Abstract
Background
PRETEXT is used to stratify risk in children with hepatoblastoma by the Liver Tumor Strategy Group (SIOPEL) of the International Society of Pediatric Oncology (SIOP). A recent analysis excluding patients that did not survive neoadjuvant chemotherapy, concluded that PRETEXT was superior to Children’s Oncology Group (COG) stage for predicting survival. Puzzled by this result, we made a similar comparison of PRETEXT and COG stage. This time, however, we include all patients, and we compare predictive value at diagnosis, instead of after neoadjuvant chemotherapy.
Methods
Hepatoblastoma patients in INT-0098 were retrospectively reviewed for PRETEXT and other potential prognostic factors including pathologic subtype, and alpha-fetoprotein (AFP).
Results
5-year overall survival by PRETEXT was 88.9%, 84.5%, 71.6%, and 30.9%, for PRETEXT I, II, III, and IV, respectively. The 5-year overall survival rates by COG Stage were 100%, 97.5%, 100%, 70.2%, and 39.3% for Stage I pure fetal histology (PFH), Stage I unfavorable histology (UH = not PFH), Stage II, Stage III, and Stage IV, respectively. PRETEXT added significant additional prognostic information within the COG Stage III, but not COG Stage IV. Additional prognostic factors statistically significant for an increased risk of death were small-cell-undifferentiated (SCU) histologic subtype and AFP<100 at diagnosis.
Conclusions
PRETEXT, COG stage, SCU histology, and AFP<100, as assessed at diagnosis, are important determinants of survival that will allow us to better develop common international criteria for risk stratification. Common risk stratification is an essential prerequisite to establish effective cooperation across the ocean in this field of rare tumors.
Keywords: hepatoblastoma, stage, PRETEXT, alpha-fetoprotein (AFP), small cell undifferentiated histology, prognostic factors, overall survival, surgical margin
Introduction
In the evolution of our treatment of childhood hepatoblastoma, many staging systems have been applied over the years. 1-5 PRETEXT (pretreatment extent of disease) was introduced in the first prospective trial of the Liver Tumor Strategy Group (SIOPEL 1) of the International Society of Pediatric Oncology (SIOP)6 and has been used to stratify pediatric liver tumors internationally for years.6-10 PRETEXT radiographically stages the tumor before surgical treatment, whereas COG stage employs the results of surgical resection prior to the administration of systemic therapy. In current SIOPEL studies, PRETEXT is one of a handful of prognostic factors (PRETEXT, distant metastasis, low AFP) that are used to stratify patients as either “standard” or “high” risk. In COG, metastatic disease has historically been the single criteria for inclusion in the highest risk category, Stage IV. PRETEXT is moderately accurate, has a tendency to over stage, is reproducible, and may have superior predictive value for patient survival when compared to alternative staging systems.11 In an attempt to define the potentially important role of PRETEXT stratification in future cooperative studies, we retrospectively assigned PRETEXT at the time of diagnosis to patients enrolled in INT-0098 (Intergroup CCG-POG American multicenter cooperative study). In contrast to the report of Aronson et al11, who compared PRETEXT and COG Stage only in patients that survived neoadjuvant chemotherapy, this study included all patients diagnosis.
