Abstract
Tendinopathies are commonly attributable to accumulation of sub-rupture fatigue damage from repetitive use. Data is limited to late stage disease from patients undergoing surgery, motivating development of animal models, such as ones utilizing treadmill running or repetitive reaching, to investigate the progression of tendinopathies. We developed an in vivo model using the rat patellar tendon that allows control of the loading directly applied to the tendon. This manuscript discusses the response of tendons to fatigue loading and applications of our model. Briefly, the fatigue life of the tendon was used to define low, moderate and high levels of fatigue loading. Morphological assessment showed a progression from mild kinks to fiber disruption, for low to high level fatigue loading. Collagen expression, 1 and 3 days post loading, showed more modest changes for low and moderate than high level fatigue loading. Protein and mRNA expression of Ineterleukin-1β and MMP-13 were upregulated for moderate but not low level fatigue loading. Moderate level (7200 cycles) and 100 cycles of fatigue loading resulted in a catabolic and anabolic molecular profile respectively, at both 1 and 7 days post loading. Results suggest unique mechanisms for different levels of fatigue loading that are distinct from laceration.
Keywords: Tendinopathy, Tendon Fatigue, Damage Accumulation, Second Harmonic Generation, Animal Model
Introduction
Tendinopathies leading to tendon rupture are common musculoskeletal injuries that are a significant source of disability. Clinical data suggests that the rotator cuff, Achilles and patellar tendons are most prone to tendinopathies1. Interestingly, these tendons are commonly engaged in repetitive loading or overuse amongst both, the athletic and non-athletic population2–4, providing evidence that the development of tendinopathy is closely associated with repetitive loading. For instance, the incidence of rotator cuff tears has been shown to increase with increase in age5 and is significantly higher in the dominant than non-dominant shoulder of elite overhead young athletes6. Similarly, patellar tendinopathy is common in sports that are characterized by repetitive jumping, such as volleyball and basketball7, and in military recruits8. Lastly, although Achilles tendon rupture may appear to be a sudden event, degenerative changes are commonly observed in ruptured tendon, supporting a pathology characterized by accumulation of damage9.
Despite the frequency of these tendon injuries, clinical management and treatment is limited by the scarcity of data on the progressive nature from early to late stage of tendinopathies. For instance, much of the data regarding tendinopathy stems from tendon biopsies, representing end-stage pathology10,11. However, an understanding of the progressive nature of tendon response to repetitive loading is necessary since the molecular, mechanical and structural response continuously changes as the loading history of the tendon is altered. In addition, while the development of tendinopathy is commonly associated with overuse injuries, some amount of exercise has been shown to result in improved tendon properties12. However, an understanding of the types and frequency of mechanical loading that can cause tendon damage or alternatively, improve the tendon’s ability to withstand loading is unclear.
Several investigators developed animal models to evaluate the response of tendons to overuse or repetitive loading. For instance, overuse injuries in the rotator cuff have been induced by subjecting rats to treadmill running13. Similarly, rats trained to perform repetitive reaching and grasping showed diminished motor performance, supporting the use of this model to induce tendon injury14. However, a limitation to these models is that the amount of load directly applied to the tendon cannot be controlled, and is likely variable between animals as a result of natural variations in gait, anatomical size or strength and temperament.
To address the knowledge gap regarding the progression in tendon response to cyclic loading, we have developed and extensively utilized an in vivo model of tendon fatigue damage accumulation using the rat patellar tendon15. Using this model, we have characterized the progression in fatigue life of the tendon during cyclic loading and found that the tendon has a unique response molecularly, mechanically and structurally that changes with different amounts of applied fatigue loading15–18. For instance, by comparing the response of a lacerated tendon, moderate level fatigue loaded tendon and a low level fatigue loaded tendon, we found that fatigue loading induces an entirely different biologic response than laceration15,17, and than nominal levels of cyclic loading (representative of exercise)17. We have utilized a multidisciplinary approach to assess the mechanical, structural, cellular and molecular changes in the tendon, to evaluate the relationship between tendon damage accumulation, and the resulting matrix damage, molecular and mechanical response.
In this manuscript, we will discuss some of our previously published work to review the main findings regarding the tendon’s response to fatigue loading. We will also introduce some of the ongoing work utilizing the patellar tendon in vivo fatigue loading model to understand damage accumulation and repair.
Patellar tendon model of fatigue damage accumulation
Some of the challenges to developing a model of damage accumulation wherein tendons can be fatigue loaded with control in survival studies, is that the tendon must be easily accessible and must not be damaged from clamping by any instrumentation. The FDL, Achilles, supraspinatus, tail and patellar tendons were all initially evaluated as potential candidates for our in vivo fatigue loading model15. The patellar tendon was chosen as the ideal candidate because it is a superficial tendon that is easily accessible and can be directly loaded without direct instrumentation with the tendon. For instance, by clamping the tibia to a fixed base and clamping the patella to a load cell, the patellar tendon can be directly loaded without direct tendon damage from the clamps. In addition, the patellar tendon commonly exhibits tendinopathy in humans (jumper’s knee)7,8,19, further justifying the use of this tendon as the site for development of our in vivo fatigue damage model.
