Abstract
Expression profiling of selected matrix remodeling genes was conducted to evaluate differences in molecular response to low-cycle (100) and high-cycle (7,200) sub-failure-fatigue loading of patellar tendons. Using our previously developed in vivo patellar tendon model, tendons were loaded for 100 or 7,200 cycles and expression of selected metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), and collagens were quantified by real-time RT-PCR at 1- and 7-day post-loading. Expression profiles were also obtained from lacerated tendons as an acute injury model. The high-cycle group showed upregulation of TIMP-1, -2, Col3a1, and Col5a1, and downregulation TIMP-4 at both time points, upregulation of MMP-2 at 7-day post-loading and downregulation of MMP-13 and -14 at 1-day post-loading, suggesting overall repair/remodeling. In contrast, the low-cycle loaded group showed upregulation of MMP-2, -3, -13, and Col12a1 at both time points, upregulation of TIMP-1, -2, -3, Col3a1, and integrin β1 and downregulation of integrin α11 at 1-day post-loading and upregulation of Col1a1 at 7-day post-loading, consistent with a hypertrophic (adaptive) pattern. Lacerated tendons showed a typical acute wound response with upregulation of all examined remodeling genes. Differences found in tendon response to high- and low-cycle loading are suggestive of the underlying mechanisms associated with a healthy or damaging response.
Keywords: patellar tendon, fatigue loading, TIMP, MMP, collagen
Tendon injuries are major clinical problems that commonly affect individuals exposed to repetitive tasks in their occupation including professional and recreational athletes.1,2 Tendinopathy, which encompasses tendinitis and tendinosis, has been suggested to result from excessive mechanical tendon loading.3,4 Numerous models of tendinopathy have been used to study the repair/remodeling responses of tendons to excessive loads.5–7 These studies revealed characteristic differences in microscopic morphology and in mRNA/protein levels for various matrix constituents and inflammatory mediators in tendinopathic tissues consistent with repair. Yet how tendons respond to varying degrees of load-induced damage remains poorly understood.
Previously, we investigated progressive damage accumulation in the rat flexor digitorum longus tendon using an ex vivo fatigue loading model8 and demonstrated repeatable, characteristic changes in tendon morphology and mechanical properties during the fatigue process. We then developed an in vivo model of tendon fatigue injury.9 We selected the rat patellar tendon (PT) because:
The rat is a small, inexpensive animal, used by many investigators for tendon studies.
The patella and tibia may be gripped to apply load without direct contact with the tendon.
A human clinical tendinopathy exists in the PT (“jumper’s knee”). Jumper’s knee results from accumulation of damage resulting from repetitive high loads experienced by the PT, a condition well modeled by our PT sub-rupture damage accumulation model.
This study examined the molecular response to two sub-failure cyclic loading regimens: either a high (7,200) or low (100) number of cycles of the same load magnitude. We examined the gene expression profile at 1-day post-loading to analyze the immediate, early gene response, but chose 7-day post-loading as the time point for both gene expression analysis and histologic assessment because our preliminary studies suggested that the tendon is at this point undergoing early matrix repair/remodeling. mRNA expression, a critical first step in a molecular response, was profiled for selected matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), and collagen genes, since they had been identified as likely mediators of tendon development, remodeling, repair, and disease.10 We hypothesized that: (1) sub-failure cyclical loading will induce distinct, cycle-dependent, mRNA expression signatures; (2) the expression profiles of the high-cycle loaded tendons will differ from that seen during healing of lacerated tendons.
METHODS
Experimental Design
Forty-eight adult female Sprague–Dawley rats (390 ± 30 g) (Charles River Laboratories Ltd, Wilmington, MA) were allotted into five groups: low-cycle fatigue (n =14), high-cycle fatigue (n =14), laceration (n =6), naïve control (n =8), and sham-operated (n =6).
Fatigue Loading of Patellar Tendons
Under IACUC approval, our previously developed fatigue loading protocol9 was modified to apply either 100 cycles or 7,200 cycles of sub-failure load to the PT for the same load magnitude (~50% maximal load (1–40 N) at 1 Hz). One hundred cycles were representative of a brief episode of low-cycle fatigue, and 7,200 cycles to simulate high-cycle fatigue. All other details are as previously described.9 Naïve controls received no experimental manipulations; sham-operated controls received a skin incision to expose the patella and tibia which were then gripped but not loaded. On postoperative days 1 (n =6/group) and 7 (n =6/group with an additional n =2/group for histological analysis), all animals were sacrificed for PT tissue harvest and processing.