Methods
Hepatoblastoma patients in the Intergroup CCG/POG study INT-0098 were independently reviewed by RLM and MHM to determine PRETEXT at diagnosis (resection or biopsy). The details regarding eligibility, treatment, evaluation and follow-up for INT-0098 have been published previously.3 All patients except those with pure fetal histology, were randomized to receive cisplatin/doxorubicin (CD) or cisplatin/5FU/vincristin (C5V). Outcomes between the two groups were not significantly different although there was less toxicity in the C5V group. Histology and COG stage were confirmed by central review of tissue slides, institutional pathology report, and operative report. CT or MRI report at diagnosis, operative report (diagnostic biopsy or resection at diagnosis), study surgical report form which included detailed drawings of the anatomic extent of the tumor by the operative surgeon, pathology report, and study pathology report form were interrogated and cross-referenced for all patients enrolled in INT-0098. Data allowed for retrospective PRETEXT assignment in 178 of 181 patients. We acknowledge that PRETEXT is best assigned prospectively by review of radiographic imaging 12,13 and in this review we rely on surrogate reports (radiographic, operative, and pathology). Additional potential prognostic variables examined include tumor histology, margin of resection at definitive surgery, surgical complications and alpha-fetoprotein level at diagnosis. INT-0098 used two histology categories for treatment stratification: pure fetal histology and unfavorable histology (unfavorable included all patients who were not pure fetal). In this analysis we retrospectively reassigned the histologic subtype as pure fetal, fetal/embryonal, small cell undifferentiated (SCU, formerly anaplastic), macrotrabecular, or mixed mesenchymal according to the pathology report and pathologist study report form. A tumor was classified as SCU if the diagnostic specimen contained any SCU elements, even a single microscopic focus.
PRETEXT (Figure 1) is based upon division of the liver into four sections”.23 The sections correspond to the traditional surgical division of the liver into left lateral and medial segments, and right anterior and posterior segments. PRETEXT designates the following: (1) left lateral (Couinaud 2 and 3); (2) left medial (Couinaud 4) (3) right anterior (Couinaud 5 and 8); and (4) right posterior (Couinaud 6 and 7). Couinaud 1, the caudate lobe, was not originally included in PRETEXT but in a recent published revision of PRETEXT by SIOPEL it was suggested that in future studies caudate lobe involvement will be denoted by an annotation of “C”23. PRETEXT is determined by the contiguous sections that are free of tumor. Extrahepatic growth is indicated by adding one or more of the following: “V”, vena cava or all three hepatic veins; “P”, main portal or both portal branches; “E”, extrahepatic contiguous growth (e.g., diaphragm or stomach), and “M”, distant metastases. Detailed information on hepatic and portal venous involvement was not consistently available and is therefore beyond the scope of this retrospective analysis. COG stage is based upon surgical findings and outcome of a primary operation (Table I).
Figure 1.
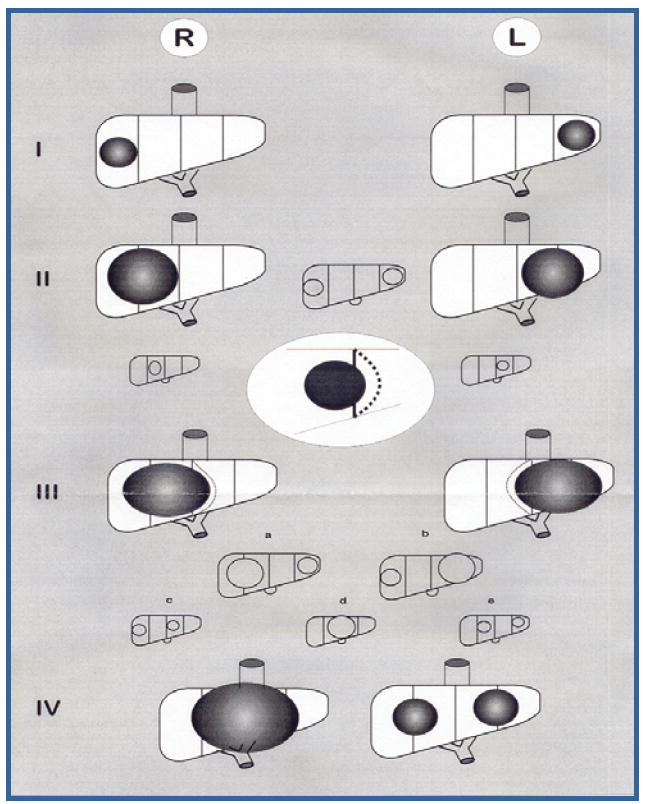
PRETEXT = pretreatment extent of disease. May be referred to as POST-TEXT (post-treatment extent of disease) if the staging is done after neoadjuvant chemotherapy. PRETEXT I = three contiguous sections free of tumor; PRETEXT II = two contiguous sections free of tumor; PRETEXT III = one contiguous section free of tumor; PRETEXT IV = no section free of tumor. In addition any group may have: “V” Invasion vena cava or all three major hepatic veins; “P” Invasion main portal vein or portal venous bifurcation; “E” Contiguous extrahepatic growth; “M” Distant metastasis; “C” Caudate lobe involvement.
