Abstract
The mitogenic dermonecrotic toxin from Pasteurella multocida (PMT) is a 1285-residue multipartite protein that belongs to the A-B family of bacterial protein toxins. Through its G-protein-deamidating activity on the α subunits of heterotrimeric Gq-, Gi- and G12/13-proteins, PMT potently stimulates downstream mitogenic, calcium, and cytoskeletal signaling pathways. These activities lead to pleiotropic effects in different cell types, which ultimately result in cellular proliferation, while inhibiting cellular differentiation, and account for the myriad of physiological outcomes observed during infection with toxinogenic strains of P. multocida.
1 Introduction
Toxinogenic strains of Pasteurella multocida, mostly serogroup D and some A, are associated with a number of epizootic and zoonotic diseases, including dermonecrosis, pasteurellosis, and atrophic rhinitis, in domestic and wild animals (DiGiacomo et al. 1991b; Foged 1992; Frymus et al. 1991; Kielstein 1986; Wilson and Ho 2006) and in humans who come in close contact with infected animals (Arashima and Kumasaka 2005; Donnio et al. 1991; Donnio et al. 2004; Garcia 1997; Iaria and Cascio 2007; Kobayaa et al. 2009; Wilson and Ho 2006). The major virulence factor responsible for the symptoms manifested during infection with these P. multocida strains is a 1285-residue (146-kDa) protein toxin (PMT), which belongs to the large, prominent group of intracellularly acting, multipartite A-B toxins that modify eukaryotic G-proteins (Wilson and Ho 2010). A-B toxins bind to host cell receptors through their binding B domains and facilitate the cellular uptake and delivery (translocation) of their toxic activity A domains into the host cell cytosol, where the A domains then interact with and modify their cellular G-protein targets to cause cellular toxicity.
The G-protein targets of A-B toxins are GTPases that regulate various cellular signal transduction pathways by cycling between an inactive GDP-bound form and an active GTP-bound form. PMT selectively deamidates a key active site Gln residue of the α subunit of its heterotrimeric G-protein targets, Gq, Gi, and G12/13 (Orth et al. 2009). This modification locks the GTPase activity of the α subunit into an active state, resulting in persistent stimulation of downstream signaling pathways modulated by the G-protein targets [reviewed in (Wilson and Ho 2010, 2011)]. While we are beginning to have a clearer picture of the molecular basis for the biochemical activity of PMT, much less is known about the molecular mechanisms of cellular intoxication or how the selective deamidation of its G-protein targets leads to the myriad of cellular outcomes observed. In this review, we focus on our current understanding of how PMT interacts with host cells to gain entry and elicit various cellular effects through its G-protein deamidase activity.
2 PMT Structure and Function
PMT is a member of the dermonecrotic toxin family, which includes the cytotoxic necrotizing factors from E. coli (CNF1, CNF2, and CNF3) and Yersinia pseudotuberculosis (CNFy) and the dermonecrotic toxin from Bordetella species (DNT) (Aktories and Barbieri 2005; Hoffmann and Schmidt 2004; Wilson and Ho 2010). Members of this family of A-B toxins share with each other sequence and structural features that enable them to enter host cells and then gain access to their G-protein targets and modify them. The N-terminus of PMT (PMT-N) has significant sequence similarity with the N-termini of the CNFs (Buys et al. 1990; Falbo et al. 1993; Kamps et al. 1990; Lockman et al. 2002; Oswald et al. 1994; Petersen and Foged 1989; Stoll et al. 2009) and to a lesser extent that of DNT (Pullinger et al. 1996). Although there is no crystal structure available for any of the full-length dermonecrotic toxins such that the actual domains responsible for receptor binding and translocation have not yet been clearly defined, there is some biochemical evidence that the N-termini of these proteins are indeed important for toxin binding and translocation (Baldwin et al. 2004; Blumenthal et al. 2007; Brothers et al. 2011; Chung et al. 2003; Kim et al. 2005; Lemichez et al. 1997; Pullinger et al. 2001).
The CNFs and DNT share over 50% sequence similarity in their C-terminal domains (residues 720–1014 in the CNFs, 1176–1464 in DNT), which have deamidase and/or transglutaminase activity (Hoffmann and Schmidt 2004). Their common G-protein targets belong to the Rho family of small GTPases, such as RhoA, Rac1, and Cdc42, involved in regulation of cytoskeletal function (Aktories and Barbieri 2005). The G-protein deamidase activity of PMT responsible for activation of mitogenic and calcium signaling pathways also resides within the C-terminal 700 amino acids of PMT (PMT-C) (Baldwin et al. 2004; Busch et al. 2001; Orth et al. 2003; Orth et al. 2009; Pullinger and Lax 2007; Pullinger et al. 2001). The crystal structures of PMT-C [PDB 2EBF] (Kitadokoro et al. 2007) and the C-terminal deamidase domain (residues 720–1014) of CNF1 [PDB 1HQ0] (Buetow et al. 2001) are available. The crystal structure of PMT-C (Kitadokoro et al. 2007) revealed three distinct domains (Fig. 1): a C1 domain (residues 575–719) that has sequence and structural homology with the membrane-targeting domains found in a number of large protein toxins (Geissler et al. 2010); a C2 domain (residues 720–1104) that is as-of-yet unknown function; and a C3 domain (residues 1105–1285) that harbors the minimal domain responsible for intracellular activity (Aminova et al. 2008). The C3 domain contains the active site Cys-His-Asp triad that is important for deamidase activity (Busch et al. 2001; Kitadokoro et al. 2007; Orth et al. 2003; Orth et al. 2009; Pullinger and Lax 2007) and has a papain-like cysteine protease structural fold that most closely resembles that of certain protein transglutaminases (Kitadokoro et al. 2007; Wilson and Ho 2010). Of particular note, however, was the surprising finding that PMT and CNF1 catalyze the same enzymatic reaction on a functionally equivalent Gln residue at the active site of their respective substrates (Gln-61 in Rac1 and Cdc42; Gln-63 in RhoA; Gln-205 in Gαi; Gln-209 in Gαq; Gln-229 in Gα12/13) (Flatau et al. 1997; Orth et al. 2009; Schmidt et al. 1997) and have essential active site catalytic His and Cys residues (Buetow et al. 2001; Busch et al. 2001; Kitadokoro et al. 2007; Orth et al. 2003; Schmidt et al. 1998). Yet, there is no discernable sequence or structural similarity between PMT-C3 and the catalytic domain of CNF1 (Wilson and Ho 2010).
Fig. 1.
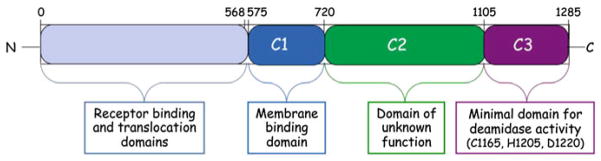
A schematic diagram of the overall structure of PMT. PMT-N (residues 1–568) and the known C-terminal structural domains (C1–C3) of PMT-C (residues 569–1285) are indicated along with their known or putative functions
3 Cellular Uptake of PMT
Little is known about the cellular intoxication mechanisms of PMT. As is the case for the other dermonecrotic toxin family members (Hoffmann and Schmidt 2004), PMT-N contains the functional receptor-binding and translocation domains. Although the precise boundaries for the binding and translocation domains have not yet been defined, PMT-N appears to be sufficient for mediating cell binding, uptake, and subsequent delivery of PMT-C into the cytosol (Baldwin et al. 2004; Brothers et al. 2011; Pullinger et al. 2001). Indeed, a fusion protein of PMT-N with GFP at the C-terminus also binds and enters cells (Repella et al. 2011). We will now consider what is known about each of these steps in PMT intoxication.
3.1 Binding to Host Cells
A-B toxins are known to employ different types of receptors to intoxicate host cells. For example, cholera and pertussis toxins utilize gangliosides alone (Merritt et al. 1994; Stein et al. 1994), diphtheria toxin uses proteins alone (Naglich et al. 1992), and botulinum and tetanus neurotoxins use both proteins and gangliosides as co-receptors (Binz and Rummel 2009; Dong et al. 2006). Earlier reports suggested that PMT utilized gangliosides, namely GM1, GM2 and GM3, as receptors (Dudet et al. 1996; Pettit et al. 1993a). However, a more recent study using several different methods, including PMT binding to membrane lipid components using TLC-overlay, liposome-pulldown, and surface plasmon resonance (SPR) experiments, showed that PMT and PMT-N do not bind gangliosides (GM1, GM2 or GM3), but instead both bound well to asialogangliosides, such as lactosylceramide, and in particular sphingomyelin (SM) and positively charged membrane phospholipids, such as phosphatidylcholine (PC) and to some extent phosphatidylethanolamine (PE) and some other lipid components (Brothers et al. 2011).
