Planning implementing, and evaluating interventions against infectious diseases depends on the nature of the infectious disease, the availability of intervention measures as well as logistical, economic and political constraints. Infectious diseases and vaccine- or drug-based interventions can be loosely categorized by how well established the infectious disease is and how well-established the intervention is. Three examples of long-known infectious diseases where global vaccination has dramatically reduced the public health burden of disease are pertussis, polio, and measles. Pertussis vaccination was introduced in the 1940s, polio vaccination in the 1950s, and measles vaccination in the 1960s, nearly eliminating these diseases in many places.
Many known infectious diseases tend to be epidemic, but exactly when and where these epidemics will occur is uncertain. For such sporadic infectious diseases, it may be most cost-effective not to have ongoing interventions, but to wait until an outbreak occurs, and implement a reactive vaccination campaign. Planning for reactive vaccination requires keeping a mobile vaccine stockpile available that can be quickly moved for emergency vaccination. This strategy could be used for epidemic cholera, such as occurs in parts of Africa [1]. In 2010, when cholera was introduced into Haiti, such a stockpile did not exist. Limited supplies of cholera vaccine were scattered in different locations, and a decision was made not to vaccinate, even though mathematical models showed that with limited quantities of vaccine, concentrating vaccination in high-risk areas would be most efficient [2]. Now that such a stockpile of 2 million doses of oral cholera vaccine exists [3], it can be used for future cholera epidemics. Mobile stockpiles, such as the oral cholera vaccine often have a finite shelf-life, and their use can be valuably reassigned in endemic locations, such as Bangladesh that experience annual cycles of high incidence.
For long-term intervention strategies, sustainability is an issue and strategies can change as the economic constraints change or new products are available. For example, a new product against meningococcal A meningitis was developed that overturned the reactive vaccination strategy in the meningitis belt of sub-Saharan Africa. An international commitment working through the Meningitis Vaccine Project developed an inexpensive vaccine against meningitis A [4] that can be used in pro-active vaccination. Initial introduction has been carried out or is planned in 26 countries of Africa with mass vaccination of people up to the age of 29 years to be followed by routine vaccination of young children.
New emerging infectious diseases sometimes lend themselves to effective vaccination. In light of the ongoing avian influenza A (H7N9) human cases in China, the USA is preparing to stockpile vaccines for H7N9 human cases. When the pandemic influenza A (H1N1) emerged suddenly in 2009, influenza vaccine manufacturers turned their production lines to developing and producing appropriate vaccines for immediate administration. Yet for other emerging diseases like HIV/AIDS, which was discovered over 30 years ago, no effective vaccine has been successfully developed for human use. For several newly emerging infectious diseases it is questionable when or if vaccines or drugs can be developed at all. One example is Middle East Respiratory Syndrome (MERS), a viral respiratory illness caused by a coronavirus and first reported in Saudi Arabia in 2012. Another example is the arbovirus chickungunya which is spreading explosively in the Americas, with 469,620 confirmed and suspected cases reported by the Pan American Health Organization as of July 25, 2014. Although antivirals, vaccines, and monoclonal antibodies are under early development, even Anthony Fauci, Director of the National Institute of Allergy and Infectious Disease wrote that “in the meantime, we can only keep our fingers crossed” [5] that the epidemic in the Americas will decline on its own before becoming more widespread. According to [5], even if a vaccine were available, chickungunya outbreaks spread too rapidly for reactive vaccination to be effective. In the current ebola outbreak in West Africa there is no pharmaceutical intervention. For these newly emerging infectious diseases, surveillance and containment, education, and avoidance are the main responses available until such time that vaccines or drugs may be developed.