TABLE I.
COG Staging System
| Stage I | Complete gross resection at diagnosis with clear margins |
| Stage II | Complete gross resection at diagnosis with microscopic residual disease at the margins of resection |
| Stage III | Biopsy only at diagnosis; or gross total resection with nodal involvement; or preoperative tumor spill/rupture; or incomplete resection with gross residual |
| Stage IV | Distant metastatic disease at diagnosis |
Outcome Definitions
EFS was defined as the period from the date chemotherapy was started until evidence of an EFS-event (progressive disease, death, diagnosis of a second malignant neoplasm) or last contact, whichever occurred first. Survival time (OS) was defined as the period from the date chemotherapy was started until death or last contact. A patient who died was considered to have experienced an OS-event, regardless of the cause of death. Patients who did not experience an event were censored on the date of last contact for the relevant outcome measure.
Statistical Methods
Life table estimates of survival time were calculated by the method of Kaplan and Meier24, and the standard deviation of the Kaplan-Meier estimate of the survivor function at selected points was calculated using Greenwood’s formula.25 Risk for death was compared across therapies and groups of patients using the log-rank statistic. For treatment comparisons, outcome was assigned to the randomized treatment, regardless of the therapy received.
Results
Table II shows PRETEXT of 178 patients enrolled on INT-0098 and subject of this analysis, according to their previous COG staging. While there was a preponderance of upfront resections for patients classified as PRETEXT I and II, and neoadjuvant chemotherapy for patients classified as PRETEXT III and IV, there were frequent exceptions to this broad theme. Sixty-one of the 178 patients reviewed, were COG Stage I or II. Of these 61 patients, 16 (26%) were PRETEXT I, 36 (59%) were PRETEXT II, and 9 (15%) were PRETEXT III. No patients with COG stage I or II disease were PRETEXT IV. Seventy-nine patients were COG Stage III. Of these 79, 14 (18%) were PRETEXT I or II, 44 (56%) were PRETEXT III, and 21 (26%) were PRETEXT IV. Of the 38 patients diagnosed with metastatic tumors (COG Stage IV), 7 (18%) were PRETEXT I or II, 24 (64%) were PRETEXT III, and 7 (18%) were PRETEXT IV. The 5-year overall survival rates by PRETEXT group were 88.9%, 84.5%, 71.6%, and 30.9%, for I, II, III, and IV, respectively. The 5-year overall survival rates by the Intergroup/COG staging system were 100%, 97.5%, 100%, 70.2%, and 39.3% for Stage I (pure fetal histology), Stage I (unfavorable histology), Stage II, Stage III, and Stage IV, respectively. The log rank test shows both systems to be significantly related to risk of death (p<0.0001) of overall survival (Figures 2 and 3). Figures 4 and 5 show the outcome of patients with COG stage III and IV according to PRETEXT grouping, respectively. PRETEXT has additional predictive value for patients with COG stage III disease, but not COG stage IV disease.
Table II.
PRETEXT of COG staged patients
| Stage I Pure Fetal | Stage I Unfavorable | Stage II | Stage III | Stage IV | |
|---|---|---|---|---|---|
| PRETEXT I | 3 | 12 | 1 | 1 | 2 |
| PRETEXT II | 4 | 26 | 6 | 13 | 5 |
| PRETEXT III | 2 | 7 | - | 44 | 24 |
| PRETEXT IV | - | - | - | 21 | 7 |
Figure 2.

Survival according to COG Stage of 178 patients with retrospective assignment of PRETEXT. COG Stage I Pure Fetal n=9; Stage I Unfavorable n=45; Stage II n=7; Stage III n=79; Stage IV n=38.