Tendon Wound Healing
PTs were exposed as above, the paratenon was released and a transverse, full-thickness midsubstance laceration was made in the tendon with a #11 blade and repaired with a modified Kessler stitch using 6-0 Proline suture. After skin closure and analgesia, animals resumed normal cage activity and sacrificed on post-operative day 7 for tissue harvest.
RNA Isolation and RT-PCR
Tendons were isolated following sacrifice and immediately frozen in liquid nitrogen. Frozen samples were pulverized and RNA isolated using the RNeasy Kit. Total RNA concentration of each sample was determined spectrophotometrically and RNA stored at −80°C. Two to 5 μg of RNA from each sample was reverse transcribed with MMLV reverse transcriptase and an oligo (dT)12–18 primer. Real-time PCR cDNA was amplified using primers designed for the targeted genes (Supplementary Table) and quantified using the ABI Prism 7900HT real-time PCR system (Applied Biosystems, Framingham, MA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin were used as control. Data analysis showed that GAPDH was more stable than β-actin, with no significant differences found between control and loading groups. Therefore, GAPDH was used as a control. Threshold cycle values (Ct) were determined to estimate differences in starting cDNA copy number. Each tissue sample was run in triplicate.
Statistical Analysis
Means and standard deviations for expression of each gene were calculated for each group. For each gene, t-tests were conducted to compare sham-operated and naive control groups, which once concluded as not significant (p ranged from 0.33 to 0.93), were pooled for subsequent analyses. For each gene, at each time point, low-cycle, high-cycle, and pooled sham-operated and naïve control groups were compared by ANOVA followed by post hoc Bonferroni. Integrin expression was evaluated separately, with one-way ANOVAs for each time point, followed by a post hoc Bonferroni to compare low-cycle and high-cycle to sham-operated. Finally, at 7 days, for each gene, laceration was compared to high-cycle fatigue using t-tests. Significance was set at p ≤0.05.
Tendon Structure Assessment
Quadriceps–patella–PT–tibia complexes were harvested and then fixed in tension in neutral-buffered formalin for 48 h, and then plastic embedded.11 Sample preparation and image acquisition were conducted as previously described.9 Briefly, mid-sagittal thick sections (200–250 μm) were prepared and second harmonic generation (SHG) imaging was performed using an upright laser-scanning multiphoton microscope (LSM 510; Carl Zeiss, Jena, Germany), with a 9-W mode-locked femtosecond Ti:Sapphire laser (170-fs pulse width, 76 MHz repetition rate; Mira 900F; Coherent, Inc., Santa Clara, CA), tuned to 840 nm. An oil immersion objective (NA =1.0; 60× magnification) was used for focusing the excitation beam and for collecting the backward SHG signals which were then directed by a dichroic mirror to an external detector through a narrow bandpass filter (450/40 nm). Images were acquired at the midsubstance at 1,024 × 1,024 pixel resolution on a field of view of 400 × 400 μm at 15 lines/s and 1 μm intervals through the thickness of the section. Tendon damage was qualitatively assessed in the thick sections, avoiding artifacts commonly associated with thin sections. Isolated kinked fiber patterns were described as “low” level damage and a further increase in matrix disruption and angulated fibers was described as “moderate” level damage.
RESULTS
The gene expression response to high-cycle loading was characterized by changes in several genes relative to naïve control and sham tendons (Fig. 1). For clarity, data are shown normalized by dividing the gene expression value of each sample by the mean value for the pooled naïve control and sham-operated groups (hereafter referred to as “control”). As previously mentioned, statistical analysis was conducted on the raw (not normalized) data.