Results from this study also implicated the potential involvement of a protein co-receptor in PMT binding to cells (Brothers et al. 2011), since no single treatment with sphingomyelinase, phospholipase D, or trypsin completely abolished PMT binding to cell membranes, and instead each treatment reduced cellular binding of PMT by about one-third. Indeed, trypsin treatment of cell membranes had minimal effect on membranes already depleted of choline or phosphocholine head groups, indicating that protein binding is only important for PMT binding when PMT is also interacting with SM and/or PC in the membrane. Based on the observed binding kinetics from the SPR studies under the different treatment conditions, a model for PMT interaction with host cells was proposed, whereby PMT initially binds nonspecifically and with low affinity to the more abundant membrane lipid components such as PC at the surface, but this is then followed by a more specific, tight-binding interaction with SM and possibly other membrane components, including a putative protein co-receptor(s).
3.2 Trafficking in Cells
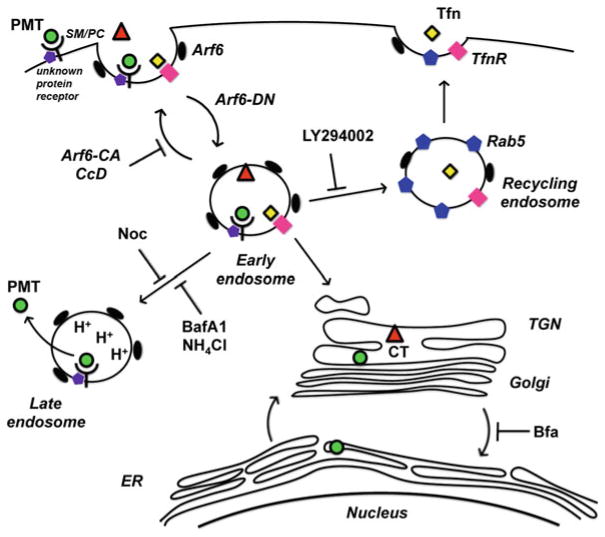
Once PMT binds to host cells, it is internalized through receptor-mediated endocytosis and then trafficked to acidic endosomes, where it is translocated across the vesicle membrane into the host cell cytosol (Baldwin et al. 2004; Repella et al. 2011; Rozengurt et al. 1990). Although knowledge of the detailed mechanism of this process is lacking, we are beginning to gain some insights. A recent study showed that receptor-mediated endocytosis and initial trafficking of PMT are dependent on the small regulatory G-protein Arf6 (Repella et al. 2011). PMT is initially internalized and trafficked to Arf6-containing vesicles, where it co-localizes with cholera toxin and transferrin, but transferrin is subsequently trafficked to recycling endosomes and cholera toxin is trafficked retrograde to the endoplasmic reticulum, while PMT-containing early endosomes are diverted to late endosomes.
Disassembly of microtubules important for trafficking from early to late endosomes by treatment with nocodazole or disruption of actin polymerization by treatment with cytochalasin D also block PMT activation of mitogenic signaling (Repella et al. 2011), suggesting that membrane translocation and cytotoxicity of PMT are dependent on trafficking to late acidic endosomes. Additional evidence that PMT trafficking to late acidic endosomes is important for PMT action comes from the finding that treatment with brefeldin A, which disrupts Golgi-endoplasmic reticulum trafficking, enhances PMT activity by over 20-fold, presumably by preventing PMT trafficking through nonproductive pathways that do not lead to translocation (Repella et al. 2011). Based on these results, a model was proposed for PMT intoxication, as depicted in Fig. 2.
Fig. 2.
Proposed model of PMT entry and trafficking. Tfn transferrin, TfnR transferrin receptor, CT cholera toxin, Rab5 GTPase marker of recycling endosomes, TGN trans-Golgi network, ER endoplasmic reticulum, SM/PC sphingomyelin/PC receptors of PMT, Arf6-CA constitutively active Arf6 GTPase, Arf6-DN dominantly negative Arf6, CcD cytochalasin D, LY294002 PI3 K inhibitor of early endosome-recycling endosome fusion, Noc nocodazole, BafA1 bafilomycin A1, Bfa brefeldin A. [Adapted from (Repella et al. 2011)]
3.3 Translocation Across Cell Membranes
The above results showing that PMT activity depends on trafficking to late acidic endosomes, where presumably translocation into the cytosol occurs, confirmed earlier findings that weak bases, such as ammonium chloride, chloroquine, or methylamine, which buffer acidification of endosomes, inhibit PMT effects on cells (Rozengurt et al. 1990). Moreover, bafilomycin A1, a potent and specific inhibitor of the vacuolar H+-ATPase pump that is responsible for acidifying early to late endosomes, likewise inhibits PMT action (Baldwin et al. 2004; Repella et al. 2011). Further evidence for a low pH-dependent membrane translocation event in PMT action was provided by experiments that showed cell surface-bound PMT could directly enter cells, even in the presence of bafilomycin A1, through a low pH pulse at 4°C, which normally blocks endocytosis (Baldwin et al. 2004).
A predicted helix-loop-helix motif, which corresponded to a similar helix-loop-helix in CNF1 (Pei et al. 2001), was identified in PMT (Baldwin et al. 2004). This motif contained two hydrophobic helices (residues 402–423 and 437–457) linked by a hydrophilic loop (residues 424–436) and was proposed to be involved in the pH-sensitive membrane translocation step (Baldwin et al. 2004). Mutation of acidic residues (Asp-373 and Asp-379) in the loop region of the helix-loop-helix motif in CNF1 resulted in complete loss of biological activity (Pei et al. 2001). However, mutation of analogous residues in the loop region of PMT (Asp-425, Asp-432, Glu-434) resulted in only partial reduction of toxin activity, whereas mutation of an acidic residue (Asp-401) just outside of the predicted motif completely abolished PMT activity (Baldwin et al. 2004). Nevertheless, these results support a model where this helix-loop-helix region of PMT is part of a putative translocation domain.
4 Effects on Cell Signaling
PMT causes a number of pleiotropic effects on targeted host cells. Most notably, PMT induces strong mitogenic (Aminova et al. 2008; Dudet et al. 1996; Mullan and Lax 1996, 1998; Rozengurt et al. 1990; Seo et al. 2000; Wilson et al. 2000; Zywietz et al. 2001) and anti-apoptotic (Aminova and Wilson 2007; Orth et al. 2007a; Preuss et al. 2010; Sabri et al. 2002) signaling in various cell lines, while simultaneously downregulating signaling pathways involved in cellular differentiation, including osteogenesis (Harmey et al. 2004; Mullan and Lax 1998; Sterner-Kock et al. 1995), adipogenesis (Aminova and Wilson 2007), and immune cell differentiation (Bagley et al. 2005; Blocker et al. 2006; Jordan et al. 2003; van Diemen et al. 1994, 1996). The cellular outcomes of these effects of PMT are manifested in the various observed disease symptoms at the different sites of infection.
Exposure to PMT through respiratory infection results in bone resorption of nasal turbinates in progressive atrophic rhinitis (DiGiacomo et al. 1991b; Foged 1992; Frymus et al. 1991; Lax and Chanter 1990; Magyar 1989; Wilson and Ho 2006) and pneumonia (a.k.a, pasteurellosis in rabbits or bovine respiratory distress in cattle) (Chrisp and Foged 1991; DiGiacomo et al. 1991a; Frymus et al. 1991; Kielstein 1986; Klein and Cunha 1997). Chronic or systemic infections also lead to testicular and splenic atrophy (Chrisp and Foged 1991; Nakai et al. 1984), liver necrosis (Cheville and Rimler 1989; Cheville et al. 1988; Chrisp and Foged 1991), kidney or bladder impairment (Hoskins et al. 1997), overall reduced weight and body fat (Cheville and Rimler 1989; Thurston et al. 1992), and growth retardation (Ackermann et al. 1996; Ackermann et al. 1995; Al-Haddawi et al. 2001). Infections in humans usually result from close contact with infected animals, particularly through respiratory exposure or bite wounds and have similar disease manifestations as those observed in animals (Arashima and Kumasaka 2005; Donnio et al. 1999; Donnio et al. 1991; Donnio et al. 2004; Frederiksen 1993; Garcia 1997; Griego et al. 1995; Henderson et al. 2010; Holst et al. 1992; Iaria and Cascio 2007; Kobayaa et al. 2009; Migliore et al. 2009; Satomura et al. 2010; Waldor et al. 1992).