Figure 3.

Survival according to retrospective assignment of PRETEXT group.
PRETEXT I n=19; PRETEXT II n=54; PRETEXT III n=77; PRETEXT IV n=28.
Figure 4.
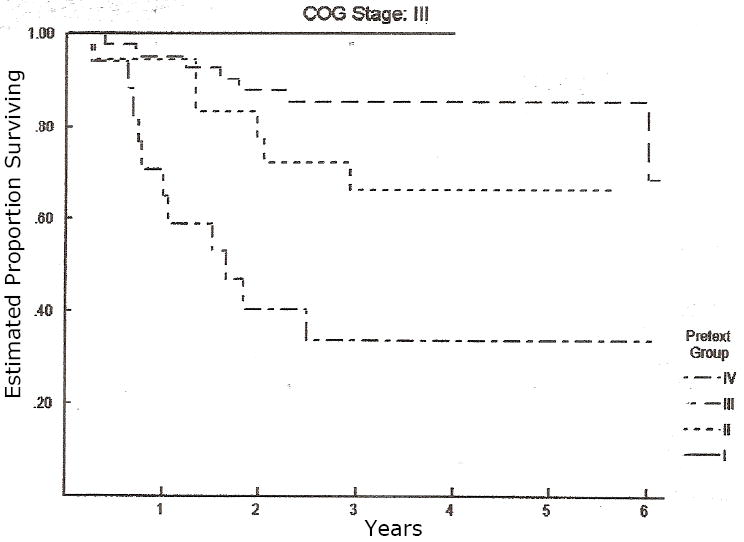
Overall survival of all patients in COG Stage III, each patient has been subclassified according to their PRETEXT group. PRETEXT adds additional predictive value for patients with COG Stage III tumors, p < 0.01 by log rank test.
PRETEXT I n=1; PRETEXT II n=13; PRETEXT III n=44; PRETEXT IV n=21.
Figure 5.
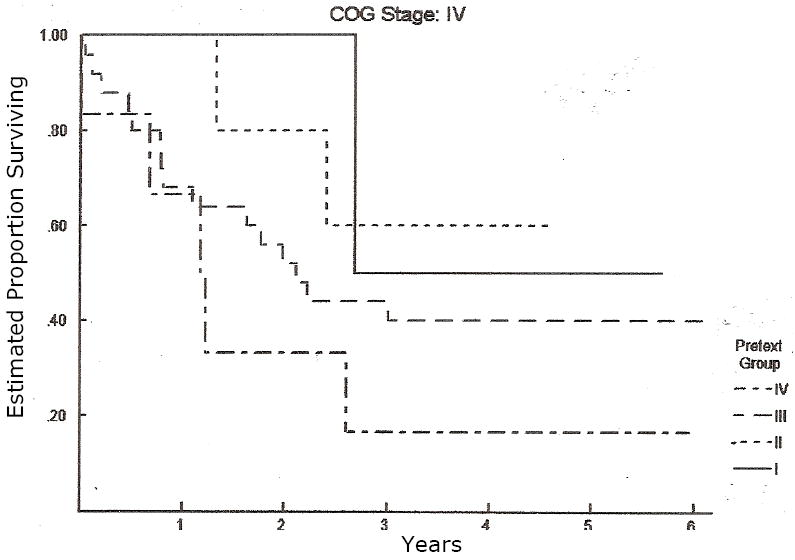
Overall survival of all patients in COG Stage IV, each patient has been subclassified according to their PRETEXT group. PRETEXT does NOT additional predictive value for patients with COG Stage IV tumors, p = 0.36 by log rank test.
PRETEXT I n=2; PRETEXT II n=5; PRETEXT III n=24; PRETEXT IV n=7.
