succeed, it is critical that they be validated in conditions that mimic the unique biochemical microenvironment of the nucleus pulposus (NP), which include low oxygen tension. Therefore, the objective of this study was to investigate the effects of oxygen tension on NP cell functional extracellular matrix elaboration in 3D culture. Bovine NP cells were encapsulated in agarose constructs and cultured for 14 or 42 days in either 20% or 2% oxygen in defined media containing transforming growth factor beta-3. At each time point, extracellular matrix composition, biomechanics and mRNA expression of key phenotypic markers were evaluated. Results showed that while bulk mechanics and composition were largely independent of oxygen level, low oxygen promoted improved restoration of the NP phenotype, higher mRNA expression of extracellular matrix and NP specific markers, and more uniform matrix elaboration. These findings indicate that culture under physiological oxygen levels is an important consideration for successful development of cell and growth factor-based regenerative strategies for the disc.
Keywords: Intervertebral disc, oxygen tension, extracellular matrix, mechanical properties, tissue engineering
Introduction
Degeneration of the lumbar intervertebral discs is common amongst the adult population, and strongly implicated as a cause of low back pain.1,2 Disc degeneration is characterized by a cascade of cellular and compositional changes that ultimately leads to structural breakdown and functional impairment.3,4 The central nucleus pulposus (NP) is implicated in the initiation of this cascade, where increasing local inflammation is associated with loss of proteoglycans.5 This compromises the ability of the NP to fulfill its most crucial mechanical role – the even distribution of compressive loads between adjacent vertebral bodies.6 Current treatment strategies for discogenic low back pain, both conservative and surgical, show relatively poor long term efficacy.7 Consequently, new, biological strategies are being actively pursued, with examples including cell, gene, and growth factor therapies.8 The broad aim of such therapies is to potentiate functional extracellular matrix elaboration within the disc space, in order to regenerate native tissue, and, in turn, facilitate long-term normalization of disc structure and mechanical function. For such therapies to succeed, it is critical that they be developed and validated in conditions which mimic the, in vivo, biochemical microenvironment of the NP.
The intervertebral disc is the largest avascular structure in the human body. During development, blood vessels penetrate deep into the annulus fibrosus, however by the second decade of life they have almost completely receded, remaining only within the outermost lamellae.9-12 At no point do blood vessels penetrate the NP itself – the primary pathway for diffusion of oxygen and nutrition to the NP is via the hyaline cartilage end plates that interface inferiorly and superiorly with the vascularized subchondral bone of the vertebral bodies.13 As a result, NP cells reside in a tissue “niche”, bound peripherally by the annulus fibrosus, and superiorly and inferiorly by the end plates, that is characterized by low oxygen tension.14 NP cells appear uniquely adapted to function within a low oxygen microenvironment. For example, NP cells, unlike many other cell types, stably express the key transcriptional regulators – hypoxia inducible factors (HIF) -1α and 2α – independent of oxygen level.15-17 Relationships between oxygen levels and the functional biosynthetic properties of NP cells, however, have not been well established. As many novel therapeutic strategies employ exogenous, anabolic stimuli such as growth factors to potentiate tissue regeneration, it is imperative that their effects be evaluated under physiological oxygen levels.
In a previous study we used an in vitro, 3D, agarose culture model to investigate the long-term, functional, biosynthetic properties of NP cells in the presence of transforming growth factor beta-3 (TGF-β3).18 After 42 days of culture, NP constructs matched or exceeded native tissue properties in terms of mechanical properties, extracellular matrix composition and mRNA expression of key phenotypic markers. This study, however, was conducted at atmospheric oxygen levels, which is not representative of the in vivo microenvironment of the NP. Therefore, the objective of this study was to investigate the effects of oxygen tension on in vitro NP cell functional extracellular matrix elaboration in 3D culture, in the presence of TGF-β3. Given that NP cells are uniquely adapted to function in low oxygen conditions and exhibit stable expression of associated transcriptional regulators independent of oxygen tension, we hypothesized that NP cells would exhibit functional biosynthetic properties that were largely independent of oxygen tension.