Direct and indirect effects of interventions
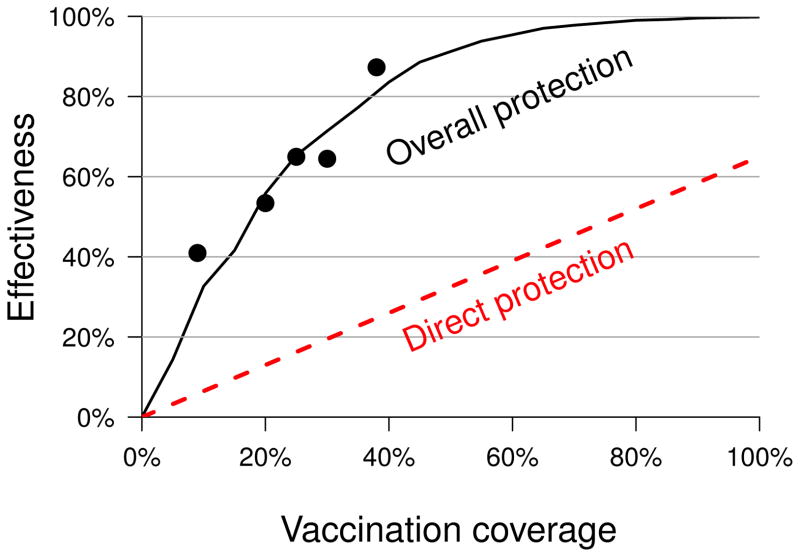
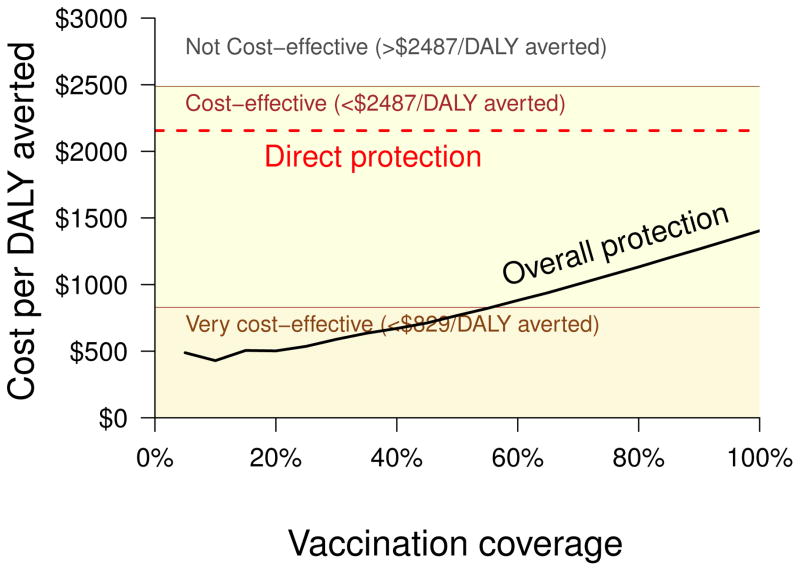
Interventions in infectious diseases can have more than just direct protective effects in the individuals receiving the intervention [6]. An intervention strategy can also have indirect protective effects in associated unvaccinated individuals. Accounting for both the direct and indirect effects will give a more accurate measure of the cost-effectiveness of a program, hence the combined direct and indirect effects need to be assessed. Dynamic mathematical transmission models offer valuable tools for planning infectious disease strategies, particularly for taking potential indirect effects into account. Especially when vaccines confer modest direct protection, the indirect and overall effects of widespread vaccination may affect the cost-effectiveness of vaccination. An example is the cholera vaccine, where indirect effects play an especially large role in the cost-effectiveness of large-scale vaccination programs. A dynamic mathematical model of cholera vaccination showed that the percent of cases expected to be averted by vaccinating different proportions of the population is much higher when both indirect and direct effects compared to just direct effects are taken into account (Figure 1) [7]. The same model showed that taking only direct protection into account, the cost per disability-adjusted life years (DALYs) averted is barely cost effective at all levels of coverage. Taking both indirect and direct protection into account, a vaccination program is much more cost effective (Figure 2).
Figure 1.
A mathematical model of cholera transmission was used to estimate the effectiveness (number of cases averted) and cost-effectiveness in disability averted life years (DALYs) when a given fraction of the population is vaccinated. The model predicts that the fraction of cases averted by mass vaccination (black solid line) exceeds the estimates when only direct protection is assumed (red dashed line). The black dots indicate levels of protection observed in a cholera vaccine trial [21]. In addition, the relationship between vaccine coverage and effectiveness is not linear when assuming overall protection, unlike direct protection [7].
Figure 2.
Mass vaccination is more cost-effective when overall protection is considered compared to calculations that only account for direct protection. According to WHO convention, the ratio of program cost to DALYs Averted in a cost-effectiveness analysis is classified by the per capita national gross domestic product (GDP) of the country of interest: less than GDP/capita classifies an intervention as very cost-effective; between 1 and 3 times GDP/capita is cost-effective; and more than 3 times the GDP/capita is cost-ineffective [7, 22].