4.1 Cellular Responses
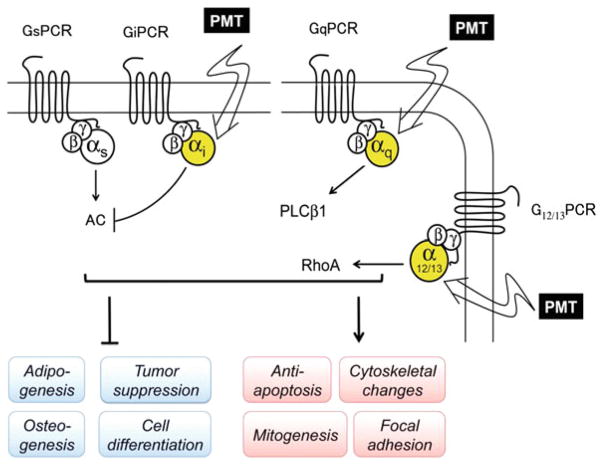
Cellular responses to PMT are induced by activation of at least three different heterotrimeric G-protein families, Gq, Gi, and G12/13 (Fig. 3). PMT-mediated activation of Gq and G12/13 signaling leads to stimulation of mitogenic responses through increased intracellular calcium and inositol phosphate levels as a result of activation of phospholipase Cβ (PLCβ) by Gq (Aminova et al. 2008; Aminova and Wilson 2007; Luo et al. 2008; Murphy and Rozengurt 1992; Staddon et al. 1991; Wilson et al. 1997) and cytoskeletal changes through activation of Rho-dependent actin signaling by Gq and G12/13 (Aepfelbacher and Essler 2001; Lacerda et al. 1996; Orth et al. 2005; Sagi et al. 2001). Simultaneous activation of Gi signaling by PMT leads to blockade of Gs-regulated adenylyl cyclase (AC) activity (Orth et al. 2008), which in turn downregulates cAMP-dependent signaling pathways involved in cellular differentiation processes. In the following sections we will summarize the known cellular effects mediated through signaling pathways activated by PMT action on its G-protein targets. It should also be noted that in addition to release of the activated Gα subunits, PMT action also releases the cognate Gβγ subunits from the heterotrimeric complexes, which concomitantly modulate other signaling pathways such as the phosphoinositide 3-kinase γ (PI3 Kγ) pathway (Preuss et al. 2009) and perhaps ion channels (Bunemann et al. 2000; Meyer et al. 2001).
Fig. 3.
Signaling pathways modulated by PMT and their cellular outcomes
4.2 Calcium Signaling
PMT strongly stimulates phospholipase Cβ1 and to a lesser extent PLCβ3, but not PLCβ2 (Wilson et al. 1997), through its selective activation of Gαq protein and not the closely related Gα11 protein (Staddon et al. 1991; Wilson et al. 1997; Zywietz et al. 2001). Although the mechanism whereby PMT discriminates between Gαq and Gα11 is not clear, this preference appears to occur through selective recognition of the helical domain of the α subunits, and not the highly conserved regions flanking the target Gln-209 residue (Orth et al. 2004). Dissociation of the Gαq subunit from the Gαβγ complex, through treatment with anti-Gβ antibodies to release the Gαq subunit or by treatment with pertussis toxin to sequester the Gβγ subunits away from the Gαq subunit, potentiates the PMT-induced PLCβ response (Wilson et al. 1997). Overexpression of Gαq protein likewise enhances the PMT-induced response. These results support the monomeric form of Gαq protein as the preferred substrate of PMT. PMT deamidation of Gαq results in constitutive activation (Orth et al. 2009), which is irreversible and persistent (Orth et al. 2007b; Wilson et al. 1997) and independent of interaction with G-protein-coupled receptors (GPCRs) (Orth et al. 2008; Orth et al. 2007b; Orth et al. 2009; Wilson et al. 1997).
Activation of PLCβ1 triggers the hydrolysis of phosphatidylinositol 4,5-bisphosphate to release inositol 1,3,5-trisphosphate (IP3) and diacylglycerol, which in turn results in mobilization of intracellular calcium pools (Staddon et al. 1991; Wilson et al. 1997) and stimulates calcium signaling pathways (Aminova et al. 2008; Aminova and Wilson 2007; Hennig et al. 2008; Luo et al. 2008) and protein kinase C (PCK)-dependent and -independent phosphorylations (Obreztchikova et al. 2006; Orth et al. 2007a; Ozgen et al. 2008; Sabri et al. 2002; Seo et al. 2000; Staddon et al. 1990; Thomas et al. 2001; Wilson et al. 2000; Zywietz et al. 2001).
4.3 Mitogenic Signaling
PMT elicits a strong mitogenic response in most cultured cell lines that leads to either proliferative or cytopathic effects. In fibroblasts (Dudet et al. 1996; Rozengurt et al. 1990; Sabri et al. 2002; Seo et al. 2000; Wilson et al. 2000; Zywietz et al. 2001), preadipocytes (Aminova and Wilson 2007), and osteoclasts (Felix et al. 1992; Gwaltney et al. 1997; Hildebrand et al. 2010a; Jutras and Martineau-Doize 1996; Martineau-Doize et al. 1993; Mullan and Lax 1996, 1998), PMT potently stimulates DNA synthesis and proliferation. PMT also strongly stimulates anti-apoptotic signaling pathways (Hildebrand et al. 2010b; Orth et al. 2007a; Ozgen et al. 2008; Preuss et al. 2010; Staddon et al. 1992). In other cells, such as embryonic bovine lung cells (Rutter and Luther 1984), Vero cells (Pennings and Storm 1984; Wilson et al. 2000), cardiomyocytes (Obreztchikova et al. 2006; Sabri et al. 2000; Sabri et al. 2002), osteoblasts, and osteosarcoma cells (Gwaltney et al. 1997; Harmey et al. 2004; Pettit et al. 1993a; Sterner-Kock et al. 1995), PMT elicits a cytopathic response characterized by stress responses, actin rearrangements and other morphological changes (see below). However, after the strong initial PMT-induced cellular response, no further stimulation occurs upon additional treatment with PMT, indicating that an uncoupling of the G-protein signaling pathways occurs (Orth et al. 2008; Wilson et al. 2000; Wilson et al. 1997).
4.4 Cytoskeletal Signaling
PMT initiates RhoA-dependent cytoskeletal signaling, including actin rearrangements, stress fiber formation, and focal adhesion assembly (Aepfelbacher and Essler 2001; Blocker et al. 2006; Dudet et al. 1996; Lacerda et al. 1996; Ohnishi et al. 1998; Orth et al. 2005; Sabri et al. 2002; Sagi et al. 2001; Thomas et al. 2001). However, PMT does not directly modify RhoA (Horiguchi 2001; Lacerda et al. 1996; Ohnishi et al. 1998); instead, PMT activation of RhoA occurs indirectly through activation of Gα12/13 (Orth et al. 2005) and to some extent Gαq (Sagi et al. 2001). PMT-induced RhoA activation leads to activation of Rho kinase and phosphorylation of focal adhesion kinase (Lacerda et al. 1996; Thomas et al. 2001) and myosin light chain (Aepfelbacher and Essler 2001; Essler et al. 1998), which in turn regulates the actin cytoskeleton, stress fiber formation, focal adhesion assembly, and endothelial cell barrier permeability. PMT disturbance of endothelial barrier function has been attributed as the cause of the observed vascular effects in dermonecrotic lesions from PMT-infected bite wounds (Aepfelbacher and Essler 2001; Elling et al. 1988).
4.5 cAMP Signaling
While potently inducing mitogenic and cytoskeletal signaling through activation of Gαq and Gα12/13 signaling, PMT simultaneously inhibits AC activity and downstream cAMP-mediated processes through activation of Gαi signaling (Orth et al. 2008). PMT-induced Gαi activation locks the Gαi subunit in its monomeric active form and overrides isoproterenol stimulation of AC by Gs-protein-coupled receptors (Orth et al. 2008). In addition, PMT deamidation of Gαi interferes with the interaction of the Gαi subunit and its cognate Gβγ subunits, and thereby converts the Gαi protein into a pertussis toxin-insensitive form (Orth et al. 2009), since pertussis toxin prefers to ADP-ribosylate the heterotrimeric Gαiβγ complex and not the monomeric Gαi protein (Katada et al. 1986). Thus, PMT treatment effectively shifts the equilibrium to dissociate the heterotrimeric complex and releases Gαi, which blocks AC and cAMP accumulation (Orth et al. 2008), and Gβγ subunits, which can interact with their cognate downstream effectors, such as PI3 Kγ (Preuss et al. 2009), which leads to inhibition of apoptotic signaling pathways (Preuss et al. 2010).