Methods
Cell Isolation and Culture Conditions
For this study we used an agarose NP cell culture model described previously.18,19 Nucleus pulposus tissue was isolated via sharp dissection from the intervertebral discs of four bovine caudal spines purchased from a local slaughterhouse according to Institutional guidelines. After dissection, the tissue was incubated overnight at 37°C in high glucose Dulbecco’s Modified Eagle Medium (DMEM) with 2% penicillin/streptomycin/fungizone (PSF). Cells were isolated from the tissue by enzymatic digestion, (1 hour in 2.5 mg/ml pronase followed by (4 hours in 0.5 mg/ml collagenase), and pooled. After digestion the cells were expanded in monolayer in high glucose DMEM containing 10% fetal bovine serum (FBS) and 1% PSF. Three aliquots of freshly isolated cells were transferred immediately to TRIzol reagent to establish baseline, native mRNA expression levels. Passage 2 cells were combined and suspended in a chemically defined medium. The complete media formulation and sources of reagents were published previously.18 The cell suspension was mixed with an equal volume of 4% sterile, low gelling temperature agarose to obtain a seeding density of 2.0×107 cells/ml in 2% agarose. Gels were cast into a slab between two glass plates (2.25 mm thickness), and individual constructs were obtained using a 4 mm biopsy punch. These NP constructs were cultured in defined media (1 ml per construct) supplemented with 10 ng/ml of TGF-β3 for either 14 or 42 days in each of two oxygen conditions: 20% (atmospheric) or 2% oxygen. Culture in 20% oxygen was performed in a standard 5% CO2 incubator. Culture in 2% oxygen was performed continuously in a hypoxic cell culture workstation (HypOxygen; Frederick, MD, USA). The culture time of 42 days was selected based on our previous work18,19, where we showed that after this period of time NP cells cultured in agarose match or exceed native tissue properties. Media changes were performed every three days. At each time point, constructs were harvested to determine cell viability, mechanical properties, composition, and mRNA expression levels of extracellular matrix and NP phenotypic markers.
Cell Viability
To quantitatively assess cell viability constructs (n=3) from each culture group were stained with a Live/Dead cell viability kit (Invitrogen; Carlsbad, USA). Constructs were halved through the mid-plane and central region imaged at 10x magnification using an inverted fluorescence microscope (TE2000U; Nikon, Tokyo, Japan). Percent viability was calculated by counting the number of dead cells (ethidium homodimer-1, red) and live cells (calcein, green) as described previously.20
Mechanical Testing
Constructs (n = 5) were tested in confined compression using previously published techniques.18,19 The testing system consisted of an acrylic chamber fixed above a porous, stainless steel platen (10 μm pore size, 50% void ratio) within a testing bath filled with culture media (without TGF-β3). To account for variability in sample diameter following agarose culture, six confinement chambers were constructed ranging in diameter from 4.0 to 4.5 mm. Prior to testing, the diameter of each construct was measured using digital calipers and matched to a confinement chamber. Compression was applied using an impermeable ceramic indenter, size-matched to the confinement chamber, attached to a mechanical testing system fitted with a 5 N load cell (Instron; Norwood MA, USA). Samples were initially subjected to a 0.02 N preload held for 500 seconds. This ensured that the indenter was uniformly in contact with the sample surface. A stress relaxation test was then performed, consisting of 10% strain calculated based on the sample thickness following preload, applied at a rate of 0.05% per second, followed by relaxation to equilibrium for 10 minutes. Aggregate modulus was calculated as the equilibrium stress (equilibrium force/sample area) divided by the applied strain. Hydraulic permeability was calculated from the relaxation data using linear biphasic theory, assuming material isotropy, as described previously.21
Compositional Analysis
Following mechanical testing, samples were weighed and digested overnight in proteinase K (0.1 mg/ml). Digests were assayed for DNA content using the PicoGreen assay (Invitrogen; Carlsbad, USA), sulfated GAG content using the dimethylmethylene blue (DMMB) assay,22 and collagen (following acid hydrolysis) using the p-diaminobenzaldehyde/chloramine-T assay for hydroxyproline.23 Collagen content was calculated assuming a ratio of hydroxyproline to collagen of 1:10.24 DNA content was normalized per construct, and GAG and collagen were normalized to both construct wet weight and DNA content. Additional samples (n = 3) were fixed in 4% paraformaldehyde and processed for paraffin histology. Eight-micron sections were stained with either Alcian blue or picrosirius red to demonstrate GAG or collagen distribution respectively. Immunohistochemical detection of collagens I (Millipore; Bellirica, USA) and II (Developmental Studies Hybridoma Bank; Iowa City, USA) was performed as described previously.25 The working dilution for both primary antibodies was 10μg/ml, and positive staining was visualized using 3,3′-Diaminobenzidine. Sections were imaged using bright field microscopy.