However, reliable mathematical models depend on good epidemiological data. Often the data required to inform the models are not collected in field trials. For example, in most vaccine field trials, cases are ascertained based on clinical symptoms, then the specific infectious agent is confirmed by a laboratory test, so efficacy against clinical disease is determined. But much more information about how vaccination affects transmission is needed for the mathematical models. ascertain on Data is needed on the effect of vaccination on asymptomatic infection and the degree to which infectiousness is reduced in of breakthrough infections. Whether the vaccine protects some people completely and others not at all, or whether it reduces the probability of becoming infected in everyone equally has important population-level consequences. The time of infection, the onset and end of infectiousness and its temporal variability, as well as contact patterns are seldom measured, but are important influences on the outcomes of simulated interventions. Uncertainty regarding these important inputs results in uncertainty of the potential epidemiological effects and cost-effectiveness of a vaccination program. Policy makers are increasingly asking for mathematical models to aid in decision making. Moving forward, vaccine studies need to include plans to measure as many of these inputs as possible for well-informed mathematical modeling, as pointed out already 20 years ago [8].
As vaccines against dengue [9] and malaria [10] become available, models can help understand how these emerging vaccines might be most effectively utilized. In a phase 3 trial, the new Sanofi Pasteur dengue vaccine has been shown to be effective against clinical dengue, and twice as effective in individuals with preexposure to natural dengue [9], providing an important input for modeling vaccination in populations with different levels of dengue exposure. However, the trial does not provide information on whether the vaccine protects against infection, or just disease, or whether it reduces the infectiousness of infected individuals for mosquitoes. The exact nature of the interaction of vaccine induced immunity with preexisting immunity and exposure to infection is also not well understood and can effect the interpretation of the vaccine effects [11]. All of these inputs are necessary to model the potential population level effects and cost-effectiveness of dengue vaccination programs in different populations.
Evaluation
In addition to modeling indirect and overall effects of vaccination, such effects of widespread vaccination can be assessed by appropriate field studies and surveillance. Either randomized or observational studies can provide assessment of indirect effects and overall effects of intervention programs. For example, studies of cholera [12] and typhoid [13] vaccination, both of which confer only modest direct protection, have been conducted to evaluate the indirect effects of widespread vaccination. Plans for evaluation should be an integral part of implementing a vaccination strategy. For example, before introducing the meningitis A vaccine in Africa, the capacity to evaluate the indirect and overall effects of the strategy on nasopharyngeal carriage was established in several countries by the MenAfriCar project. But studies can also be undertaken after introducing vaccination. A study evaluating direct, indirect, total and overall effectiveness of rotavirus vaccines in the USA using insurance data compared the pre-vaccination baseline years with the years after vaccination [14]. The study revealed important indirect effects that need to be taken into account when estimating the sustained impact of the vaccination program. Delivery of a new dengue vaccine would offer the opportunity for a well-planned phased introduction to evaluate not only the direct effect of vaccination, but also the indirect and overall effects. In all instances, good field epidemiology and surveillance are required.
Changing fronts
Even when a good intervention is available, problems can arise, requiring ongoing careful epidemiological assessment of apparently excellent interventions. Interventions can put selective evolutionary pressure on pathogens resulting in drug resistance and immune escape. The emergence of drug resistant strains such as methycillin-resistant Staphylococcus aureus (MRSA), multi-drug-resistant tuberculosis (MDR-TB), and drug-resistant malaria parasites are the result of evolutionary pressure. The USA is experiencing a resurgence of pertussis cases despite high coverage and booster vaccination of teenagers. The reasons are poorly understood, but it is possible the current acellular pertussis vaccines do not produce long lasting immunity [15]. In addition, pertussis strains that lack one of the important vaccine antigens have evolved because of immune escape and spread in recent years [16]. Careful epidemiological assessment of the changing pertussis situation is required to guide development of new vaccines and intervention strategies [17].
Influenza viruses evolve antigenically over time, escaping both natural and vaccine-induced immunity. Challenges are faced in producing an influenza vaccine each year, and constant monitoring of the influenza viruses is needed. Global influenza virological surveillance has been conducted through World Health Organization’s (WHO) Global Influenza Surveillance and Response System for over half a century. Twice a year, once for the northern hemisphere, and once for the southern hemisphere, a decision is made by the advisory panel on what viruses will be incorporated in the next seasonal influenza vaccine [18].