After evaluating mice, rats, rabbits and dogs, the rat was chosen for our model because it is a small animal that has previously been extensively used to investigate tendon biomechanics but is sufficiently large for investigation of local, region-specific effects of damage accumulation in future studies15.
Experimental setup
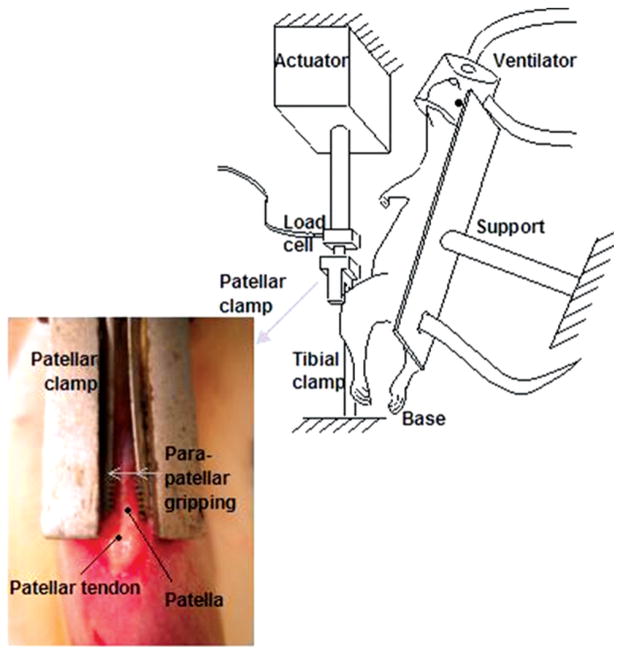
As previously described by Fung et al., to induce fatigue damage in the tendon rats were anesthetized throughout the experiment with isoflurane (2–3% by volume, 0.4 L/minute) and their left patellar tendons were surgically exposed for fatigue loading15. The experimental setup is shown in Figure 1. Under aseptic conditions, a clamp was used to fix the tibia at ~30° knee flexion. A custom clamp was then used to grip the patella and connect it to a 50-lb load cell and actuator of a servo-hydraulic loading system, allowing loading of the patellar tendon without direct contact with the tendon15.
Figure 1.
Experimental setup from Fung et al15. Rats are anesthetized and fixed onto a support. The patella and the tibia are exposed and clamped to connect the patella to a load cell and fix the tibia to a base allowing tendon loading without direct instrumentation with the tendon.
The previously described fatigue loading protocol is shown in Figure 215. Briefly, a diagnostic test was applied before fatigue loading (Diag1) to assess the initial state of the tendon. Fatigue loading was then applied for x cycles that typically ranged from 1N to 40% of the ultimate load of the tendon. Ultimate load was defined from preliminary monotonic load to failure experiments as the highest load reached prior to onset of failure as indicated by inability to reach a higher load level despite further applied loading. The number of fatigue loading cycles (x) was guided by our understanding of the amount of damage induced in the tendon throughout the fatigue life (refer to the sub-section on ‘Assessment of mechanical parameters’ for details regarding the characterization of the fatigue life of the tendon) and was determined for each experiment by the scientific question being evaluated A second diagnostic test (Diag2) was then applied immediately post fatigue loading to assess the initial combined recoverable and non-recoverable effect of fatigue loading. The tendon was allowed to recover for 45 minutes (the longest amount of time possible in conjunction with our longest fatigue loading protocol that satisfied IACUC constraints), and then a final diagnostic test (Diag3) was applied to deduce the non-recoverable initial damage. Diagnostic tests typically ranged from 1N to 15% of the ultimate load. All data discussed in this manuscript was collected using a loading frequency of 1Hz. The tendon was continuously moistened with phosphate buffered saline throughout the experiment.
Figure 2.
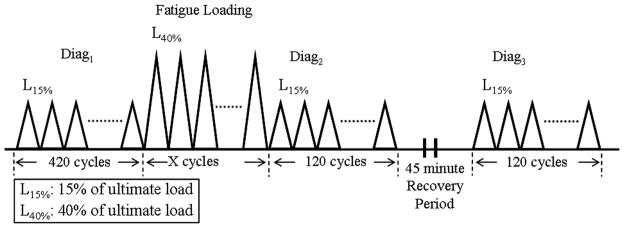
Fatigue loading protocol. Diagnostic tests are applied before (Diag1), immediately after (Diag2) and 45 minutes (Diag3) after fatigue loading.
Clamps were then removed and skin incisions were sutured with 6-0 prolene. Analgesia (Buprenex) was administered and the animal resumed cage activity.