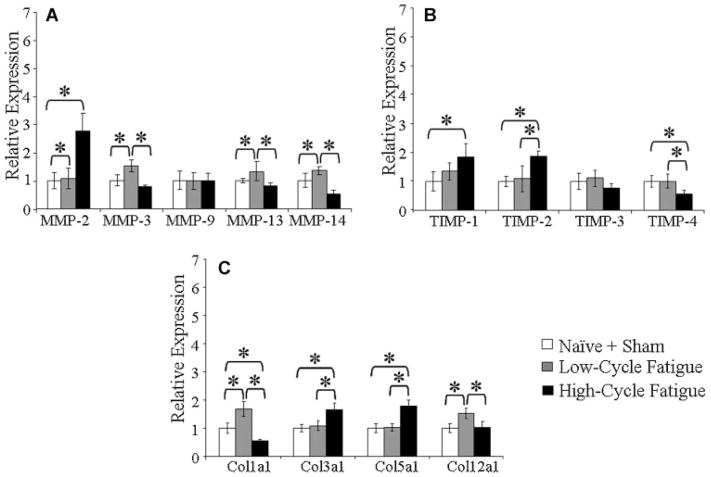
Figure 1.
(A,B,C) At 7-day post-loading, high-cycle loaded and low-cycle fatigue-loaded tendons have distinct expression signatures. (A) MMP expression, (B) TIMP expression, and (C) Col expression relative to pooled sham-operated and naive control. A significant difference of p <0.05 is denoted by *.
In the PT at 7-day after high-cycle loading, MMP-2 was upregulated, relative to control, by 2.8-fold (p <0.001), MMP-14 was downregulated by 0.5-fold (p <0.001) and MMP-3, -9, and -13 did not significantly differ (p ≥ 0.05). TIMPs 1 and 2 were upregulated to 1.9-fold (p <0.001), but TIMP-4 was downregulated to 0.6-fold (p ≤ 0.01) and TIMP-3 was not significantly different relative to control. Cols III and V were upregulated to 1.7- and 1.8-fold, respectively (p <0.001), Col1a1 was downregulated to 0.6-fold (p <0.001), and Col12a1 did not significantly differ from control.
The response at 7-day post-low-cycle-loading was distinct from high-cycle-loading and also from control (Fig. 1). Relative to control, MMP-3, -13, and -14, and Cols I and XII upregulated to 1.6-fold (p <0.001), 1.3-fold (p <0.01), 1.4-fold (p =0.01), 1.8-fold (p <0.001), and 1.6-fold (p <0.001), respectively. MMP-2, TIMP-2, Col3a1, and Col5a1 were significantly more upregulated relative to the high-cycle than in the low-cycle fatigue-loaded group (p <0.001, p =0.001, p <0.001, p <0.001). MMP-3, -13, -14, TIMP-4, and Col12a1 were signifi-cantly more upregulated for the low-cycle than the high-cycle fatigue-loaded group (p <0.001, p <0.001, p <0.001, p <0.01, and p <0.001).
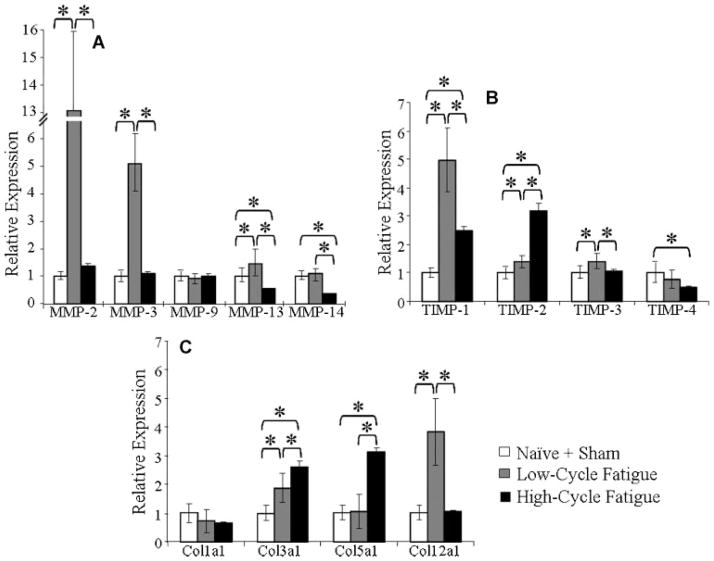
The responses in PTs at 1-day post-fatigue loading are distinguished from that at 7 days in a cycle-dependent manner (Fig. 2). In response to low-cycle loading TIMP-1, -2, -3, and Col3a1 were only upregulated at 1 day but not at 7 days, suggesting the responses were transient. MMP-14 and Col1a1 were upregulated at 7-day but not 1-day post-fatigue loading, likely as a later phase or secondary response. Similarities between the profile at 1 and 7 days were observed for the low-cycle fatigue group for MMP-2, -3, -13, and Col12a1 (upregulated), although with different levels of induction. In response to high-cycle fatigue loading, MMP-13 and -14 were downregulated at 1 day only, indicating a transient response. MMP-2 was upregulated and Col1a1 was downregulated at 7 days indicating a secondary response. Notably, similarities between the response at 1 and 7 days were observed for the high-cycle fatigue group for MMP-13, -14, and TIMP-4 (downregulated) and TIMP-1, -2, Col3a1, and Col5a1 (upregulated), although the levels of the induction were different.