Messenger RNA Expression Levels
Four constructs from each oxygen condition and time point, and the three samples of freshly isolated cells, were analyzed to determine mRNA expression levels. RNA was isolated via two sequential extractions in TRIZOL/chloroform (Invitrogen). RNA quantity and quality (260/280 > 1.8) were spectrophotometrically quantified (ND-1000; Nanodrop Technologies, Wilmington, USA). Reverse transcription was performed on 1 μg of RNA with random hexamers using a Superscript II kit (Invitrogen Corp; Carlsbad, USA) in a 20 μl volume. Expression levels of aggrecan (ACAN), collagens I and II (COL1A1 and COL2A1), solute carrier family 2 facilitated glucose transporter member 1 (SLC2A1, a marker of anaerobic metabolism), cytokeratin 8 (KRT8, an NP specific marker) were determined by quantitative real-time PCR (StepOne Plus; Applied Biosystems, Carlsbad, CA, USA). Expression levels were calculated using the comparative ct method, normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT1), and presented as a percent of freshly isolated levels. The primer sequence (direction 5′ => 3′) for SLC2A1 was F: AAGAGAGTCGCAGACGGAGT, R: CTGTCAGCTTCTTGCTGGTG; the primer sequence for HPRT1 was F: TGATGAAGGAGATGGGTGGC, R: TCGGCAAAGAACTTATAGCCCC. Sequences for all other genes were published previously.18
Statistical Analysis
All results are presented as mean ± standard deviation. Statistical analyses were undertaken using SYSTAT (Systat Software Inc; Chicago, IL, USA). Differences between study groups were established via 2-way ANOVAs, with culture time (14 and 42 days) and oxygen condition (20 or 2%) as independent variables. Where significance was found, post-hoc Tukey’s comparisons were conducted to establish pairwise significance between groups. Significance was defined as two-tailed p<0.05. The results are representative of three complete study replicates.
Results
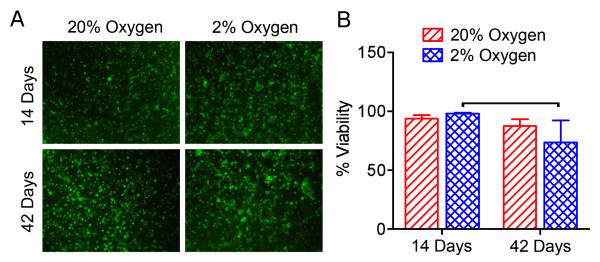
To determine the effects of oxygen tension on cell viability, Live/Dead staining was performed (Figure 1). After 42 days of culture, viability was 25% lower for 2% oxygen compared to 2% oxygen after 14 days of culture (p<0.05). There were no other significant differences in viability between groups.
Figure 1. Cell viability.
A. Representative images of live/dead stained samples from each culture group (central region of sample mid-plane, 10x magnification). B. Quantification of cell viability for each culture group. Bars indicates significant difference between groups (p<0.05).