Other potential prognostic factors are shown in Table III by COG stage, and in Table IV by PRETEXT. No patients in this cohort had both AFP < 100 and the presence of SCU elements in the diagnostic specimen. The presence of either AFP <100 at diagnosis or SCU elements were statistically significant predictors of increased risk of death (Figure 6). Prognostic factors not included in the above tables include surgical complications, multifocal tumor, and surgical resection margin. The number and distribution of patients with multifocal tumor, positive microscopic surgical resection margin, or surgical complication was not sufficient to reach a meaningful statistical conclusion. While not statistically significant, the data on surgical complications is nonetheless important and is detailed below.
Table III.
3-Year Event-Free-Survival by Prognostic Factors and COG Stage
| Stage I & II N=61 (ESD)1 | Stage III N=79 (ESD)1 | Stage IV N=38 (ESD)1 | p-value | |
|---|---|---|---|---|
| Multifocal Tumor | 0.86 | |||
| Yes | 100% (0%) | 46.2 % (13.8%) | 37.5% (17.1%) | |
| No | 90% (4.6%) | 69.6% (5.7%) | 23.3% (7.7%) | |
| Alpha Fetal Protein at Diagnosis | 0.0042 | |||
| < 100 at DX2 | NA | 25.0% (21.7%) | 0% (0%) | |
| >= 100 at DX | 91.1% (4.2%) | 67.6% (5.6%) | 28.6% (7.6%) | |
| Small Cell Undifferentiated Histology | 0.0007 | |||
| Yes2 | NA | 0% (0%) | 0% (0%) | |
| No | 90.7% (4.4%) | 69.3% (5.3%) | 28.1% (8.0%) | |
| Macrotrabecular Histology | 0.90 | |||
| Yes | 85.7% (13.2%) | 83.3% (15.2%) | 22.2% (13.9%) | |
| No | 91.7% (4.6%) | 64.8% (5.7%) | 28.0% (9.0%) |
Estimated Standard Deviation of the Kaplan-Meier estimate of 3-Year EFS;
No patient had AFP < 100 and SCU histology at time of diagnosis.
Figure 6.
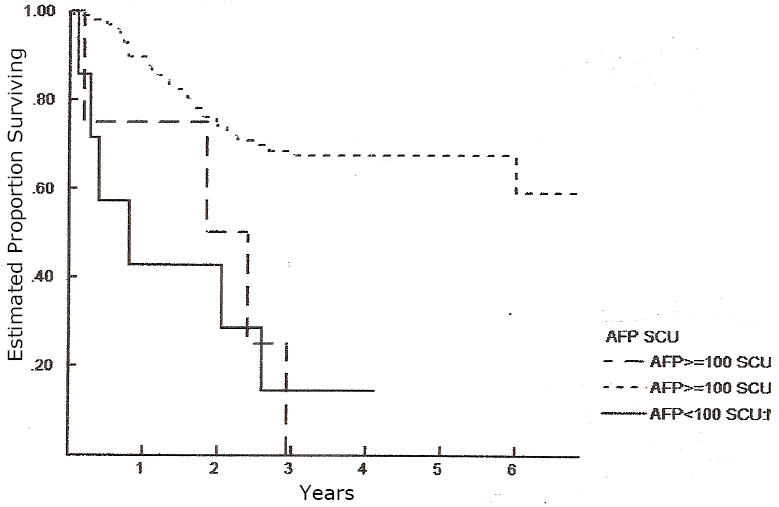
Overall survival according to the diagnostic risk factors “AFP at diagnosis” and “small cell undifferentiated (SCU) histology”. Both of these risk factors are independent predictors of overall survival (p < 0.0001) by log rank test. AFP > 100 and no SCU n=165; AFP >100 and presence of SCU n=6; AFP <100 and no SCU n=7.
There were a total of 35 surgical complications noted, most were minor but 8 were considered to be potential contributing factors to the patient’s death: Stage I Unfavorable Histology: one patient with bile stricture and septic cholangitis; Stage II: one patient with diaphragm injury and postoperative hemorrhage; Stage III: one patient with hepatic artery thrombosis after liver transplant; one patient with portal hypertension and variceal bleeding after postoperative hepatic outflow occlusion; two patients with liver failure at least partially caused by vascular injury at the time of surgery; Stage IV: two patients with liver failure at least partially caused by vascular injury at the time of surgery.