4.6 Adipogenic Signaling
PMT treatment was shown to prevent adipocyte differentiation and block adipogenesis in cultured 3T3-L1 cells under differentiation-inducing conditions (Aminova and Wilson 2007). In this study, PMT prevented expression of key adipocyte-specific markers, C/EBPα and PPARγ, in 3T3-L1 preadipocytes and downregulated these markers in mature adipocytes. PMT also prevented the downregulation of Pref1 (also called Dlk1), an EGF-like transmembrane protein that is strongly downregulated during adipocyte differentiation (Boney et al. 1996; Garces et al. 1999; Sul 2009). PMT was further shown to completely downregulate Notch1 mRNA and protein expression, while stabilizing β-catenin protein levels (Aminova and Wilson 2007). Notch1 and Wnt/β-catenin signaling pathways are involved in pivotal cell fate decisions (Andersson et al. 2011). Interestingly, the inhibitory effects of PMT on adipocyte differentiation and Notch1 could not be reversed by treatment with cyclosporine A (CsA) (Aminova and Wilson 2007), an inhibitor of calcium-calmodulin-dependent calcineurin signaling that is known to reverse Gq-PLCβ1-mediated inhibition of adipogenesis (Liu and Clipstone 2007; Neal and Clipstone 2002). These results suggest that PMT-induced Gq–PLCβ1 activation of calcium signaling is not the only signaling pathway mediated by PMT to block adipocyte differentiation, which leaves the possibility that PMT blockade of adipogenesis might also be mediated through PMT activation of either Gi or G12/13 signaling.
4.7 Osteogenic Signaling
PMT is the primary etiological agent responsible for progressive atrophic rhinitis in pigs, rabbits, and other animals (Deeb et al. 1990; DiGiacomo et al. 1993; Foged 1992; Magyar 1989; Wilson and Ho 2006). Atrophic rhinitis is characterized by destruction of the nasal turbinate bones through disruption of bone biogenesis by osteoblasts and bone degradation processes (resorption) by macrophage-like osteoclasts (Kimman and Kamp 1986; Kimman et al. 1987; Mullan and Lax 1996, 1998). In vivo, PMT treatment appears to promote bone resorption through differentiation of preosteoclasts into osteoclasts, which then proliferate (Jutras and Martineau-Doize 1996; Martineau-Doize et al. 1993). In cell culture, PMT also promotes bone resorption by osteoclasts (Felix et al. 1992; Gwaltney et al. 1997; Mullan and Lax 1996) and inhibits bone biogenesis by inhibiting osteoblast differentiation (Gwaltney et al. 1997; Harmey et al. 2004; Mullan and Lax 1998; Sterner-Kock et al. 1995).
4.8 Immune Signaling
Although immunization with PMT protein toxoid affords protection against atrophic rhinitis (Bourdon et al. 2007; Chanter and Rutter 1990; Foged et al. 1989; Frymus et al. 1989; Pettit et al. 1993b; Suckow 2000; Suckow et al. 1995; Thurston et al. 1992), natural infection with toxinogenic P. multocida is characterized by an overall lack of immune response against PMT (Bagley et al. 2005; Hamilton et al. 1998; Jordan et al. 2003; van Diemen et al. 1996). Indeed, in vivo PMT is a poor immunogen and appears to suppress antibody responses to PMT and other antigens (Bagley et al. 2005; Hamilton et al. 1998; Jordan et al. 2003), suggesting a possible in vivo role for PMT as an immunomodulator during infection.
While PMT activates human monocyte-derived and murine bone marrow-derived dendritic cells in vitro, it inhibits migration of the dendritic cells (Bagley et al. 2005; Blocker et al. 2006). PMT treatment results in Gq-dependent phosphorylation and activation of Janus tyrosine kinases (JAK1 and JAK2), which leads to activation of downstream STAT signaling and consequent upregulation of proinflammatory responses (Orth et al. 2007a) and cytokine signaling (Hildebrand et al. 2010b). Clearly, additional studies are needed to clarify the contrasting effects observed regarding PMT action on immune signaling in vitro versus in vivo.
5 Perspective
Initial studies of how bacterial protein toxins modulate host cells focused primarily on the structural organization and mode of action of the toxins. Recently, the focus of toxin studies has shifted more toward understanding the molecular interactions of the toxins with host cells, specifically how they are taken up, trafficked, and translocated into the cytosol, and how they modulate various cellular signaling pathways that lead to changes in cellular function and physiology. There are still a number of large gaps in our understanding of the intoxication process, not only for PMT but also for many other large AB toxins as well.
Since the active site Gln residue of the Gα-protein targeted by PMT is highly conserved throughout the heterotrimeric Gα-proteins, it remains to be determined which of the other Gα-proteins are also substrates for PMT and what the substrate recognition determinants are that discriminate one substrate from the others. Another area that remains unclear is the consequences to the Gα-protein once it has been modified by PMT, particularly the mechanism of its subsequent downregulation. Defining more clearly the substrate preferences of PMT and the ultimate consequences that toxin modification have on the various Gα-protein targets will assist in deciphering the differential effects on the signaling pathways elicited in different cell types and the overall cellular outcomes. Understanding toxin-induced changes in modulation of various cellular processes, including processes that facilitate actin rearrangements, proliferation, and cellular differentiation, will illuminate the critical role of toxins for the successful survival of the pathogen in the animal host. Certainly, reconciling in vitro observations with in vivo outcomes of toxin exposure is high on the list of tasks for future studies.
Acknowledgments
Some of the work reported here was supported by grants from the National Institutes of Health (NIH/NIAID AI038396) and the US Department of Agriculture (NRI 1999-02295) (to B.A.W.).
References
- Ackermann MR, Register KB, Stabel JR, Gwaltney SM, Howe TS, Rimler RB. Effect of Pasteurella multocida toxin on physeal growth in young pigs. Am J Vet Res. 1996;57:848–852. [PubMed] [Google Scholar]
- Ackermann MR, Stabel JR, Pettit RK, Jacobson CD, Elmquist JK, Register KB, Rimler RB, Hilton JH. Reduced physeal area and chondrocyte proliferation in Pasteurella multocida toxin-treated rats. Vet Pathol. 1995;32:674–682. doi: 10.1177/030098589503200609. [DOI] [PubMed] [Google Scholar]
- Aepfelbacher M, Essler M. Disturbance of endothelial barrier function by bacterial toxins and atherogenic mediators: a role for Rho/Rho kinase. Cell Microbiol. 2001;3:649–658. doi: 10.1046/j.1462-5822.2001.00145.x. [DOI] [PubMed] [Google Scholar]
- Aktories K, Barbieri JT. Bacterial cytotoxins: targeting eukaryotic switches. Nat Rev Microbiol. 2005;3:397–410. doi: 10.1038/nrmicro1150. [DOI] [PubMed] [Google Scholar]
- Al-Haddawi MH, Jasni S, Israf DA, Zamri-Saad M, Mutalib AR, Sheikh-Omar AR. Ultrastructural pathology of nasal and tracheal mucosa of rabbits experimentally infected with Pasteurella multocida serotype D:1. Res Vet Sci. 2001;70:191–197. doi: 10.1053/rvsc.2001.0459. [DOI] [PubMed] [Google Scholar]
- Aminova LR, Luo S, Bannai Y, Ho M, Wilson BA. The C3 domain of Pasteurella multocida toxin is the minimal domain responsible for activation of Gq-dependent calcium and mitogenic signaling. Protein Sci. 2008;17:945–949. doi: 10.1110/ps.083445408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminova LR, Wilson BA. Calcineurin-independent inhibition of 3T3-L1 adipogenesis by Pasteurella multocida toxin: suppression of Notch1, stabilization of beta-catenin and preadipocyte factor 1. Cell Microbiol. 2007;9:2485–2496. doi: 10.1111/j.1462-5822.2007.00975.x. [DOI] [PubMed] [Google Scholar]
- Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- Arashima Y, Kumasaka K. Pasteurellosis as zoonosis. Intern Med. 2005;44:692–693. doi: 10.2169/internalmedicine.44.692. [DOI] [PubMed] [Google Scholar]