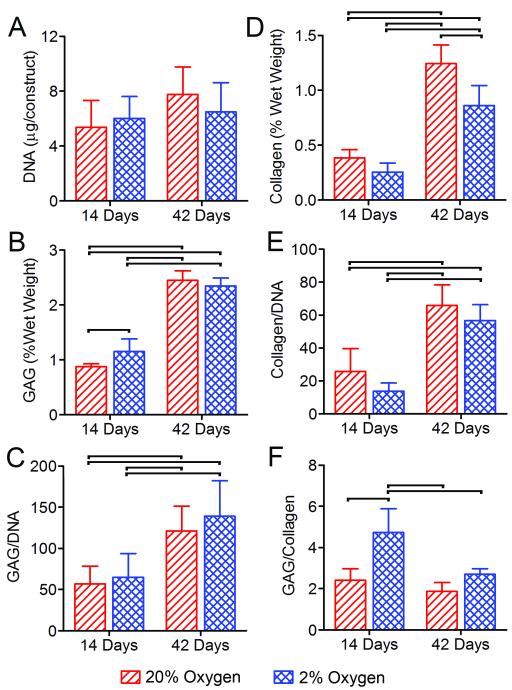
To determine the effects of oxygen tension on cell proliferation and extracellular matrix elaboration, construct DNA, GAG and collagen contents were determined at each time point and for each culture condition (Figure 2). DNA content was not significantly affected by either culture time or oxygen condition (Figure 2A). Glycosaminoglycan and collagen contents were analyzed both normalized to construct wet weight and DNA content (Figures 2B-E). Both GAG and collagen were significantly higher at 42 days than 14 days for both oxygen conditions. These results were true both as a percent of construct wet weight (Figures 2B and D) and normalized to DNA content (Figures 2C and E). After 14 days of culture GAG/wet weight was significantly (1.3-fold) greater for 2% than 20% oxygen, and after 42 days collagen/wet weight was significantly (1.3-fold) greater for 20% compared to 2% oxygen. Normalized to DNA, neither of these differences were significant. Additionally, after 14 days of culture, the GAG/collagen ratio was significantly (1.6-fold) higher for 2% compared to 20% oxygen (Figure 2F).
Figure 2. Composition.
A. DNA content. B. GAG content normalized by construct wet weight. C. GAG content normalized by DNA content. D. Collagen content normalized by construct wet weight. E. Collagen content normalized by DNA content. F. GAG to collagen ratio. Bars indicate significant differences between groups (p<0.05).
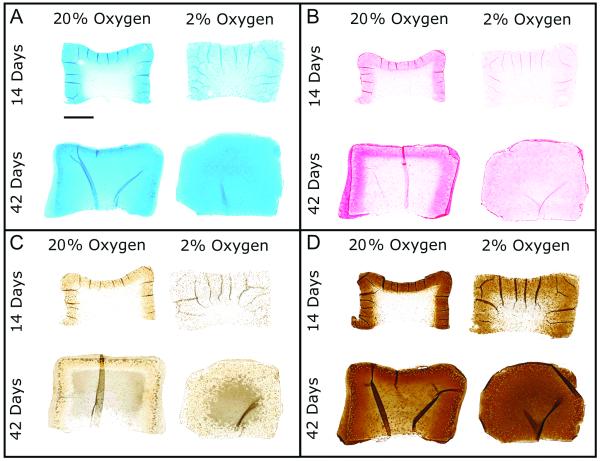
Histological evaluation revealed progressive increases in total GAG and collagen deposition as demonstrated by Alcian blue and picrosirius red staining respectively, for both oxygen conditions with culture time (Figures 3A and B). There were marked differences in matrix distribution between 20 and 2% oxygen at both time points. For 20% oxygen, greater staining intensity (for both GAG and collagen) was present closer to the construct periphery compared to the construct center. For 2% oxygen, staining intensity was more homogeneous throughout the construct expanse. With respect to collagen sub-types, low levels of collagen I staining were evident at 14 and 42 days for 20% oxygen groups, and localized to the periphery (Figure 3C). In comparison, collagen I staining was largely absent for 2% oxygen groups. Collagen II staining was markedly more intense than collagen I for all culture groups, with staining showing similar distributions to picrosirius red and Alcian blue (Figure 3D).
Figure 3. Histology.
A. Representative Alcian blue staining for glyosaminoglycans. B. Representative picrosirius red staining for collagens. C. Representative immunostaining for collagen I. D. Representative immunostaining for collagen II. Scale = 1mm. Sections taken from the construct mid-plane.