Discussion
Children’s Oncology Group (COG) has used a hepatoblastoma staging system based upon the outcome of upfront surgical intervention (resection or biopsy) and prior to any systemic treatment. Despite the fact that a staging classification should ideally be done prior to any therapeutic intervention and that it should be independent of the results of any one therapeutic modality, the COG system has been proven to have prognostic relevance.3, 14 In Japan and Germany, a modified TNM staging system has been used in the past2 but has since been abandoned in favor of PRETEXT based staging systems. In considering whether to adopt a PRETEXT based staging system in future studies, COG felt it was important to validate this approach as rigorously as possible. PRETEXT (pretreatment extent of disease) was developed by the Liver Tumor Strategy Group (SIOPEL) of the International Society of Pediatric Oncology (SIOP) and is based upon the number of contiguous tumor-free surgical sections of the liver with annotations V, P, E, and/or M for extrahepatic tumor involvement according to radiographic findings. 6, 11,23 Thus, PRETEXT is independent of therapeutic strategies and, most importantly, independent of the individual surgeon’s judgment regarding surgical resectability and it can be used at time of diagnosis or after neo-adjuvant chemotherapy.
A report specifically aimed at validation of the ability of PRETEXT (without the V,P,E,M annotations) to predict survival was published recently.11 Retrospective review of SIOPEL-1 compared PRETEXT after neoadjuvant chemotherapy with COG and TNM stage after neoadjuvant chemotherapy. Predictive value of PRETEXT was roughly equivalent to the TNM system but superior to the COG stage. They did NOT include the PRETEXT annotations of V, P, E, or M in the analysis or stratify the patients by the current SIOPEL risk stratification system. Aronson et al11 speculated that COG stage was not significantly related to survival “probably because most patients had Stage I disease”. This peculiar finding results from the assignment of COG stage after neoadjuvant chemotherapy, while COG stage is designed to be applied at diagnosis. By neglecting to analyze patients who either died during neoadjuvant chemotherapy, or who never had a definitive resection, they excluded many of the patients that would have been COG Stage IV. The authors acknowledge this, saying that further research is necessary to evaluate the predictive value of PRETEXT “not only in patients who receive surgical resection, but in all patients.” In Aronson et al11, the comparative study concludes with the statement that the “predictive value for survival of PRETEXT and of the tumor-node-metastasis based system was highly significant in contrast to the predictive value of the CCG/POG based system which was poor”. Because of this, we did not feel that their study validated the use of PRETEXT assignment at diagnosis for risk stratification. In this study we classified patients according to the PRETEXT system at the time of diagnosis and prior to chemotherapy. Our results showed that both the COG Stage and PRETEXT were good predictors of risk of death and long term survival. Additionally, we believe that PRETEXT may be used to identify patients who may be amenable to upfront surgical resection (PRETEXT I and II) and who may then achieve long term survival with less intensive chemotherapy and less chemotherapy related toxicity.
For patients with non-metastatic disease who did not have a complete tumor resection at diagnosis (COG stage III) the further stratification of patients according to PRETEXT identified a group of patients at high-risk of death. These patients in particular should be identified early in their course of treatment as potential candidates for liver transplantation.
As has been shown in several prior studies, but not the recent report by Aronson et al, this analysis shows that the presence of metastatic disease (COG Stage IV) is associated with high-risk of death regardless of the extent of liver involvement as quantified by PRETEXT. SIOPEL 1 and SIOPEL 2 designate metastasis at diagnosis as a “+M” annotation to the PRETEXT. These patients are currently placed in the SIOPEL 4 “high risk” treatment stratum. Due to the poor outcome in many of these patients, all study groups continue in search of new therapeutic approaches.