Multi-disciplinary approach for evaluation of tendon damage and repair
Tendons are complex structures that exhibit a close relationship between structural changes and a molecular response through mechanotransduction. Accumulation of damage is expected to ultimately compromise tendon function and mechanical strength. It is likely that as damage is accumulated, structural damage alters the loading environment of the cells resulting in extracellular matrix changes that lead to changes in tendon function. It is unclear whether increase in amount of damage accumulation induces different levels of one mechanism or induces entirely different mechanisms. A comprehensive assessment that includes molecular, mechanical and structural evaluation of the effect of damage accumulation on the tendon is necessary.
Assessment of molecular changes in response to fatigue loading
Several studies have evaluated the biologic healing response of tendons to laceration20,21. It was expected that the typical healing response that includes overt inflammation may only occur in response to fiber disruption, and therefore was unlikely to occur with sub-rupture damage accumulation until fiber disruption occurred. In addition, tissue biopsies representing late stage tendinopathies provide insight into late stage but not early stage disease leaving little known about the biologic response to early fatigue damage accumulation.
Using our model, we have begun to investigate the progressive nature of the response of the tendon to damage accumulation15,17,18. Since the role of inflammation in chronic tendinopathy is of much debate, studies by Sun et al. evaluated the mRNA and protein expression and of Interleukin-1β (IL-1β) and Matrix Metalloproteinase (MMP)-13, 1 and 3 days post low and moderate level fatigue loading18. Results showed a similar trend for both genes at both timepoints18. For instance, 1 and 3 days post fatigue loading, low fatigue loaded tendons exhibited suppressed expression of MMP-13 and IL-1β by 70%18. Similarly, at both timepoints, moderate fatigue loaded tendons expressed a 5–6 fold upregulation of MMP-13 and IL-1β18. Changes in mRNA expression were always paralleled by changes in protein expression, as confirmed by western blots18.
Fung et al. utilized real time PCR to evaluate early phase (1 and 3 days) mRNA expression of Collagen (Col)-I, Col-III and Col-V of tendons in response to low, moderate and high level fatigue15. Small but significant changes were observed following low and moderate level fatigue loading but significant large changes were observed following high level fatigue loading at both time-points15. More specifically, in comparison to sham controls (animals that experienced identical surgical protocol without fatigue loading), Col-I was slightly downregulated at 1 (1.8 fold) and 3 days (1.5 fold) following low level fatigue loading and was unchanged following moderate level fatigue loading but was highly upregulated at both time points following high level fatigue loading15. Similarly, 1 day post low and moderate level fatigue loading, Col-III was slightly upregulated (2.8 and 4.0 fold respectively) with no difference observed following high level fatigue loading15. At 3 days post fatigue loading, no difference was observed following low and a modest decrease (2.9 fold) was observed following moderate level fatigue loading, but a significant increase was observed for high level damage (9.5 fold). Lastly, at 1 day post low and moderate level fatigue loading, Col-V was upregulated by approximately 3.5 fold, and then by 3.2 and 5.8 fold at 3 day post fatigue loading15. In contrast, a much higher upregulation of 16 and 21 fold was observed for high level fatigue loading at 1 and 3 days respectively15.
Finally, mRNA expression was profiled for select Collagens, MMPs and their inhibitor (TIMPs), 1 and 7 days post 100 (representative of exercise) and 7200 cycles (falls within the range of moderate level fatigue loading) fatigue loading17. The trends observed for Col-I, Col-III and Col-V for 7200 cycles 7 days post fatigue loading were similar to those observed for moderate level fatigue loading as described by Fung et al15. A hypertrophic response, indicative of tendon adaptation was observed for 100 cycles of fatigue loading, as demonstrated by upregulation of MMP-3 (critical to tendon remodeling), MMP-13 and 14 (necessary for degradation of damaged matrix and remodeling), Col-I, and Col-XII17. In contrast, at 7200 cycles, a catabolic response was observed that was denoted by up-regulation of Col-III and Col-V but downregulation of Col-I17. The ratio of mRNA expression of Col-III to Col-I increased from 3:10 to 9:10 for 100 to 7200 cycles of fatigue loading17. A high ratio of Col-III to Col-I is expected in tendinopathy and may be reflective of poor matrix organization22. In addition, no significant change in TIMP activity was observed for 100 cycle fatigue loading, while TIMP-1 and 2 were upregulated, and TIMP-4 was downregulated for 7200 cycle fatigue loading, reflective of altered homeostasis17.