- Bagley KC, Abdelwahab SF, Tuskan RG, Lewis GK. Pasteurella multocida toxin activates human monocyte-derived and murine bone marrow-derived dendritic cells in vitro but suppresses antibody production in vivo. Infect Immun. 2005;73:413–421. doi: 10.1128/IAI.73.1.413-421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MR, Lakey JH, Lax AJ. Identification and characterization of the Pasteurella multocida toxin translocation domain. Mol Microbiol. 2004;54:239–250. doi: 10.1111/j.1365-2958.2004.04264.x. [DOI] [PubMed] [Google Scholar]
- Binz T, Rummel A. Cell entry strategy of clostridial neurotoxins. J Neurochem. 2009;109:1584–1595. doi: 10.1111/j.1471-4159.2009.06093.x. [DOI] [PubMed] [Google Scholar]
- Blocker D, Berod L, Fluhr JW, Orth J, Idzko M, Aktories K, Norgauer J. Pasteurella multocida toxin (PMT) activates RhoGTPases, induces actin polymerization and inhibits migration of human dendritic cells, but does not influence macropinocytosis. Int Immunol. 2006;18:459–464. doi: 10.1093/intimm/dxh386. [DOI] [PubMed] [Google Scholar]
- Blumenthal B, Hoffmann C, Aktories K, Backert S, Schmidt G. The cytotoxic necrotizing factors from Yersinia pseudotuberculosis and from Escherichia coli bind to different cellular receptors but take the same route to the cytosol. Infect Immun. 2007;75:3344–3353. doi: 10.1128/IAI.01937-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boney CM, Fiedorek FT, Jr, Paul SR, Gruppuso PA. Regulation of preadipocyte factor-1 gene expression during 3T3-L1 cell differentiation. Endocrinology. 1996;137:2923–2928. doi: 10.1210/endo.137.7.8770915. [DOI] [PubMed] [Google Scholar]
- Bourdon A, Minai L, Serre V, Jais JP, Sarzi E, Aubert S, Chretien D, de Lonlay P, Paquis-Flucklinger V, Arakawa H, Nakamura Y, Munnich A, Rotig A. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet. 2007;39:776–780. doi: 10.1038/ng2040. [DOI] [PubMed] [Google Scholar]
- Brothers MC, Ho M, Maharjan R, Clemons NC, Bannai Y, Waites MA, Faulkner MJ, Kuhlenschmidt TB, Kuhlenschmidt MS, Blanke SR, Rienstra CM, Wilson BA. Membrane interaction of Pasteurella multocida toxin involves sphingomyelin. FEBS J. 2011;278:4633–4648. doi: 10.1111/j.1742-4658.2011.08365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetow L, Flatau G, Chiu K, Boquet P, Ghosh P. Structure of the Rho-activating domain of Escherichia coli cytotoxic necrotizing factor 1. Nat Struct Biol. 2001;8:584–588. doi: 10.1038/89610. [DOI] [PubMed] [Google Scholar]
- Bunemann M, Meyer T, Pott L, Hosey M. Novel inhibition of gbetagamma-activated potassium currents induced by M(2) muscarinic receptors via a pertussis toxin-insensitive pathway. J Biol chem. 2000;275:12537–12545. doi: 10.1074/jbc.275.17.12537. [DOI] [PubMed] [Google Scholar]
- Busch C, Orth J, Djouder N, Aktories K. Biological activity of a C-terminal fragment of Pasteurella multocida toxin. Infect Immun. 2001;69:3628–3634. doi: 10.1128/IAI.69.6.3628-3634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys WE, Smith HE, Kamps AM, Kamp EM, Smits MA. Sequence of the dermonecrotic toxin of Pasteurella multocida ssp. multocida. Nucleic Acids Res. 1990;18:2815–2816. doi: 10.1093/nar/18.9.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanter N, Rutter JM. Colonisation by Pasteurella multocida in atrophic rhinitis of pigs and immunity to the osteolytic toxin. Vet Microbiol. 1990;25:253–265. doi: 10.1016/0378-1135(90)90082-7. [DOI] [PubMed] [Google Scholar]
- Cheville NF, Rimler RB. A protein toxin from Pasteurella multocida type D causes acute and chronic hepatic toxicity in rats. Vet Pathol. 1989;26:148–157. doi: 10.1177/030098588902600208. [DOI] [PubMed] [Google Scholar]
- Cheville NF, Rimler RB, Thurston JR. A toxin from Pasteurella multocida type D causes acute hepatic necrosis in pigs. Vet Pathol. 1988;25:518–520. doi: 10.1177/030098588802500617. [DOI] [PubMed] [Google Scholar]
- Chrisp CE, Foged NT. Induction of pneumonia in rabbits by use of a purified protein toxin from Pasteurella multocida. Am J Vet Res. 1991;52:56–61. [PubMed] [Google Scholar]
- Chung JW, Hong SJ, Kim KJ, Goti D, Stins MF, Shin S, Dawson VL, Dawson TM, Kim KS. 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J Biol Chem. 2003;278:16857–16862. doi: 10.1074/jbc.M301028200. [DOI] [PubMed] [Google Scholar]
- Deeb BJ, DiGiacomo RF, Bernard BL, Silbernagel SM. Pasteurella multocida and Bordetella bronchiseptica infections in rabbits. J Clin Microbiol. 1990;28:70–75. doi: 10.1128/jcm.28.1.70-75.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiacomo RF, Allen V, Hinton MH. Naturally acquired Pasteurella multocida subsp. multocida infection in a closed colony of rabbits: characteristics of isolates. Lab Anim. 1991a;25:236–241. doi: 10.1258/002367791780808365. [DOI] [PubMed] [Google Scholar]
- DiGiacomo RF, Deeb BJ, Brodie SJ, Zimmerman TE, Veltkamp ER, Chrisp CE. Toxin production by Pasteurella multocida isolated from rabbits with atrophic rhinitis. Am J Vet Res. 1993;54:1280–1286. [PubMed] [Google Scholar]
- DiGiacomo RF, Xu YM, Allen V, Hinton MH, Pearson GR. Naturally acquired Pasteurella multocida infection in rabbits: clinicopathological aspects. Can J Vet Res. 1991b;55:234–238. [PMC free article] [PubMed] [Google Scholar]
- Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- Donnio PY, Allardet-Servent A, Perrin M, Escande F, Avril JL. Characterisation of dermonecrotic toxin-producing strains of Pasteurella multocida subsp. multocida isolated from man and swine. J Med Microbiol. 1999;48:125–131. doi: 10.1099/00222615-48-2-125. [DOI] [PubMed] [Google Scholar]
- Donnio PY, Avril JL, Andre PM, Vaucel J. Dermonecrotic toxin production by strains of Pasteurella multocida isolated from man. J Med Microbiol. 1991;34:333–337. doi: 10.1099/00222615-34-6-333. [DOI] [PubMed] [Google Scholar]
- Donnio PY, Lerestif-Gautier AL, Avril JL. Characterization of Pasteurella spp. strains isolated from human infections. J Comp Pathol. 2004;130:137–142. doi: 10.1016/j.jcpa.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Dudet LI, Chailler P, Dubreuil JD, Martineau-Doize B. Pasteurella multocida toxin stimulates mitogenesis and cytoskeleton reorganization in Swiss 3T3 fibroblasts. J Cell Physiol. 1996;168:173–182. doi: 10.1002/(SICI)1097-4652(199607)168:1<173::AID-JCP21>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Elling F, Pedersen KB, Hogh P, Foged NT. Characterization of the dermal lesions induced by a purified protein from toxigenic Pasteurella multocida. APMIS. 1988;96:50–55. doi: 10.1111/j.1699-0463.1988.tb05267.x. [DOI] [PubMed] [Google Scholar]
- Essler M, Hermann K, Amano M, Kaibuchi K, Heesemann J, Weber PC, Aepfelbacher M. Pasteurella multocida toxin increases endothelial permeability via Rho kinase and myosin light chain phosphatase. J Immunol. 1998;161:5640–5646. [PubMed] [Google Scholar]
- Falbo V, Pace T, Picci L, Pizzi E, Caprioli A. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect Immun. 1993;61:4909–4914. doi: 10.1128/iai.61.11.4909-4914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R, Fleisch H, Frandsen PL. Effect of Pasteurella multocida toxin on bone resorption in vitro. Infect Immun. 1992;60:4984–4988. doi: 10.1128/iai.60.12.4984-4988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- Foged NT. Pasteurella multocida toxin. The characterisation of the toxin and its significance in the diagnosis and prevention of progressive atrophic rhinitis in pigs. APMIS Suppl. 1992;25:1–56. [PubMed] [Google Scholar]
- Foged NT, Nielsen JP, Jorsal SE. Protection against progressive atrophic rhinitis by vaccination with Pasteurella multocida toxin purified by monoclonal antibodies. Vet Rec. 1989;125:7–11. doi: 10.1136/vr.125.1.7. [DOI] [PubMed] [Google Scholar]
- Frederiksen W. Ecology and significance of Pasteurellaceae in man–an update. Zentralbl Bakteriol. 1993;279:27–34. doi: 10.1016/s0934-8840(11)80488-3. [DOI] [PubMed] [Google Scholar]