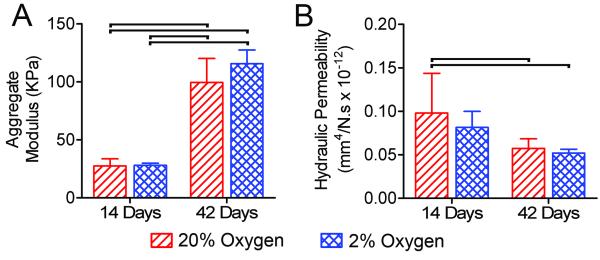
To evaluate functional maturation of NP constructs as a function of oxygen condition, aggregate modulus and hydraulic permeability were determined at each time point using confined compression tests (Figure 4). Modulus was significantly higher at 42 days than 14 days for both oxygen conditions. Permeability was significantly lower at day 42 for both oxygen conditions, compared to 20%, but not 2% oxygen, at 14 days. There was no significant effect of oxygen condition on modulus or permeability at either time point.
Figure 4. Mechanical properties.
A. Aggregate modulus. B. Hydraulic permeability. Bars indicate significant differences between groups (p<0.05).
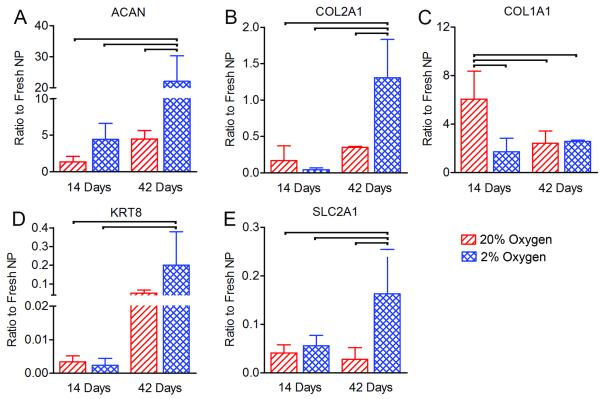
Finally, mRNA expression of key extracellular matrix and NP cell specific markers was assessed (Figure 5). Both ACAN and COL2A1 expression were significantly greater for the 2% oxygen group after 42 days of culture compared to all other groups (4.9 and 3.7-fold greater than 20% oxygen at 42 days, respectively, p<0.05). In contrast, COL1A1 expression was significantly higher for the 20% oxygen group after 14 days of culture compared to all other groups (3.5-fold greater than 2% oxygen at 14 days, p<0.05). Expression of the NP marker KRT8 for the 2% oxygen group after 42 days was significantly greater than both the 2% and 20% oxygen groups after 14 days. Expression of SLC2A1 (glucose transporter 1, a marker of anaerobic glycolysis) was significantly higher for the 2% oxygen group after 42 days of culture compared to all other groups (5.8-fold higher than 20% oxygen after 42 days of culture, p<0.05).
Figure 5. Messenger RNA expression levels of extracellular matrix and NP markers.
A. Aggrecan (ACAN). B. Collagen II (COL2A1). C. Collagen I (COL1A1). D. Cytokeratin 8 (KRT8). E. Glucose transporter 1 (SLC2A1). Bars indicate significant differences between groups (p<0.05).
Discussion
In this study we investigated the effects of oxygen tension on the functional biosynthetic properties of NP cells. We used a 3D agarose culture model that was previously shown to promote restoration of phenotype of NP cells following monolayer expansion, and subsequent deposition of a GAG and collagen-rich extracellular matrix at atmospheric oxygen.18 Here we extend those findings, and report the response of NP cells cultured under more physiological oxygen levels. Our results suggest that, while overall bulk mechanical and compositional properties are largely independent of oxygen levels, low oxygen tension combined with growth factor stimulation promotes improved restoration of the native NP phenotype, higher mRNA expression of key extracellular matrix and NP specific markers, and more uniform matrix elaboration throughout the construct expanse.