Assessment of mechanical parameters
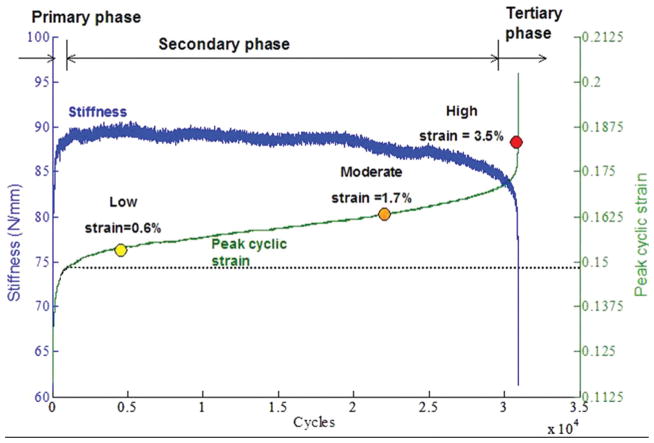
To evaluate the immediate effect of fatigue loading on the load bearing capacity of tendons, real time measures were determined from the fatigue loading curves15. Fung et al. showed that during fatigue loading to failure, peak cyclic strain ((dmax−do)/Lo where dmax: actuator position at maximum load, do: initial actuator position, Lo: initial tendon length), followed 3 phases (Figure 3)15. During the primary phase, an increase in peak cyclic strain and stiffness was observed15. During the secondary phase, the rate of peak cyclic strain increase decreased significantly from that experienced during the primary phase15. Concurrently, no change in stiffness was induced during the secondary phase. Finally, a steep rate of peak cyclic strain increase and stiffness decrease was induced in the tertiary phase15.
Figure 3.
Representative fatigue life of the tendon adapted from Fung et al15. Stiffness and peak cyclic strain followed 3 phases. Low, moderate and high level fatigue loading were defined by a peak cyclic strain of 0.6% (Low), 1.7% (Moderate) and 3.5% (High) beyond initial measurement determined at cycle 500 (end of the primary phase).
Baseline hysteresis and tangent stiffness were compared at an initial cycle (cycle 15) and endpoint cycle during fatigue loading to assess the effect of fatigue loading15. Hystersis was defined as the area between the loading and unloading curves15. Interestingly, at the end of the fatigue loading protocol, a significant increase in stiffness (~18%) was observed for low and moderate level fatigue loading but a significant decrease was observed for high level fatigue loading (~18%)15. Similarly, a significant decrease in hysteresis was observed for low and moderate level fatigue loading (~30%), but an increase was observed for high level fatigue loading (~25%)15.
In addition to real time measures of induced damage deduced from the fatigue loading portion of the curve, current work has separated the recoverable and non-recoverable changes by first calculating the average of certain mechanical parameters from the last 10 cycles of each diagnostic test and comparing these average values between diagnostics23,24. Parameters that are being evaluated include tendon elongation, hysteresis and stiffness. Comparing the value of any mechanical parameter between Diag1 (pre-fatigue) and Diag3 (post-recovery) reflects the non-recoverable changes in the tendon. Similarly, comparing the value of any mechanical parameter between Diag2 (post-fatigue) and Diag3 (post-recovery) reflects the recoverable damage in the tendon. Whether these parameters can serve as indices of damage and can predict the molecular and mechanical response of the tendon over time is being evaluated in current work. These measures, evaluated over a time course, can be used to determine whether tendons recover, further degenerate or are slow to experience any change over time.
Structural analysis of tendon damage
Tendons primarily function in tension and their tensile properties are predominantly attributable to their high content of Col-I. Several investigators have shown that Col-I content is directly affected by tendinopathy10 and exercise25 emphasizing the importance of inclusion of collagen analysis in assessment of tendon damage. In addition, since damage is not expected to accumulate uniformly in the tendon26,27, it is expected that damaged and undamaged regions will exhibit a different cellular response. Therefore, an ability to visualize tendon structural damage, with the long term goal of correlating it with protein expression and mechanical function is integral to understanding the response of tendons to fatigue loading.
Conventional histological techniques utilizing thin paraffin or plastic sections with polarized light, bright-field, scanning electron and confocal microscopy provide insight into collagen structure. To better retain tendon morphology and minimize thin section cutting artifacts, Laudier et al. developed a novel procedure for tendon histology that utilized Methyl Methacrylate (MMA) instead of the typically used paraffin28. Further advancement was then made in visualization of collagen structure damage by extending the application of second harmonic generation (SHG) imaging for use with tendon embedded in thick plastic (MMA) sections16. SHG imaging allows visualization of collagen microstructure by invoking the second-order optical property of collagen using a laser tuned to the near-infrared range. The ability of this imaging modality to penetrate into the depth of the tissue allows evaluation of tendon structure through thick plastic sections (~40 thick), minimizing the effect of sectioning artifact16. In addition, current work has expanded utilization of SHG imaging for tendon structure visualization on non-fixed mouse patellar tendons and rat tail tendons, extending the potential application of this technique to in vivo studies29.