Additional risk factors including AFP<100 at diagnosis,13,15 histologic subtype,16,17 surgical margin,4,18-20 and surgical complications21,22 have been reported to have potential prognostic value in hepatoblastoma. The distribution of some of these risk factors is reported in Tables III. No patients in this cohort had both AFP < 100 and the presence of SCU elements in the diagnostic specimen. The presence of either AFP <100 at diagnosis or SCU elements were statistically significant predictors of increased risk of death. The numbers of patients with these risk factors were small preventing us from analyzing their independent prognostic value when compared with that of the presence of metastatic disease. However, patients with these factors may also be considered for treatment with new therapeutic approaches. The number and distribution of patients with multifocal tumors, positive surgical margin, or a surgical complication did not suggest prognostic value for these risk factors. However, by examining a larger number of patients, and combining these results with that of the subsequent COG study, COG 9645, and perhaps other multigroup studies, we might find them to be of prognostic value. Just such an international collaboration, termed the CHIC project (Children’s Hepatoblastoma International Consortium) has been proposed at a recent hepatoblastoma international symposium.
This study demonstrated the value of both staging systems in predicting risk of death. We propose for future COG studies that PRETEXT be used as an objective tool to monitor the effect of neoadjuvant chemotherapy and to determine the timing and extent of tumor surgical resection. Specifically, we believe that a majority of PRETEXT I and II tumors without major venous involvement can be safely resected at diagnosis. PRETEXT IV tumors, the majority of which are extensively multifocal, should undergo early referral for potential liver transplant.
This analysis also allowed us to identify important prognostic factors that can be assessed in all patients prior to neoadjuvant therapy. Adjustment for these prognostic factors in the analysis of studies of this disease will allow us to better understand these patient populations, develop criteria for risk stratification and develop new therapeutic approaches which hold the most potential for decreasing toxicity while advancing cure. Most importantly, these prognostic factors will allow us to better develop common international criteria for risk stratification , an essential prerequisite to establish effective cooperation across the ocean in this field of very rare tumors.
References
- 1.Evans AE, Land VJ, Newton WA, et al. Combination chemotherapy (vincristine, Adriamycin, Cyclophosphamide, and 5-fluorouracil) in the treatment of children with malignant hepatoma. Cancer. 1982;50:821–826. doi: 10.1002/1097-0142(19820901)50:5<821::aid-cncr2820500502>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki F, Matsunaga T, Iwafuchi M, et al. Outcome of hepatoblastoma treated with JPLT-1 (Japanese Study Group for Pediatric Liver Tumor) Protocol-1: A report from the Japanese Study Group for Pediatric Liver Tumor. J Pediatr Surg. 2002;37:851–856. doi: 10.1053/jpsu.2002.32886. [DOI] [PubMed] [Google Scholar]
- 3.Ortega JA, Douglass EC, Feusner JH, et al. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children’s Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 2000;18:2665–75. doi: 10.1200/JCO.2000.18.14.2665. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds M, Douglass EC, Finegold M, et al. Chemotherapy can convert unresectable hepatoblastoma. J Pediatr Surg. 1992;27:1080–1084. doi: 10.1016/0022-3468(92)90564-n. [DOI] [PubMed] [Google Scholar]
- 5.Von Schweinitz D, Hecker H, Schmidt-von-Arndt G, et al. Prognostic factors and staging systems in childhood hepatoblastoma. Int J Cancer. 1997;74:593–599. doi: 10.1002/(sici)1097-0215(19971219)74:6<593::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Brown J, Perilongo G, Shafford E, et al. Pretreatment prognostic factors for children with hepatoblastoma: Results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 2000;36:1418–1425. doi: 10.1016/s0959-8049(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 7.Czauderna P, Otte JB, Roebuck DJ, et al. Surgical treatment of hepatoblastoma in children. Pediatr Radiol. 2006;36:187–191. doi: 10.1007/s00247-005-0067-0. [DOI] [PubMed] [Google Scholar]