Figure 2.
(A,B,C) At 1-day post-loading, high-cycle loaded and low-cycle fatigue-loaded tendons have distinct expression signatures. (A) MMP expression, (B) TIMP expression, and (C) Col expression relative to pooled sham-operated and naive control. A significant difference of p <0.05 is denoted by *.
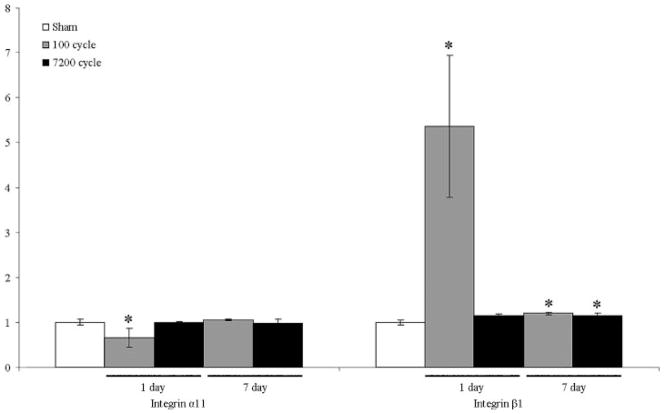
Interestingly, integrin α11 was slightly downregu-lated, and integrin β1 upregulated, in response to 100 but not 7,200 cycles of loading 1-day post-loading. Changes in gene expression of integrin β1 were observed 7 days post-loading for both loading groups (Fig. 3).
Figure 3.
mRNA expression of integrin α11 and β1 for low-cycle and high-cycle loaded groups at 1- and 7-day post-loading.
We then compared the high-cycle loading induced gene expression response with that induced by laceration in rat PT. The gene expression response in the tendon at 7-day after laceration was distinctly different from the high-cycle fatigue loading group (Table 1). MMP-3, -9, -13, and -14, TIMP-1, -3, and -4, and all collagen types evaluated were upregulated at a higher level in the lacerated than the high-cycle fatigue. MMP-2 and TIMP-2 were not significantly different for the lacerated than the high-cycle fatigue-loaded group.
Table 1.
Fold Difference in Gene Expression between Laceration and High-Cycle Fatiguea
| Gene | Laceration = (High-Cycle) × (x) | p-Value | Gene | Laceration = (High-Cycle) × (x) | p-Value | Gene | Laceration = (High-Cycle) × (x) | p-Value |
|---|---|---|---|---|---|---|---|---|
| MMP-2 | 0.8x | ns | ||||||
| MMP-3 | 2.99x | p ≤0.001 | TIMP-1 | 3.63x | p ≤0.0001 | Col1a1 | 14.85x | p ≤0.0001 |
| MMP-9 | 2.34x | p ≤0.001 | TIMP-2 | 0.79x | ns | Col3a1 | 3.45x | p ≤0.0001 |
| MMP-13 | 5.49x | p ≤0.0001 | TIMP-3 | 2.40x | p ≤0.001 | Col5a1 | 1.27x | p =0.02 |
| MMP-14 | 19.72x | p ≤0.0001 | TIMP-4 | 5.01x | p ≤0.0001 | Col12a1 | 2.61x | p ≤0.0001 |
p-Values are shown with “ns” denoting nonsignificant.