Analysis techniques to visualize tendon structural changes have been developed to analyze SHG images16,30. As described previously, using Fast Fourier Transform based analysis (FFT), a method indicative of the disorganization of the entire tendon was first developed16. Briefly, FFT was performed on the entire image, to convert it from spatial to frequency domain. It was expected that fiber disorganization that results form fatigue damage would be reflected in changes in the frequency components16. A graphical representation of the distribution of the frequency components was obtained from a plot of the power spectrum (amplitude of the transform equation)16. The field of view (FOV) was divided into 30 × 30 pixel windows, and a power spectrum was generated for each window16. The distribution of angular deviations from the principal axis of the tendon was graphed and served as an indicator of overall image anisotropy16. As expected, results showed greater anisotropy with each level of increased damage16. Building on this work, quantification of structural damage was further advanced through the development of a method to quantify the damage area fraction (DAF, the fractional area of a 2D SHG image of tendon determined to be damaged)30. First, manual analysis was conducted by a trained blinded user and confirmed that DAF increases with increased level of fatigue loading30. Since manual image analysis is time intensive and subject to user variability, development of an automated method became clearly valuable. In this method developed by Sereysky et al., Fourier transform was calculated, its power spectrum (ps) was plotted, and an ellipse was fitted to the filtered power spectrum30. The orientation of the collagen fibers within each image subsection was determined from the short axis of the fitted ellipse30. The two user-defined input variables, image subsection size and fiber orientation angle difference necessary for two adjacent subsections to be considered damaged were then adjusted to optimize the correlation between manual and automated analysis. Results showed that damage measured manually and automatically colocalized 78±13% of the time, supporting the use of this automated method30.
Qualitative assessment of SHG images of the tendon mid-substance showed discernible differences in images representative of different levels of damage (Figure 4)15. More specifically, control tendons exhibited aligned collagen fibers without matrix disruption whereas fatigue loaded tendons exhibited progressive structural damage. Tendons that were loaded to low level fatigue exhibited isolated fiber kinks that were more abundant for moderate level fatigue loaded tendons15. Moderate level fatigue loaded tendons also exhibited widening of interfiber space15. High level fatigue loaded tendons exhibited severe matrix disruption15. The qualitatively observed manifestation of structural damage was reflected in our automated methods16,30.
Figure 4.
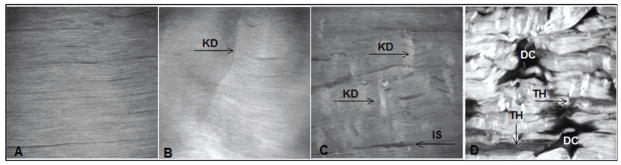
Adapted from Fung et al.15 fatigue loading to increasing levels of fatigue loading resulted in discernibly progressive changes in tendon structure. (A) Control, non-fatigue loaded tendons, exhibited aligned collagen fibrils. (B) Low level fatigue loaded tendons exhibited kinked fiber deformations (KD). (C) Moderate level fatigue loaded tendons exhibited kinked fiber deformations with widening of the inter fiber space (IS). (D) High level fatigue loaded tendons exhibited severe matrix disruption with fiber thinning (TH) and matrix discontinuities (DC). FOV=400 mm.
Discussion
Despite the prevalence of tendinopathy, its clinical management is limited by the scarcity of information about progression from the early, more clinically manageable stage, to the late, less manageable stage. An in vivo model of tendon fatigue loading that allows for accurate control over the input experimental parameters without damage to the tendon from the experimental setup has tremendous potential applications for the study of progression of tendon damage. In this manuscript, we reviewed early applications using this model. Studies were conducted to characterize the fatigue life of the tendon. In agreement with findings from ex vivo evaluation, during fatigue loading to failure, creep elongation and secant stiffness exhibited 3 distinct phases15,31. The steep increase in peak elongation during the primary phase is likely attributable to a complex combination of fibers uncrimping, and others being elastically loaded while water is being exuded. We expect that in the secondary phase, most fibers are elastically responding, accounting for the slow increase in peak elongation. Finally, in the third phase, overt fiber rupture (decrease in number of load bearing fibers) results in steep increases in creep elongation. Evaluation of low, moderate and high level fatigue loading supports a progressive nature to damage accumulation, evidenced by morphological and molecular changes.
It is unclear how early in the fatigue life the tendon begins to accumulate damage. Although there is an established association between prolonged, strenuous exercise and the development of tendinopathies32, exercise has also been shown to improve the ability of the tendon to withstand loads. For instance, eccentric calf muscle training has been shown to increase Achilles tendon stiffness33, a mechanical property that typically decreases with the development of tendinopathy34. The increase in tendon stiffness observed with exercise likely results in decreased strain in response to loading, reducing the risk of tendon injury35. In contrast, increased strains have been measured in tendons of patients suffering from tendinopathy36, suggesting that they may be at increased risk of further injury. Other studies have shown that exercise increases vascularization, upregulates vascular endothelial growth factor (VEGF) and increases cellular proliferation37–40. Comparison of concentric and eccentric running showed upregulation of angiogenic factors for both groups, with earlier downregulation in eccentric loading, which is likely more clinically favorable to avoid scar tissue build-up39. Using our in vivo model of fatigue damage accumulation, we compared the molecular profile of 100 cycles (representative of exercise) and moderate level fatigue loading (7200 cycles), and found an anabolic response to 100 cycles, and a catabolic response to 7200 cycles17. These data suggest that there is a threshold of loading frequency and magnitude that once overcome, changes the response of the tendon to loading from beneficial to degenerative. In addition, current work is evaluating whether exercise (treadmill running) can protect a tendon from accumulating damage or improve the functional ability of fatigue damaged tendons. Preliminary data showed a distinctly different response to exercise for fatigue damaged tendons than healthy tendons, suggesting that a short bout of exercise can induce an adaptive response and improve overall function of fatigue damaged tendons41. An understanding of the types and frequency of mechanical loading that results in tendon damage or improve the tendon’s functional ability is unclear. In addition, while it is expected that the tendon’s attempt to repair may be more successful with lower than higher amounts of accumulated damage, data to establish these relationships is scarce and is the focus of our ongoing work.