- Frymus T, Bielecki W, Jakubowski T. Toxigenic Pasteurella multocida in rabbits with naturally occurring atrophic rhinitis. Zentralbl Veterinarmed B. 1991;38:265–268. doi: 10.1111/j.1439-0450.1991.tb00870.x. [DOI] [PubMed] [Google Scholar]
- Frymus T, Muller E, Franz B, Petzoldt K. Protection by toxoid-induced antibody of gnotobiotic piglets challenged with the dermonecrotic toxin of Pasteurella multocida. Zentralbl Veterinarmed B. 1989;36:674–680. doi: 10.1111/j.1439-0450.1989.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Garces C, Ruiz-Hidalgo MJ, Bonvini E, Goldstein J, Laborda J. Adipocyte differentiation is modulated by secreted delta-like (dlk) variants and requires the expression of membrane-associated dlk. Differentiation; research in biological diversity. 1999;64:103–114. doi: 10.1046/j.1432-0436.1999.6420103.x. [DOI] [PubMed] [Google Scholar]
- Garcia VF. Animal bites and Pasturella infections. Pediatr Rev. 1997;18:127–130. doi: 10.1542/pir.18-4-127. [DOI] [PubMed] [Google Scholar]
- Geissler B, Tungekar R, Satchell KJ. Identification of a conserved membrane localization domain within numerous large bacterial protein toxins. Proc Natl Acad Sci U S A. 2010;107:5581–5586. doi: 10.1073/pnas.0908700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griego RD, Rosen T, Orengo IF, Wolf JE. Dog, cat, and human bites: a review. J Am Acad Dermatol. 1995;33:1019–1029. doi: 10.1016/0190-9622(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Gwaltney SM, Galvin RJ, Register KB, Rimler RB, Ackermann MR. Effects of Pasteurella multocida toxin on porcine bone marrow cell differentiation into osteoclasts and osteoblasts. Vet Pathol. 1997;34:421–430. doi: 10.1177/030098589703400506. [DOI] [PubMed] [Google Scholar]
- Hamilton TD, Roe JM, Hayes CM, Webster AJ. Effect of ovalbumin aerosol exposure on colonization of the porcine upper airway by Pasteurella multocida and effect of colonization on subsequent immune function. Clin Diagn Lab Immunol. 1998;5:494–498. doi: 10.1128/cdli.5.4.494-498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmey D, Stenbeck G, Nobes CD, Lax AJ, Grigoriadis AE. Regulation of osteoblast differentiation by Pasteurella multocida toxin (PMT): a role for Rho GTPase in bone formation. J Bone Miner Res. 2004;19:661–670. doi: 10.1359/JBMR.040105. [DOI] [PubMed] [Google Scholar]
- Henderson SR, Shah A, Banford KB, Howard LS. Pig trotters lung–novel domestic transmission of Pasteurella multocida. Clin Med. 2010;10:517–518. doi: 10.7861/clinmedicine.10-5-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Orth J, Aktories K, Diener M. Anion secretion evoked by Pasteurella multocida toxin across rat colon. Eur J Pharmacol. 2008;583:156–163. doi: 10.1016/j.ejphar.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Hildebrand D, Heeg K, Kubatzky KF. Pasteurella multocida toxin-stimulated osteoclast differentiation is B cell dependent. Infect Immun. 2010a;79:220–228. doi: 10.1128/IAI.00565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand D, Walker P, Dalpke A, Heeg K, Kubatzky KF. Pasteurella multocida toxin-induced Pim-1 expression disrupts suppressor of cytokine signalling (SOCS)-1 activity. Cell Microbiol. 2010b;12:1732–1745. doi: 10.1111/j.1462-5822.2010.01504.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Schmidt G. CNF and DNT. Rev Physiol Biochem Pharmacol. 2004;152:49–63. doi: 10.1007/s10254-004-0026-4. [DOI] [PubMed] [Google Scholar]
- Holst E, Rollof J, Larsson L, Nielsen JP. Characterization and distribution of Pasteurella species recovered from infected humans. J Clin Microbiol. 1992;30:2984–2987. doi: 10.1128/jcm.30.11.2984-2987.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi Y. Escherichia coli cytotoxic necrotizing factors and Bordetella dermonecrotic toxin: the dermonecrosis-inducing toxins activating Rho small GTPases. Toxicon. 2001;39:1619–1627. doi: 10.1016/s0041-0101(01)00149-0. [DOI] [PubMed] [Google Scholar]
- Hoskins IC, Thomas LH, Lax AJ. Nasal infection with Pasteurella multocida causes proliferation of bladder epithelium in gnotobiotic pigs. Vet Rec. 1997;140:22. doi: 10.1136/vr.140.1.22. [DOI] [PubMed] [Google Scholar]
- Iaria C, Cascio A. Please, do not forget Pasteurella multocida. Clin Infect Dis. 2007;45:940. doi: 10.1086/521247. [DOI] [PubMed] [Google Scholar]
- Jordan RW, Hamilton TD, Hayes CM, Patel D, Jones PH, Roe JM, Williams NA. Modulation of the humoral immune response of swine and mice mediated by toxigenic Pasteurella multocida. FEMS Immunol Med Microbiol. 2003;39:51–59. doi: 10.1016/S0928-8244(03)00201-3. [DOI] [PubMed] [Google Scholar]
- Jutras I, Martineau-Doize B. Stimulation of osteoclast-like cell formation by Pasteurella multocida toxin from hemopoietic progenitor cells in mouse bone marrow cultures. Can J Vet Res. 1996;60:34–39. [PMC free article] [PubMed] [Google Scholar]
- Kamps AM, Kamp EM, Smits MA. Cloning and expression of the dermonecrotic toxin gene of Pasteurella multocida ssp. multocida in Escherichia coli. FEMS Microbiol Lett. 1990;55:187–190. doi: 10.1016/0378-1097(90)90192-s. [DOI] [PubMed] [Google Scholar]
- Katada T, Oinuma M, Ui M. Two guanine nucleotide-binding proteins in rat brain serving as the specific substrate of islet-activating protein, pertussis toxin. Interaction of the alpha subunits with beta gamma-subunits in development of their biological activities. J Biol Chem. 1986;261:8182–8191. [PubMed] [Google Scholar]
- Kielstein P. On the occurrence of toxin-producing Pasteurella multocida strains in atrophic rhinitis and in pneumonias of swine and cattle. Zentralbl Veterinarmed B. 1986;33:418–424. doi: 10.1111/j.1439-0450.1986.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Chung JW, Kim KS. 67-kDa laminin receptor promotes internalization of cytotoxic necrotizing factor 1-expressing Escherichia coli K1 into human brain microvascular endothelial cells. J Biol Chem. 2005;280:1360–1368. doi: 10.1074/jbc.M410176200. [DOI] [PubMed] [Google Scholar]
- Kimman TG, Kamp EM. Induced atrophic rhinitis in rats. Am J Vet Res. 1986;47:2426–2430. [PubMed] [Google Scholar]
- Kimman TG, Lowik CW, van de Wee-Pals LJ, Thesingh CW, Defize P, Kamp EM, Bijvoet OL. Stimulation of bone resorption by inflamed nasal mucosa, dermonecrotic toxin-containing conditioned medium from Pasteurella multocida, and purified dermonecrotic toxin from P. multocida. Infect Immun. 1987;55:2110–2116. doi: 10.1128/iai.55.9.2110-2116.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitadokoro K, Kamitani S, Miyazawa M, Hanajima-Ozawa M, Fukui A, Miyake M, Horiguchi Y. Crystal structures reveal a thiol protease-like catalytic triad in the C-terminal region of Pasteurella multocida toxin. Proc Natl Acad Sci U S A. 2007;104:5139–5144. doi: 10.1073/pnas.0608197104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein NC, Cunha BA. Pasteurella multocida pneumonia. Semin Respir Infect. 1997;12:54–56. [PubMed] [Google Scholar]
- Kobayaa H, Souki RR, Trust S, Domachowske JB. Pasteurella multocida meningitis in newborns after incidental animal exposure. Pediatr Infect Dis J. 2009;28:928–929. doi: 10.1097/INF.0b013e3181a81f0f. [DOI] [PubMed] [Google Scholar]
- Lacerda HM, Lax AJ, Rozengurt E. Pasteurella multocida toxin, a potent intracellularly acting mitogen, induces p125FAK and paxillin tyrosine phosphorylation, actin stress fiber formation, and focal contact assembly in Swiss 3T3 cells. J Biol Chem. 1996;271:439–445. doi: 10.1074/jbc.271.1.439. [DOI] [PubMed] [Google Scholar]
- Lax AJ, Chanter N. Cloning of the toxin gene from Pasteurella multocida and its role in atrophic rhinitis. J Gen Microbiol. 1990;136:81–87. doi: 10.1099/00221287-136-1-81. [DOI] [PubMed] [Google Scholar]
- Lemichez E, Flatau G, Bruzzone M, Boquet P, Gauthier M. Molecular localization of the Escherichia coli cytotoxic necrotizing factor CNF1 cell-binding and catalytic domains. Mol Microbiol. 1997;24:1061–1070. doi: 10.1046/j.1365-2958.1997.4151781.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Clipstone NA. Prostaglandin F2alpha inhibits adipocyte differentiation via a G alpha q-calcium-calcineurin-dependent signaling pathway. J Cell Biochem. 2007;100:161–173. doi: 10.1002/jcb.21044. [DOI] [PubMed] [Google Scholar]