Although global polio vaccination has eliminated polio disease in many parts of the world, choosing between live-attenuated oral polio vaccine (OPV) and inactivated polio vaccine (IPV) is a crucial policy decision once elimination has been achieved. OPV is less expensive, easy to administer, can be transmitted to close contacts and in the environment helping to spread vaccination, induces more natural immunity in the gut, preventing onward transmission, but can revert causing very occasional polio cases. IPV is more expensive, produces antibodies that can prevent paralytic disease, but does not induce gut immunity, thus does not prevent onward transmission, but it also does not have the same safety considerations. When polio has been essentially eliminated and the vaccine is affordable, then the switch to IPV from OPV is a sensible policy decision. However, this can also be fraught with difficulties. Israel switched to IPV for nine years, but through intense environmental surveillance discovered circulation of type 1 wild poliovirus, and reintroduced OPV [19, 20]. The excellent environmental surveillance made possible the rapid response to the changing situation.
The changing landscape of infectious diseases and interventions poses challenges. Focused field epidemiology and surveillance are needed to provide the information required to make informed policy decisions about interventions to further global public health.
Acknowledgments
The authors are funded by grants from: NIH/NIAID R37-AI032042 and NIH/NIGMS U01-GM070749. Christopher Troeger and Dennis Chao kindly provided figures 1 and 2.
References
- 1.Azman A, et al. PLoS Negl Trop Dis. 2012;6:e1901. doi: 10.1371/journal.pntd.0001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao D, Halloran M, Longini I., Jr Proceedings of the National Academy of Sciences. 2011;108:7081–5. doi: 10.1073/pnas.1102149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [Accessed: July 31, 2014];Oral cholera vaccine stockpile. http://www.who.int/cholera/vaccines/ocvstockpile2013/en/
- 4.LaForce F, Konde K, Viviani S, Préziosi M. Vaccine. 2007;3(Suppl1):A97–100. doi: 10.1016/j.vaccine.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Morens D, Fauci A. New England Journal of Medicine. 2014 doi: 10.1056/NEJMp1408509. [DOI] [Google Scholar]
- 6.Halloran M, Longini I, Struchiner C. Design and Analysis of Vaccine Studies. Springer; New York: 2010. [Google Scholar]
- 7.Troeger C, Sack D, Chao D. American Journal of Tropical Medicine and Hygiene. 2014 under revision. [Google Scholar]
- 8.Halloran M, Cochi S, Lieu T, Wharton M, Fehrs L. Am J Epidemiol. 1994a;140:81–104. doi: 10.1093/oxfordjournals.aje.a117238. [DOI] [PubMed] [Google Scholar]
- 9.Capeding MR, Tran NH, Hadinegoro SR, et al. Lancet. 2014 doi: 10.1016/50140.6736(14)61060.6. [DOI] [PubMed] [Google Scholar]
- 10.Agnandji S, Lell B, Soulanoudjingar S, et al. N Eng J Med. 2011;365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 11.Struchiner C, Halloran M. Epidemiol Infect. 2007;135:181–194. doi: 10.1017/S0950268806006716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali M, et al. Lancet. 2005;366:44–49. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- 13.Sur D, et al. N Engl J Med. 2009;361:335–44. doi: 10.1056/NEJMoa0807521. [DOI] [PubMed] [Google Scholar]
- 14.Panozzo C, et al. American Journal of Epidemiology. 2014:x. doi: 10.1093/aje/kwu001. [DOI] [Google Scholar]
- 15.Tartof S, Lewis M, Kenyon C, et al. Pediatrics. 2013 doi: 10.1542/peds.2012-1928. [DOI] [PubMed] [Google Scholar]
- 16.Pawloski L, Queenan A, Cassiday P, et al. Clinical and Vaccine Immunology. 2014;21:119–125. doi: 10.1128/CVI.00717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson D, Rohani P. Epidemiol Infect. 2013;16:1–13. doi: 10.1017/S0950268812003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klimov A, et al. Vaccine. 2012;30:6461–6471. doi: 10.1016/j.vaccine.2012.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anis E, Kopel E, Singer S, et al. Euro Surveillance. 2013;18:pli=20586. doi: 10.2807/1560-7917.es2013.18.38.20586. [DOI] [PubMed] [Google Scholar]
- 20.Shulman L, Gavrilin E, Jorba J, et al. Euro Surveillance. 2014;19:pii=20709. doi: 10.2807/1560-7917.es2014.19.7.20709. [DOI] [PubMed] [Google Scholar]
- 21.Longini I, et al. PLoS Med. 2007b;4:e336. doi: 10.1371/journal.pmed.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Vaccine introduction guidelines: adding a vaccine to a national immunization programme: decision and implementation. 2005 [Google Scholar]