The unexpected increase in stiffness and decrease in hysteresis that was observed for low and moderate levels of fatigue loading is likely attributable to redistribution of loads from non-load bearing damaged fibers to undamaged fibers15. It is not until high level fatigue loading where the majority of load bearing fibers are damaged that the expected stiffness loss and increase in hysteresis are observed. In our previous studies, we have evaluated the bulk tissue molecular response and concluded that molecular response for low and moderate level fatigue loading significantly differed from that of 100 cycle (representative of exercise), high level fatigue loading, and laceration. It remains unclear how the bulk tissue response relates to the local tissue response. For instance, cells in damaged regions of the matrix may all express the same genes with the difference in observed molecular profiles associated with different levels of fatigue loading being attributed to the difference in number of cells in damaged regions. Alternatively, the difference in profile observed between different levels of damage may be attributable to a different cellular response associated with different severities of matrix changes. The relationship between matrix damage and cellular response in the context of damage accumulation and repair is being further evaluated in current work.
Inflammation is known to be a key component of the tendon healing response to laceration. However, the role of inflammation in tendinopathy is far from established and is continuously debated. The commonly reported absence of inflammatory cells in tendons from patients suffering from late stage tendinopathy42 has led many investigators to discount inflammation as a component of chronic tendinopathy. Millar et al. found inflammatory cells in intact subscapularis tendons exhibiting early to moderate tendinopathy in patients with supraspinatus tendon tears.
Interestingly, in these patients, the severe degenerative changes associated with the torn supraspinatus tendon were associated with a much lower presence of inflammation than was observed in the intact subscapularis43. While inflammatory cell infiltration may not be associated with late stage tendinopathy, the presence of molecular mediators of inflammation in early tendinopathy may implicate its role in the progression of the disease. We found that inducing moderate level fatigue resulted in a 5–6 fold upregulation of IL-1β, a pro-inflammatory cytokine, that is paralleled by a similar increase in MMP-1318. Archambault et al. found an increase in MMP activity to result from introducing exogenous IL-1β or stretch, with a greater effect due to the combination of both, IL-1β and stretch44. These findings suggest that upregulation of IL-12 that results in response to moderate level fatigue loading may initiate a cascade of molecular inflammation that results in stimulation of MMPs leading to matrix degeneration. Interestingly, a suppressed expression of MMP-13 and IL-1β was observed for low fatigue loaded tendons18. Results suggest that a certain amount of accumulated damage is necessary to induce molecular inflammation, which once induced, may play a role in altering the response of the tendon to further loading.
Our model of fatigue damage accumulation allows us to induce injury from fatigue loading that results in degenerative changes, as is commonly seen in tendinopathy. Our model is specifically conducive for investigation of early stage tendinopathy; the area of tendinopathy research exhibiting the greatest knowledge gap. While our current applications of the model have focused on fatigue loading for 1 bout, the model can be used to apply multiple bouts of fatigue loading provided that the animal is given sufficient recovery time from surgery between bouts. Since skin incisions are made each time fatigue loading is applied, a finite number of separate bouts will ultimately be ethically possible. The loading frequencies and loading force chosen can be adjusted in our model to mirror different clinical scenarios that are expected to lead to development of tendinopathy. For instance, applying 1 bout of moderate level fatigue loading simulates a very important and common clinical scenario such as the effect of 1 episode of intense military training, or 1 session of very intense exercise. Per our goals, our model allows us to investigate the development of early stage tendinopathy and exhibits some limitations in its application for long term chronic tendinopathy.