- 8.Plaschkes J, Vos A, Czauderma P, et al. Into the year 2000 with SIOPEL 3: Cisplatin alone or in combination for standard and high risk hepatoblastoma. Med Pediatr Oncol. 1998;31:240. [Google Scholar]
- 9.Schnater JM, Aronson DC, Plaschkes J, et al. Surgical view of the treatment of patients with hepatoblastoma: Results from the first prospective trial of the International Society of Paediatric Oncology Liver Tumor Study Group (SIOPEL 1) Cancer. 2002;94:1111–1120. [PubMed] [Google Scholar]
- 10.Stringer MD. Liver tumors. Semin Pediatr Surg. 2000;9:196–208. doi: 10.1053/spsu.2000.18844. [DOI] [PubMed] [Google Scholar]
- 11.Aronson DC, Schnater JM, Staalman CR, et al. Predictive value of the pretreatment extent of disease system in hepatoblastoma: Results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol. 2005;23:1245–52. doi: 10.1200/JCO.2005.07.145. [DOI] [PubMed] [Google Scholar]
- 12.Roebuck DJ, Olsen O, Pariente D. Radiological staging in children with hepatoblastoma. Pediatr Radiol. 2005;36:176–182. doi: 10.1007/s00247-005-0029-6. [DOI] [PubMed] [Google Scholar]
- 13.Czauderna P, Otte JB, Aronson DC, et al. Guidelines for surgical treatment of hepatoblastoma in the modern era – recommendations from the Childhood Liver Tumor Strategy Group of the International Society of Pediatric Oncology (SIOPEL) Eur J Cancer. 2005;41:1031–1036. doi: 10.1016/j.ejca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Von Schweinitz D. Management of liver tumors in childhood. Semin Pediatr Surg. 2006;15:17–14. doi: 10.1053/j.sempedsurg.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Von Schweinitz D. Identification of risk groups in hepatoblastoma: Another step in optimizing therapy. Eur J Cancer. 2000;36:1343–1346. doi: 10.1016/s0959-8049(00)00138-6. [DOI] [PubMed] [Google Scholar]
- 16.Davies JQ, de la Hall PM, Kaschula RO, et al. Hepatoblastoma – evolution and outcome and significance of histology of the resected tumor. A 31-year experience with 40 cases. J Pediatr Surg. 2004;39:1321–1327. doi: 10.1016/j.jpedsurg.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Schnater JM, Kuijper CF, Zsiros J, et al. Pre-operative diagnostic biopsy and surgery in pediatric liver tumors – the Amsterdam experience. Eur J Surg Oncol. 2005;31:1160–1165. doi: 10.1016/j.ejso.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Tiao GM, Bobey N, Allen S, et al. The current management of hepatoblastoma: a combination of chemotherapy, conventional resection and liver transplantation. J Pediatr Surg. 2005;146:204–211. doi: 10.1016/j.jpeds.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Brugieres L, Plaschkes J, MacKinlay G, et al. Hepatoblastoma: Microscopic residual disease after delayed surgery, prognostic implications. Med Pediatr Oncol. 2000;35:177. [Google Scholar]
- 20.Dicken BJ, Bigam DL, Lees GM. Association between surgical margins and long-term outcome in advanced hepatoblastoma. J Pediatr Surg. 2004;39:721–725. doi: 10.1016/j.jpedsurg.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs J, Rydzynski J, Hecker H, et al. The influence of preoperative chemotherapy and surgical technique in the treatment of hepatoblastoma: A report from the German cooperative liver tumor studies HB 89 and HB 94. Eur J Pediatr Surg. 2002;12:255–261. doi: 10.1055/s-2002-34484. [DOI] [PubMed] [Google Scholar]
- 22.Towu E, Kiely E, Pierro A, et al. Outcome and complications after resection of hepatoblastoma. J Pediatr Surg. 2004;39:199–202. doi: 10.1016/j.jpedsurg.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Roebuck DJ, Aronson D, Clapuyt P, et al. 2005 PRETEXT: a revised staging system for primary malignant tumors of childhood developed by the SIOPEL group. Pediatr Radiol. 2007;37:123–132. doi: 10.1007/s00247-006-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 25.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. John Wiley and Sons; New York: 1980. [Google Scholar]