A number of previous studies have examined effects of oxygen tension on NP cell phenotypic stability and extracellular matrix production, utilizing a variety of culture conditions and with contrasting results.26-30 Ishihara and Urban described a bi-modal response to varying oxygen tension, with NP cell biosynthesis peaking at 5% oxygen.30 Mwale et al. found that NP cells cultured in alginate beads in serum-containing media exhibit relatively little dependence on oxygen tension with respect to both GAG synthesis and aggrecan expression. This study did note however that low oxygen tension produced a higher GAG to collagen ratio, suggesting promotion of a more NP like extracellular matrix expression profile. In contrast, in two recent studies, Feng et al. found that NP cells cultured on the surface of poly-L-lactide scaffolds in serum-containing media exhibited significantly greater mRNA expression of aggrecan and collagen II after 1 and 3 weeks, and significantly higher GAG synthesis after 3 weeks, in 2% oxygen compared to 20% oxygen.26,27 In a very recent study, and in further contrast, Naqvi et al. found that NP cells cultured in alginate in defined media exhibited lower GAG and collagen (per DNA) synthesis in 5% oxygen compared to 20% oxygen, while the GAG to collagen ratio was significantly higher for 5% oxygen.29 Contrasting results amongst these studies can most likely be attributable to variables such as media formulation, scaffold material, cell source and culture duration.
In the current study we selected culture conditions we considered well-suited to elucidate the effects of oxygen on regenerative strategies for the NP, such as growth factor therapy and tissue engineering. Under these conditions we further extended the results of previous studies by measuring, in addition to matrix synthesis and mRNA expression, functional outcome parameters and matrix distribution in long-term culture. With respect to composition and mRNA expression of key phenotypic markers, our results demonstrated both similarities and differences compared to previous studies. These included higher mRNA expression of aggrecan, collagen II, cytokeratin 8 and glucose transporter I after 42 days of culture for 2% oxygen compared to 20%, but no significant differences in GAG or collagen (per DNA) with oxygen level at either time point. The apparent disparity between mRNA expression and actual extracellular matrix elaboration suggests that co-factors required for collagen and proteoglycan assembly and elaboration may not be concomitantly upregulated. Future studies will explore this possibility.
While bulk mechanical and compositional analysis did not reveal substantial dependence on oxygen tension, histological analysis revealed oxygen-dependent differences in the pattern of extracellular matrix deposition. Specifically, for 20% oxygen, greater staining intensity (for both GAG and collagen) was present closer to the construct periphery compared to the construct center, whereas for 2% oxygen, staining intensity was more homogeneous throughout the construct expanse. A possible explanation for this may be that NP cells are initially more biosynthetically active in the presence of higher oxygen, and oxygen is more readily available to cells near the construct periphery. The consequence of this would be more robust matrix synthesis in this region in 20% oxygen. This outer layer of dense matrix may then limit subsequent diffusion of vital nutrients to inner regions of the construct, impairing matrix elaboration in those regions. Absence of a significant overall increase in DNA content suggests cell proliferation in the central regions may have also be impeded. In 2% oxygen, the absence of this outer layer of dense matrix suggests that NP cell metabolism is initially slower and more homogenous, enabling matrix elaboration to proceed uniformly through the construct expanse. Importantly, robust matrix deposition at the construct center was evident for the 2% oxygen condition after 42 days of culture despite no significant increase in DNA content and a 25% decrease in cell viability. Overall, these findings suggests that low oxygen tension maybe more appropriate than atmospheric oxygen for generating tissue-engineered NP constructs of uniform matrix distribution.
Another important variable likely to influence NP cell response to oxygen tension is the rate of re-differentiation following monolayer expansion. It has been well established that monolayer expansion of NP cells results in loss of expression of phenotypic markers such as aggrecan and collagen II, and increased expression of fibroblastic markers such as collagen I.18,31,32 Low oxygen tension has previously been shown to both enhance re-differentiation of monolayer-expanded articular cartilage chondrocytes33 and the chondrogenic differentiation potential of mesenchymal stem cells.34,35 In previous work, we showed that our agarose culture model effectively restores the native NP phenotype.18 Our results here suggest that 2% oxygen may promote more robust re-differentiation of NP cells than 20% oxygen. Specifically, after 14 days, constructs cultured in 2% oxygen exhibited a higher ratio of GAG to collagen, and lower collagen I mRNA expression compared to 20% oxygen, and after 42 days, mRNA expression of ACAN, COL2A1, KRT8 and SLC2A1 were all significantly higher for 2% oxygen. Expression of KRT8, together with other cytokeratins, and other NP markers such as cadherin 2 and sclerostin domain-containing protein 1 have been shown previously to distinguish NP cells from articular chondrocytes and annulus fibrosus cells, and likely reflects the notochordal origin of NP cells.36 While KRT8 expression was enhanced by culture in 2% oxygen, expression remained only a small fraction of native levels. In another recent study we measured KRT8 mRNA expression, as well as that of other putative NP makers KRT18, KRT19 and CDH2, following culture in hyaluronic acid hydrogels and found that expression of all four of these markers remained an order of magnitude less than that for native NP cells after long term culture.37 This suggests that additional microenvironmental mediators may be required to effectively restore expression of these molecules to native levels.