Futures studies will investigate the underlying cellular mechanisms associated with the distinctly different responses to different amounts of accumulated fatigue loading. The studies described in this manuscript focused on the early in vivo response to different levels of fatigue loading by evaluating the molecular response of the tendon 1, 3, and 7 days post fatigue loading. Current studies are evaluating the molecular, mechanical and structural response of the tendon up to 6 weeks post fatigue loading to determine if fatigue loaded tendons repair or further degenerate overtime. In addition, to investigating the mechanisms of development and progression of tendon sub-rupture damage accumulation, future studies will investigate the ability of surgically repaired fatigue loaded tendons to heal, mimicking the confounding degeneration observed in conjunction with a clinical tendon tear. Finally, we have recently developed a mouse model of in vivo fatigue damage accumulation that parallels our model in the rat to investigate the effect of variation in genetic background on the tendon’s response to fatigue loading45. By comparing inbred genetic strains or mice with specific genes knocked out, insight will be gained into the underlying molecular mechanisms associated with the biological response to damage accumulation.
Acknowledgments
The authors acknowledge Dr. David Fung, Dr. Herb Sun, Dr. Karl Jepsen, Jedd Sereysky, Stephen Ros, Daniel Leong, and Damien Laudier for their contributions.
Footnotes
The authors have no conflict of interest.
References
- 1.Abate M, Gravare Silbernagel K, Siljeholm C, et al. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther. 2009;11:235. doi: 10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- 3.Maffulli N, Testa V, Capasso G, et al. Similar histopathological picture in males with Achilles and patellar tendinopathy. Med Sci Sports Exerc. 2004;36:1470–1475. doi: 10.1249/01.mss.0000139895.94846.8d. [DOI] [PubMed] [Google Scholar]
- 4.Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37:1855–1867. doi: 10.1177/0363546508324283. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi K, Ditsios K, Middleton WD, et al. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699–1704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 6.Connor PM, Banks DM, Tyson AB, et al. Magnetic resonance imaging of the asymptomatic shoulder of overhead athletes: a 5-year follow-up study. Am J Sports Med. 2003;31:724–727. doi: 10.1177/03635465030310051501. [DOI] [PubMed] [Google Scholar]
- 7.Tiemessen IJ, Kuijer PP, Hulshof CT, Frings-Dresen MH. Risk factors for developing jumper’s knee in sport and occupation: a review. BMC Res Notes. 2009;2:127. doi: 10.1186/1756-0500-2-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linenger JM, West LA. Epidemiology of soft-tissue/musculoskeletal injury among U.S. Marine recruits undergoing basic training. Mil Med. 1992;157:491–493. [PubMed] [Google Scholar]
- 9.Maffulli N, Wong J. Rupture of the Achilles and patellar tendons. Clin Sports Med. 2003;22:761–776. doi: 10.1016/s0278-5919(03)00009-7. [DOI] [PubMed] [Google Scholar]
- 10.Ireland D, Harrall R, Curry V, et al. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–169. doi: 10.1016/s0945-053x(01)00128-7. [DOI] [PubMed] [Google Scholar]
- 11.Riley GP, Curry V, DeGroot J, et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- 12.Foure A, Nordez A, Cornu C. Plyometric training effects on Achilles tendon stiffness and dissipative properties. J Appl Physiol. 2002;109:849–854. doi: 10.1152/japplphysiol.01150.2009. [DOI] [PubMed] [Google Scholar]
- 13.Soslowsky LJ, Carpenter JE, DeBano CM, et al. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 14.Barbe MF, Barr AE, Gorzelany I, et al. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J Orthop Res. 2003;21:167–176. doi: 10.1016/S0736-0266(02)00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung DT, Wang VM, Andarawis-Puri N, et al. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech. 2010;43:274–279. doi: 10.1016/j.jbiomech.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung DT, Sereysky JB, Basta-Pljakic J, et al. Second harmonic generation imaging and Fourier transform spectral analysis reveal damage in fatigue-loaded tendons. Ann Biomed Eng. 2010;38:1741–1751. doi: 10.1007/s10439-010-9976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun HB, Andarawis-Puri N, Li Y, et al. Cycle-dependent matrix remodeling gene expression response in fatigue-loaded rat patellar tendons. J Orthop Res. 2010;28:1380–1386. doi: 10.1002/jor.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun HB, Li Y, Fung DT, et al. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res. 2008;466:1555–1561. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561–567. doi: 10.1177/0363546504270454. [DOI] [PubMed] [Google Scholar]
- 20.Oshiro W, Lou J, Xing X, et al. Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg Am. 2003;28:814–823. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Cao Y, Wu YF, et al. Tendon healing in vivo: gene expression and production of multiple growth factors in early tendon healing period. J Hand Surg Am. 2008;33:1834–1842. doi: 10.1016/j.jhsa.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Lui PP, Chan LS, Lee YW, et al. Sustained expression of proteoglycans and collagen type III/type I ratio in a calcified tendinopathy model. Rheumatology (Oxford) 49:231–239. doi: 10.1093/rheumatology/kep384. [DOI] [PubMed] [Google Scholar]