Representative images of the tendon midsubstance acquired using SHG are shown in Figure 4. At 7-day post-fatigue, damage was observed in both the 100- and 7,200-cycle-loaded images but not in naïve control images. Angulated fibers were present in the 7,200 but not the 100-cycle group indicating a higher level of matrix damage for the 7,200-cycle group.
Figure 4.
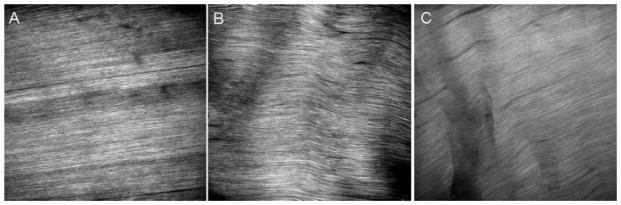
Representative images of the tendon midsubstance acquired using SHG at 7 days post-fatigue for (A) control, (B) 100-cycle loaded group, and (C) 7,200-cycle loaded group.
DISCUSSION
While mechanisms underlying the pathogenesis of tendinopathy remain unknown, tendon overuse has been proposed as a critical inciting factor. Repeated mechanical loading at levels within physiologic range can lead to accumulation of microscopic damage in tendon; continued loading may then result in clinically evident tendinosis, tendinitis, and tendon rupture.12
In this study, we assessed the molecular response to low-cycle and high-cycle fatigue loading in rat PTs. Changes in in vivo mechanical/material properties resulting from high-cycle tendon fatigue resembled those of ex vivo fatigue-loaded tendons previously shown to recapitulate microstructural changes seen in tendinopathy.8,9,13 Low-cycle fatigue loading, chosen to reflect the early fatigue process, simulated a different, possibly adaptive, response.
Gene expression profiling demonstrated distinct, cycle-dependent tissue responses in the two loaded tendon groups. Since sub-failure damage to ligaments in vivo (Grade II sprain) was reported to induce a different molecular response than tissue rupture,14 and since loading in our tendon overuse model was applied acutely to healthy tendons, a PT laceration group was included and compared to the high-cycle fatigue to assess if the response of the tendon to high-cycle fatigue resembled that of an acute wound healing response. The increased expression of 11 of 13 genes in lacerated tendons relative to the high-cycle fatigue group indicates that wound repair responses in tendon are distinct from the response to cyclic loading.
MMPs play various roles in ECM development, degradation, and remodeling. Our data suggest that high-cycle fatigue loading may impair the ability of ECM to maintain homeostasis, potentially resulting in a catabolic response to overuse. In contrast, the gene expression profile of the low-cycle fatigue group is consistent with an adaptive (hypertrophic) response to loading. For example, MMP-3, which is downregulated in tendinopathic tendons and may be critical to maintenance and remodeling of tendons,15,16 was upregulated in low-cycle (but not high-cycle) fatigue loading at both 1-and 7-day post-loading. In contrast, MMP-2, which was found upregulated in human peritendinous tissue after exercise17 and in Achilles tendinopathy,18,19 was increased 1-day post-low-cycles fatigue loading and 7-day post-high-cycle fatigue loading. Finally, MMP-13 and -14, which may be implicated in damaged matrix degradation and ECM remodeling,20 showed opposite expression profiles in low-cycle (upregulated) and high-cycle loading (downregulated), further highlighting the disparate response at the two loading levels.