A potential limitation of this study is the fact that a high glucose media formulation was used, which may not accurately represent the nutrient-poor in vivo microenvironment of the NP, and NP cell metabolism has previously been shown to be sensitive to glucose availability.38,39 Our own previous work has highlighted the sensitivity of mesenchymal stem cells (MSCs) to glucose concentration,20 and ongoing work will investigate how glucose availability modulates the biosynthetic properties of both MSCs and NP cells in the context of regenerative therapeutics.
In summary, while our results with respect to overall bulk mechanical and compositional properties support our hypothesis that NP cell functional extracellular matrix elaboration with growth factor stimulation is largely independent of oxygen tension, low oxygen tension was important for promoting more rapid restoration of the native NP phenotype, and a more uniform matrix elaboration throughout the construct. These results support the contention that culture under physiological oxygen levels is an important consideration for future cell and growth factor-based therapeutic approaches for disc regeneration.
Acknowledgements
This project was funded by grants from the Department of Veterans Affairs (I01RX000211 and I01RX001321), and was supported by the histology and biomechanics cores of the Penn Center for Musculoskeletal Disorders (National Institutes of Health program grant P30AR050950). The authors thank Minwook Kim for performing immunohistochemistry, and have no conflicts of interest to disclose.
References
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Bogduk N. The lumbar disc and low back pain. Neurosurg Clin N Am. 1991;2:791–806. [PubMed] [Google Scholar]
- 3.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology. 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 4.Vernon-Roberts B. Disc Pathology and Disease States. In: Ghosh P, editor. The Biology of the Intervertebral Disc. CRC Press; 1988. pp. 73–119. [Google Scholar]
- 5.Le Maitre CL, Pockert A, Buttle DJ, et al. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652–655. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 6.Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965–972. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- 7.Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine. 2007;32:816–823. doi: 10.1097/01.brs.0000259225.37454.38. [DOI] [PubMed] [Google Scholar]
- 8.Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Smith LJ, Elliott DM. Formation of lamellar cross bridges in the annulus fibrosus of the intervertebral disc is a consequence of vascular regression. Matrix Biol. 2011;30:267–274. doi: 10.1016/j.matbio.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith LJ, Nerurkar NL, Choi KS, et al. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4:31–41. doi: 10.1242/dmm.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nerlich AG, Schaaf R, Walchli B, et al. Temporo-spatial distribution of blood vessels in human lumbar intervertebral discs. Eur Spine J. 2007;16:547–555. doi: 10.1007/s00586-006-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudert M, Tillmann B. Detection of lymph and blood vessels in the human intervertebral disc by histochemical and immunohistochemical methods. Ann Anat. 1993;175:237–242. doi: 10.1016/s0940-9602(11)80009-9. [DOI] [PubMed] [Google Scholar]
- 13.Grunhagen T, Shirazi-Adl A, Fairbank JC, et al. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42:465–477. vii. doi: 10.1016/j.ocl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Risbud MV, Schipani E, Shapiro IM. Hypoxic regulation of nucleus pulposus cell survival: from niche to notch. Am J Pathol. 2010;176:1577–1583. doi: 10.2353/ajpath.2010.090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrawal A, Guttapalli A, Narayan S, et al. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–631. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- 16.Rajpurohit R, Risbud MV, Ducheyne P, et al. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–407. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- 17.Risbud MV, Guttapalli A, Stokes DG, et al. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 18.Smith LJ, Chiaro JA, Nerurkar NL, et al. Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine treatment following long term agarose culture. Eur Cells Mater. 2011;20:291–301. doi: 10.22203/ecm.v022a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorth DJ, Mauck RL, Chiaro JA, et al. IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1beta-mediated degradation of nucleus pulposus in vitro. Arthritis Res Ther. 2012;14:R179. doi: 10.1186/ar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrell MJ, Shin JI, Smith LJ, et al. Functional consequences of glucose and oxygen deprivation on engineered mesenchymal stem cell-based cartilage constructs. Osteoarthritis Cartilage. 2014 doi: 10.1016/j.joca.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 22.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 23.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 24.Nimni ME. Collagen: structure, function, and metabolism in normal and fibrotic tissues. Seminars in Arthritis & Rheumatism. 1983;13:1–86. doi: 10.1016/0049-0172(83)90024-0. [DOI] [PubMed] [Google Scholar]
- 25.Farrell MJ, Fisher MB, Huang AH, et al. Functional properties of bone marrow-derived MSC-based engineered cartilage are unstable with very long-term in vitro culture. J Biomech. 2013 doi: 10.1016/j.jbiomech.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng G, Li L, Hong Y, et al. Hypoxia promotes nucleus pulposus phenotype in 3D scaffolds in vitro and in vivo. J Neurosurg Spine. 2014;21:303–309. doi: 10.3171/2014.4.SPINE13870. [DOI] [PubMed] [Google Scholar]
- 27.Feng G, Li L, Liu H, et al. Hypoxia differentially regulates human nucleus pulposus and annulus fibrosus cell extracellular matrix production in 3D scaffolds. Osteoarthritis Cartilage. 2013;21:582–588. doi: 10.1016/j.joca.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Mwale F, Ciobanu I, Giannitsios D, et al. Effect of oxygen levels on proteoglycan synthesis by intervertebral disc cells. Spine (Phila Pa 1976) 2011;36:E131–138. doi: 10.1097/BRS.0b013e3181d52b9e. [DOI] [PubMed] [Google Scholar]
- 29.Naqvi SM, Guillaume O, Buckley CT. Orthopaedic Research Society Transactions. New Orleans: 2014. Oxygen is a more potent regulator than glucose concentration for matrix production of nucleus pulposus and bone marrow derived stem cells. [Google Scholar]
- 30.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829–835. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- 31.Kluba T, Niemeyer T, Gaissmaier C, et al. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine (Phila Pa 1976) 2005;30:2743–2748. doi: 10.1097/01.brs.0000192204.89160.6d. [DOI] [PubMed] [Google Scholar]
- 32.Wang JY, Baer AE, Kraus VB, et al. Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture. Spine (Phila Pa 1976) 2001;26:1747–1751. doi: 10.1097/00007632-200108150-00003. [DOI] [PubMed] [Google Scholar]
- 33.Domm C, Schunke M, Christesen K, et al. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthritis Cartilage. 2002;10:13–22. doi: 10.1053/joca.2001.0477. [DOI] [PubMed] [Google Scholar]
- 34.Meyer EG, Buckley CT, Thorpe SD, et al. Low oxygen tension is a more potent promoter of chondrogenic differentiation than dynamic compression. J Biomech. 2010;43:2516–2523. doi: 10.1016/j.jbiomech.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Sheehy EJ, Buckley CT, Kelly DJ. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun. 2012;417:305–310. doi: 10.1016/j.bbrc.2011.11.105. [DOI] [PubMed] [Google Scholar]
- 36.Minogue BM, Richardson SM, Zeef LA, et al. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12:R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DH, Martin JT, Elliott DM, et al. Phenotypic stability, matrix elaboration and functional maturation of nucleus pulposus cells encapsulated in photocrosslinkable hyaluronic acid hydrogels. Acta Biomaterialia. 2014 doi: 10.1016/j.actbio.2014.10.030. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephan S, Johnson WE, Roberts S. The influence of nutrient supply and cell density on the growth and survival of intervertebral disc cells in 3D culture. Eur Cell Mater. 2011;22:97–108. doi: 10.22203/ecm.v022a08. [DOI] [PubMed] [Google Scholar]
- 39.Bibby SR, Jones DA, Ripley RM, et al. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976) 2005;30:487–496. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]