- Lockman HA, Gillespie RA, Baker BD, Shakhnovich E. Yersinia pseudotuberculosis produces a cytotoxic necrotizing factor. Infect Immun. 2002;70:2708–2714. doi: 10.1128/IAI.70.5.2708-2714.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Ho M, Wilson BA. Application of intact cell-based NFAT-beta-lactamase reporter assay for Pasteurella multocida toxin-mediated activation of calcium signaling pathway. Toxicon. 2008;51:597–605. doi: 10.1016/j.toxicon.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar T. Study of the toxin-producing ability of Pasteurella multocida in mice. Acta Vet Hung. 1989;37:319–325. [PubMed] [Google Scholar]
- Martineau-Doize B, Caya I, Gagne S, Jutras I, Dumas G. Effects of Pasteurella multocida toxin on the osteoclast population of the rat. J Comp Pathol. 1993;108:81–91. doi: 10.1016/s0021-9975(08)80230-7. [DOI] [PubMed] [Google Scholar]
- Merritt EA, Sarfaty S, van den Akker F, L’Hoir C, Martial JA, Hol WG. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 1994;3:166–175. doi: 10.1002/pro.5560030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Wellner-Kienitz MC, Biewald A, Bender K, Eickel A, Pott L. Depletion of phosphatidylinositol 4,5-bisphosphate by activation of phospholipase C-coupled receptors causes slow inhibition but not desensitization of G protein-gated inward rectifier K+ current in atrial myocytes. J Biol Chem. 2001;276:5650–5658. doi: 10.1074/jbc.M009179200. [DOI] [PubMed] [Google Scholar]
- Migliore E, Serraino C, Brignone C, Ferrigno D, Cardellicchio A, Pomero F, Castagna E, Osenda M, Fenoglio L. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of literature. Adv Med Sci. 2009;54:109–112. doi: 10.2478/v10039-009-0005-8. [DOI] [PubMed] [Google Scholar]
- Mullan PB, Lax AJ. Pasteurella multocida toxin is a mitogen for bone cells in primary culture. Infect Immun. 1996;64:959–965. doi: 10.1128/iai.64.3.959-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan PB, Lax AJ. Pasteurella multocida toxin stimulates bone resorption by osteoclasts via interaction with osteoblasts. Calcif Tissue Int. 1998;63:340–345. doi: 10.1007/s002239900537. [DOI] [PubMed] [Google Scholar]
- Murphy AC, Rozengurt E. Pasteurella multocida toxin selectively facilitates phosphatidylinositol 4,5-bisphosphate hydrolysis by bombesin, vasopressin, and endothelin. Requirement for a functional G protein. J Biol Chem. 1992;267:25296–25303. [PubMed] [Google Scholar]
- Naglich JG, Metherall JE, Russell DW, Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992;69:1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- Nakai T, Sawata A, Tsuji M, Kume K. Characterization of dermonecrotic toxin produced by serotype D strains of Pasteurella multocida. Am J Vet Res. 1984;45:2410–2413. [PubMed] [Google Scholar]
- Neal JW, Clipstone NA. Calcineurin mediates the calcium-dependent inhibition of adipocyte differentiation in 3T3-L1 cells. J Biol Chem. 2002;277:49776–49781. doi: 10.1074/jbc.M207913200M207913200. [DOI] [PubMed] [Google Scholar]
- Obreztchikova M, Elouardighi H, Ho M, Wilson BA, Gertsberg Z, Steinberg SF. Distinct signaling functions for Shc isoforms in the heart. J Biol Chem. 2006;281:20197–20204. doi: 10.1074/jbc.M601859200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Horiguchi Y, Masuda M, Sugimoto N, Matsuda M. Pasteurella multocida toxin and Bordetella bronchiseptica dermonecrotizing toxin elicit similar effects on cultured cells by different mechanisms. J Vet Med Sci. 1998;60:301–305. doi: 10.1292/jvms.60.301. [DOI] [PubMed] [Google Scholar]
- Orth JH, Aktories K, Kubatzky KF. Modulation of host cell gene expression through activation of STAT transcription factors by Pasteurella multocida toxin. J Biol Chem. 2007a;282:3050–3057. doi: 10.1074/jbc.M609018200. [DOI] [PubMed] [Google Scholar]
- Orth JH, Blocker D, Aktories K. His1205 and His1223 are essential for the activity of the mitogenic Pasteurella multocida toxin. Biochemistry. 2003;42:4971–4977. doi: 10.1021/bi0272959. [DOI] [PubMed] [Google Scholar]
- Orth JH, Fester I, Preuss I, Agnoletto L, Wilson BA, Aktories K. Activation of Galpha (i) and subsequent uncoupling of receptor-Galpha(i) signaling by Pasteurella multocida toxin. J Biol Chem. 2008;283:23288–23294. doi: 10.1074/jbc.M803435200. [DOI] [PubMed] [Google Scholar]
- Orth JH, Lang S, Aktories K. Action of Pasteurella multocida toxin depends on the helical domain of Galphaq. J Biol Chem. 2004;279:34150–34155. doi: 10.1074/jbc.M405353200. [DOI] [PubMed] [Google Scholar]
- Orth JH, Lang S, Preuss I, Milligan G, Aktories K. Action of Pasteurella multocida toxin on Galpha(q) is persistent and independent of interaction with G-protein-coupled receptors. Cell Signal. 2007b;19:2174–2182. doi: 10.1016/j.cellsig.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Orth JH, Lang S, Taniguchi M, Aktories K. Pasteurella multocida toxin-induced activation of RhoA is mediated via two families of G{alpha} proteins, G{alpha}q and G{alpha}12/13. J Biol Chem. 2005;280:36701–36707. doi: 10.1074/jbc.M507203200. [DOI] [PubMed] [Google Scholar]
- Orth JH, Preuss I, Fester I, Schlosser A, Wilson BA, Aktories K. Pasteurella multocida toxin activation of heterotrimeric G proteins by deamidation. Proc Natl Acad Sci U S A. 2009;106:7179–7184. doi: 10.1073/pnas.0900160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald E, Sugai M, Labigne A, Wu HC, Fiorentini C, Boquet P, O’Brien AD. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc Natl Acad Sci U S A. 1994;91:3814–3818. doi: 10.1073/pnas.91.9.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgen N, Obreztchikova M, Guo J, Elouardighi H, Dorn GW, 2nd, Wilson BA, Steinberg SF. Protein kinase D links Gq-coupled receptors to cAMP response element-binding protein (CREB)-Ser133 phosphorylation in the heart. J Biol Chem. 2008;283:17009–17019. doi: 10.1074/jbc.M709851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei S, Doye A, Boquet P. Mutation of specific acidic residues of the CNF1 T domain into lysine alters cell membrane translocation of the toxin. Mol Microbiol. 2001;41:1237–1247. doi: 10.1046/j.1365-2958.2001.02596.x. [DOI] [PubMed] [Google Scholar]
- Pennings AM, Storm PK. A test in vero cell monolayers for toxin production by strains of Pasteurella multocida isolated from pigs suspected of having atrophic rhinitis. Vet Microbiol. 1984;9:503–8. doi: 10.1016/0378-1135(84)90071-3. 0378-1135(84)90071-3. [DOI] [PubMed] [Google Scholar]
- Petersen SK, Foged NT. Cloning and expression of the Pasteurella multocida toxin gene, toxA, in Escherichia coli. Infect Immun. 1989;57:3907–3913. doi: 10.1128/iai.57.12.3907-3913.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit RK, Ackermann MR, Rimler RB. Receptor-mediated binding of Pasteurella multocida dermonecrotic toxin to canine osteosarcoma and monkey kidney (vero) cells. Lab Invest. 1993a;69:94–100. [PubMed] [Google Scholar]
- Pettit RK, Rimler RB, Ackermann MR. Protection of Pasteurella multocida dermonecrotic toxin-challenged rats by toxoid-induced antibody. Vet Microbiol. 1993b;34:167–173. doi: 10.1016/0378-1135(93)90170-c. [DOI] [PubMed] [Google Scholar]
- Preuss I, Hildebrand D, Orth JH, Aktories K, Kubatzky KF. Pasteurella multocida toxin is a potent activator of anti-apoptotic signalling pathways. Cell Microbiol. 2010;12:1174–1185. doi: 10.1111/j.1462-5822.2010.01462.x. [DOI] [PubMed] [Google Scholar]