MMP activity is irreversibly inhibited by TIMPs. Disturbance in the balance between MMP and TIMP activities, likely leading to altered matrix turnover, may play a critical role in the pathogenesis of tendinopathy.21 Although no significant changes in TIMP expression were observed with low-cycle fatigue, high-cycle fatigue-loaded tendons upregulated TIMP-1 and -2 and down-regulated TIMP-4, again highlighting altered ECM homeostasis in response to high-cycle fatigue loading. TIMP-1 and -2 upregulation might be expected to occur in conjunction with MMP-2, reflecting the 1:1 stoichiometry of TIMP interaction with MMPs.22 However, TIMP-4 downregulation may indicate a distinct function for this TIMP in tendon repair. TIMP-4 has been shown to be downregulated in healing ligaments and in tendinopathy15,23 and may have anti-angiogenic and anti-apoptotic activities, among others.24
Upregulation of Col3a1 and Col5a1 and downregulation of Col1a1 expression in high-cycle fatigue loading are consistent with a repair/remodeling response. Type III collagen has been identified as a major component of newly synthesized ECM in early tendon healing,25 while type V collagen is found in the vascular walls of tendons,26 developing embryonic tendons,27 and degenerated adult tendons.28 Moreover, type V collagen has been suggested to play a critical role in regulating collagen fibril assembly.29 Additionally, it should be noted that the Col3a1/Col1a1 mRNA expression ratio was increased 3-fold from 3:10 in low-cycle fatigue-loaded tendons to 9:10 in high-cycle fatigue-loaded tendons, consistent with elevated type III to type I collagen protein and mRNA ratios in tendino-pathy.30,31
In contrast to the high-cycle fatigue group, the low-cycle fatigue group was characterized by upregulation of Col1a1 and Col12a1. Col I (95% of collagen in mature tendon)32 is decreased in tendons and ligaments of immobilized rat and rabbit hindlimbs.33 Furthermore, Col I synthesis is increased in the anabolic response to physical exercise34 and may indicate a hypertrophic adaptive response to the load magnitude used in our loading protocol. Col XII interacts with Col I and proteoglycans, acting as a molecular cross-link within and between collagen fibrils and between fibrils and other ECM components,35 and may play a role in stabilizing tissues components during normal ECM maintenance. Therefore, upregulation of Col1a1 and Col12a1 expression may represent a hypertrophic adaptive response. Furthermore, MMP and TIMPs expression in low-cycle loading demonstrated an overall balanced ratio which may provide sufficient proteolytic activity for collagen such as Col I synthesis and fibro-genesis while protecting the tissue. Therefore, these data support that low-cycle loading may be beneficial. Interestingly, in this study we found that expression of integrinα11 and integrinβ1 differentially respond to loading in a cycle- and time-dependent fashion. Integrins play important roles in mediating matrix–cellular interactions, collagen and metalloproteinase synthesis, and mechanotransduction.36,37 The α11 integrin, associated with β1 integrin is a receptor for fibril collagens, such as Col I, and is involved in cell migration and collagen reorganization on mesenchymal cells including periodontal ligament fibroblasts.38 In vivo and in vitro studies also showed that MMP-13 and -14 syntheses were disturbed in α11−/− cells.37 Further study of the differential responses of integrins to low and high cycles of fatigue loading may provide insights into the mechanism behind its regulation of tendon matrix homeostasis, repair, and regeneration.
There are limitations to this study. First, mRNA expression is not necessarily directly translated into protein level. MMPs are initially synthesized as an inactive pro-enzyme and require various factors to convert the pro-MMP into its active form. Although evaluation at the mRNA level is important, analysis at the protein level may provide further insight into the underlying mechanisms of tendinopathy. Second, our bulk tissue analysis showed progressive and repeatable changes in the mechanical properties and morphology; however, the tissue response to fatigue is likely localized to areas of cellular and matrix damage. Third, analysis in this study was limited to 1- and 7-day post-loading. Additional time points may be required to fully understand the biologic response of tendon to fatigue loading. Finally, in this study we compared two cycle numbers (100 and 7,200) and did not relate the biologic response to tendon mechanical response. Future studies are planned to further investigate all of these areas.