- 23.Andarawis-Puri N, Sereysky JB, Sun HB, et al. Non-recoverable changes in mechanical properties immediately after a single bout of loading of rat patellar tendon predict the temporal molecular response. Transaction of the 57th Annual Orthopaedic Research Society Meeting. 2011;36:0220. [Google Scholar]
- 24.Andarawis-Puri N, Sereysky JB, Ros SJ, et al. Cycle-dependent fatigue loading changes in immediate mechanical properties of the tendon correlate with the in vivo temporal mechanical response. Transaction of the 56th Annual Orthopaedic Research Society Meeting. 2011;35:0023. [Google Scholar]
- 25.Miller BF, Olesen JL, Hansen M, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bey MJ, Song HK, Wehrli FW, Soslowsky LJ. Intratendinous strain fields of the intact supraspinatus tendon: the effect of glenohumeral joint position and tendon region. J Orthop Res. 2002;20:869–874. doi: 10.1016/S0736-0266(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 27.Williams LN, Elder SH, Horstemeyer MF, Harbarger D. Variation of diameter distribution, number density, and area fraction of fibrils within five areas of the rabbit patellar tendon. Ann Anat. 2008;190:442–451. doi: 10.1016/j.aanat.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Laudier D, Schaffler MB, Flatow EL, Wang VM. Novel procedure for high-fidelity tendon histology. J Orthop Res. 2007;25:390–395. doi: 10.1002/jor.20304. [DOI] [PubMed] [Google Scholar]
- 29.Sereysky JB, Andarawis-Puri N, Kochhar D, et al. Accumulation of tendon fatigue damage detected in situ using automated histological quantification. Transaction of the 57thAnnual Orthopaedic Research Society Meeting. 2011;36:0042. [Google Scholar]
- 30.Sereysky JB, Andarawis-Puri N, Ros SJ, et al. Automated image analysis method for quantifying damage accumulation in tendon. J Biomech. 2010;43:2641–2644. doi: 10.1016/j.jbiomech.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fung DT, Wang VM, Laudier DM, et al. Subrupture tendon fatigue damage. J Orthop Res. 2009;27:264–273. doi: 10.1002/jor.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hreljac A, Marshall RN, Hume PA. Evaluation of lower extremity overuse injury potential in runners. Med Sci Sports Exerc. 2000;32:1635–1641. doi: 10.1097/00005768-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Morrissey D, Roskilly A, Twycross-Lewis R, et al. The effect of eccentric and concentric calf muscle training on Achilles tendon stiffness. Clin Rehabil. 2011;25:238–47. doi: 10.1177/0269215510382600. [DOI] [PubMed] [Google Scholar]
- 34.Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108:670–675. doi: 10.1152/japplphysiol.00259.2009. [DOI] [PubMed] [Google Scholar]
- 35.Andarawis-Puri N, Ricchetti ET, Soslowsky LJ. Rotator cuff tendon strain correlates with tear propagation. J Biomech. 2009;42:158–163. doi: 10.1016/j.jbiomech.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Child S, Bryant AL, Clark RA, Crossley KM. Mechanical properties of the achilles tendon aponeurosis are altered in athletes with achilles tendinopathy. Am J Sports Med. 2010;38:1885–1893. doi: 10.1177/0363546510366234. [DOI] [PubMed] [Google Scholar]
- 37.Breen EC, Johnson EC, Wagner H, et al. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol. 1996;81:355–361. doi: 10.1152/jappl.1996.81.1.355. [DOI] [PubMed] [Google Scholar]
- 38.Gavin TP, Wagner PD. Effect of short-term exercise training on angiogenic growth factor gene responses in rats. J Appl Physiol. 2001;90:1219–1226. doi: 10.1152/jappl.2001.90.4.1219. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Kitaoka K, Tomita K. Effect of eccentric exercise on the healing process of injured patellar tendon in rats. J Orthop Sci. 2008;13:371–378. doi: 10.1007/s00776-008-1242-6. [DOI] [PubMed] [Google Scholar]
- 40.Skovgaard D, Bayer ML, Mackey AL, et al. Increased Cellular Proliferation in Rat Skeletal Muscle and Tendon in Response to Exercise: Use of FLT and PET/CT. Mol Imaging Biol. 2010;12:626–34. doi: 10.1007/s11307-010-0316-y. [DOI] [PubMed] [Google Scholar]
- 41.Andarawis-Puri N, Sereysky JB, Ros SJ, et al. Treadmill running modulates the molecular profile of fatigue damages tendons. Transaction of the 57thAnnual Orthopaedic Research Society Meeting. 2010;36:0399. [Google Scholar]
- 42.Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. J Orthop Res. 2001;19:881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- 43.Millar NL, Hueber AJ, Reilly JH, et al. Inflammation is present in early human tendinopathy. Am J Sports Med. 38:2085–2091. doi: 10.1177/0363546510372613. [DOI] [PubMed] [Google Scholar]
- 44.Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002;20:36–39. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 45.Sereysky JB, Andarawis-Puri N, Jepsen KJ, Flatow EL. Genetic variation in murine patellar tendon mechanical properties. Transaction of the 56th Annual Orthopaedic Research Society Meeting. 2010;35:1065. [Google Scholar]