- Preuss I, Kurig B, Nurnberg B, Orth JH, Aktories K. Pasteurella multocida toxin activates Gbetagamma dimers of heterotrimeric G proteins. Cell Signal. 2009;21:551–558. doi: 10.1016/j.cellsig.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Pullinger GD, Adams TE, Mullan PB, Garrod TI, Lax AJ. Cloning, expression, and molecular characterization of the dermonecrotic toxin gene of Bordetella spp. Infect Immun. 1996;64:4163–4171. doi: 10.1128/iai.64.10.4163-4171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullinger GD, Lax AJ. Histidine residues at the active site of the Pasteurella multocida toxin. Open Biochem J. 2007;1:7–11. doi: 10.2174/1874091X00701010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullinger GD, Sowdhamini R, Lax AJ. Localization of functional domains of the mitogenic toxin of Pasteurella multocida. Infect Immun. 2001;69:7839–7850. doi: 10.1128/IAI.69.12.7839-7850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repella TL, Ho M, Chong TP, Bannai Y, Wilson BA. Arf6-dependent intracellular trafficking of pasteurella multocida toxin and pH-dependent translocation from late endosomes. Toxins. 2011;3:218–241. doi: 10.3390/toxins3030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E, Higgins T, Chanter N, Lax AJ, Staddon JM. Pasteurella multocida toxin: potent mitogen for cultured fibroblasts. Proc Natl Acad Sci U S A. 1990;87:123–127. doi: 10.1073/pnas.87.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter JM, Luther PD. Cell culture assay for toxigenic Pasteurella multocida from atrophic rhinitis of pigs. Vet Rec. 1984;114:393–396. doi: 10.1136/vr.114.16.393. [DOI] [PubMed] [Google Scholar]
- Sabri A, Pak E, Alcott SA, Wilson BA, Steinberg SF. Coupling function of endogenous alpha(1)- and beta-adrenergic receptors in mouse cardiomyocytes. Circ Res. 2000;86:1047–1053. doi: 10.1161/01.res.86.10.1047. [DOI] [PubMed] [Google Scholar]
- Sabri A, Wilson BA, Steinberg SF. Dual actions of the Galpha(q) agonist Pasteurella multocida toxin to promote cardiomyocyte hypertrophy and enhance apoptosis susceptibility. Circ Res. 2002;90:850–857. doi: 10.1161/01.RES.0000016165.23795.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi SA, Seasholtz TM, Kobiashvili M, Wilson BA, Toksoz D, Brown JH. Physical and functional interactions of Galphaq with Rho and its exchange factors. J Biol Chem. 2001;276:15445–15452. doi: 10.1074/jbc.M008961200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satomura A, Yanai M, Fujita T, Arashima Y, Kumasaka K, Nakane C, Ito K, Fuke Y, Maruyama T, Maruyama N, Okada K, Nakayama T, Matsumoto K. Peritonitis associated with Pasteurella multocida: molecular evidence of zoonotic etiology. Ther Apher Dial. 2010;14:373–376. doi: 10.1111/j.1744-9987.2009.00788.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Selzer J, Lerm M, Aktories K. The Rho-deamidating cytotoxic necrotizing factor 1 from Escherichia coli possesses transglutaminase activity. Cysteine 866 and histidine 881 are essential for enzyme activity. J Biol Chem. 1998;273:13669–13674. doi: 10.1074/jbc.273.22.13669. [DOI] [PubMed] [Google Scholar]
- Seo B, Choy EW, Maudsley S, Miller WE, Wilson BA, Luttrell LM. Pasteurella multocida toxin stimulates mitogen-activated protein kinase via G(q/11)-dependent transactivation of the epidermal growth factor receptor. J Biol Chem. 2000;275:2239–2245. doi: 10.1074/jbc.275.3.2239. [DOI] [PubMed] [Google Scholar]
- Staddon JM, Barker CJ, Murphy AC, Chanter N, Lax AJ, Michell RH, Rozengurt E. Pasteurella multocida toxin, a potent mitogen, increases inositol 1,4,5-trisphosphate and mobilizes Ca2+ in Swiss 3T3 cells. J Biol Chem. 1991;266:4840–4847. [PubMed] [Google Scholar]
- Staddon JM, Bouzyk MM, Rozengurt E. Interconversion of GRP78/BiP. A novel event in the action of Pasteurella multocida toxin, bombesin, and platelet-derived growth factor. J Biol Chem. 1992;267:25239–25245. [PubMed] [Google Scholar]
- Staddon JM, Chanter N, Lax AJ, Higgins TE, Rozengurt E. Pasteurella multocida toxin, a potent mitogen, stimulates protein kinase C-dependent and -independent protein phosphorylation in Swiss 3T3 cells. J Biol Chem. 1990;265:11841–11848. [PubMed] [Google Scholar]
- Stein PE, Boodhoo A, Armstrong GD, Heerze LD, Cockle SA, Klein MH, Read RJ. Structure of a pertussis toxin-sugar complex as a model for receptor binding. Nat Struct Biol. 1994;1:591–596. doi: 10.1038/nsb0994-591. [DOI] [PubMed] [Google Scholar]
- Sterner-Kock A, Lanske B, Uberschar S, Atkinson MJ. Effects of the Pasteurella multocida toxin on osteoblastic cells in vitro. Vet Pathol. 1995;32:274–279. doi: 10.1177/030098589503200309. [DOI] [PubMed] [Google Scholar]
- Stoll T, Markwirth G, Reipschlager S, Schmidt G. A new member of a growing toxin family–Escherichia coli cytotoxic necrotizing factor 3 (CNF3) Toxicon. 2009;54:745–753. doi: 10.1016/j.toxicon.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Suckow MA. Immunization of rabbits against Pasteurella multocida using a commercial swine vaccine. Lab Anim. 2000;34:403–408. doi: 10.1258/002367700780387769. [DOI] [PubMed] [Google Scholar]
- Suckow MA, Bowersock TL, Nielsen K, Chrisp CE, Frandsen PL, Janovitz EB. Protective immunity to Pasteurella multocida heat-labile toxin by intranasal immunization in rabbits. Lab Anim Sci. 1995;45:526–532. [PubMed] [Google Scholar]
- Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol. 2009;23:1717–1725. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W, Pullinger GD, Lax AJ, Rozengurt E. Escherichia coli cytotoxic necrotizing factor and Pasteurella multocida toxin induce focal adhesion kinase autophosphorylation and Src association. Infect Immun. 2001;69:5931–5935. doi: 10.1128/IAI.69.9.5931-5935.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston JR, Rimler RB, Ackermann MR, Cheville NF. Use of rats to compare atrophic rhinitis vaccines for protection against effects of heat-labile protein toxin produced by Pasteurella multocida serogroup D. Vet Immunol Immunopathol. 1992;33:155–162. doi: 10.1016/0165-2427(92)90042-o. [DOI] [PubMed] [Google Scholar]
- van Diemen PM, de Vries Reilingh G, Parmentier HK. Immune responses of piglets to Pasteurella multocida toxin and toxoid. Vet Immunol Immunopathol. 1994;41:307–321. doi: 10.1016/0165-2427(94)90104-x. [DOI] [PubMed] [Google Scholar]
- van Diemen PM, de Vries Reilingh G, Parmentier HK. Effect of Pasteurella multocida toxin on in vivo immune responses in piglets. Vet Q. 1996;18:141–146. doi: 10.1080/01652176.1996.9694636. [DOI] [PubMed] [Google Scholar]
- Waldor M, Roberts D, Kazanjian P. In utero infection due to Pasteurella multocida in the first trimester of pregnancy: case report and review. Clin Infect Dis. 1992;14:497–500. doi: 10.1093/clinids/14.2.497. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Aminova LR, Ponferrada VG, Ho M. Differential modulation and subsequent blockade of mitogenic signaling and cell cycle progression by Pasteurella multocida toxin. Infect Immun. 2000;68:4531–4538. doi: 10.1128/iai.68.8.4531-4538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BA, Ho M. Pasteurella multocida toxin. In: Alouf JE, Popoff MR, editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. Elsevier Science Publishers B. V; Amsterdam: 2006. pp. 430–447. [Google Scholar]
- Wilson BA, Ho M. Recent insights into Pasteurella multocida toxin and other G-protein-modulating bacterial toxins. Future Microbiol. 2010;5:1185–1201. doi: 10.2217/fmb.10.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BA, Ho M. Cellular and molecular action of the mitogenic protein-deamidating toxin from Pasteurella multocida. FEBS J. 2011;278:4616–4632. doi: 10.1111/j.1742-4658.2011.08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BA, Zhu X, Ho M, Lu L. Pasteurella multocida toxin activates the inositol triphosphate signaling pathway in Xenopus oocytes via G(q)alpha-coupled phospholipase C-beta1. J Biol Chem. 1997;272:1268–1275. doi: 10.1074/jbc.272.2.1268. [DOI] [PubMed] [Google Scholar]
- Zywietz A, Gohla A, Schmelz M, Schultz G, Offermanns S. Pleiotropic effects of Pasteurella multocida toxin are mediated by Gq-dependent and -independent mechanisms. Involvement of Gq but not G11. J Biol Chem. 2001;276:3840–3845. doi: 10.1074/jbc.M007819200. [DOI] [PubMed] [Google Scholar]