In summary, using an in vivo sub-failure fatigue loading model, we showed that high-cycle fatigue loading (7,200 cycles of loading) of rat PTs induced changes in gene expression in MMPs, TIMPs, and collagens that differed from low-cycle fatigue loading (100 cycles of loading) and laceration. The former suggested a repair/remodeling response while the latter was consistent with tendon hypertrophy (adaptation). These gene expression profiles, coupled with imaging analysis and previous morphological findings, support the hypothesis that molecular responses of tendons induced by sub-failure loading are cycle dependent.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institutes of Health to E. L. F. (AR052743) and H. B. S. (AR050968 and AR04768). The authors thank Dr. Majeska and Dr. Rajan for their contributions.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Khan K, Cook J. The painful nonruptured tendon: clinical aspects. Clin Sports Med. 2003;22:711–725. doi: 10.1016/s0278-5919(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 2.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43:131–142. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 4.Williams JG. Achilles tendon lesions in sport. Sports Med. 1986;3:114–135. doi: 10.2165/00007256-198603020-00003. [DOI] [PubMed] [Google Scholar]
- 5.Nakama LH, King KB, Abrahamsson S, et al. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Pike AV, Ker RF, Alexander RM. The development of fatigue quality in high- and low-stressed tendons of sheep (Ovis aries) J Exp Biol. 2000;203:2187–2193. doi: 10.1242/jeb.203.14.2187. [DOI] [PubMed] [Google Scholar]
- 7.Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 8.Fung DT, Wang VM, Laudier DM, et al. Subrupture tendon fatigue damage. J Orthop Res. 2009;27:264–273. doi: 10.1002/jor.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung DT, Wang VM, Andarawis-Puri N, et al. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech. 2009;43:274–279. doi: 10.1016/j.jbiomech.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466:1528–1538. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laudier D, Schaffler MB, Flatow EL, et al. Novel procedure for high-fidelity tendon histology. J Orthop Res. 2007;25:390–395. doi: 10.1002/jor.20304. [DOI] [PubMed] [Google Scholar]
- 12.Ker RF. The implications of the adaptable fatigue quality of tendons for their construction, repair and function. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:987–1000. doi: 10.1016/s1095-6433(02)00171-x. [DOI] [PubMed] [Google Scholar]
- 13.Sun HB, Li Y, Fung DT, et al. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res. 2008;466:1555–1561. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provenzano PP, Alejandro-Osorio AL, Valhmu WB, et al. Intrinsic fibroblast-mediated remodeling of damaged collagenous matrices in vivo. Matrix Biol. 2005;23:543–555. doi: 10.1016/j.matbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Lo IK, Marchuk LL, Hollinshead R, et al. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32:1223–1229. doi: 10.1177/0363546503262200. [DOI] [PubMed] [Google Scholar]
- 16.Riley GP, Curry V, DeGroot J, et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- 17.Koskinen SO, Heinemeier KM, Olesen JL, et al. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J Appl Physiol. 2004;96:861–864. doi: 10.1152/japplphysiol.00489.2003. [DOI] [PubMed] [Google Scholar]
- 18.Alfredson H, Lorentzon M, Backman S, et al. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. J Orthop Res. 2003;21:970–975. doi: 10.1016/S0736-0266(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 19.Choi HR, Kondo S, Hirose K, et al. Expression and enzymatic activity of MMP-2 during healing process of the acute supraspinatus tendon tear in rabbits. J Orthop Res. 2002;20:927–933. doi: 10.1016/S0736-0266(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 20.Oshiro W, Lou J, Xing X, et al. Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg Am. 2003;28:814–823. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]
- 21.Dalton S, Cawston TE, Riley GP, et al. Human shoulder tendon biopsy samples in organ culture produce procollagenase and tissue inhibitor of metalloproteinases. Ann Rheum Dis. 1995;54:571–577. doi: 10.1136/ard.54.7.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bramono DS, Richmond JC, Weitzel PP, et al. Matrix metalloproteinases and their clinical applications in orthopaedics. Clin Orthop Relat Res. 2004;428:272–285. doi: 10.1097/01.blo.0000144166.66737.3a. [DOI] [PubMed] [Google Scholar]
- 23.Reno C, Boykiw R, Martinez ML, et al. Temporal alterations in mRNA levels for proteinases and inhibitors and their potential regulators in the healing medial collateral ligament. Biochem Biophys Res Commun. 1998;252:757–763. doi: 10.1006/bbrc.1998.9734. [DOI] [PubMed] [Google Scholar]
- 24.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 25.Eriksen HA, Pajala A, Leppilahti J, et al. Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res. 2002;20:1352–1357. doi: 10.1016/S0736-0266(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 26.Jozsa LG, Kannus P. Human tendons: anatomy, physiology, and pathology. 1. Champaign, Illinois: Human Kinetics Publishers; 1997. [Google Scholar]
- 27.Birk DE, Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol. 1997;72:352–361. [PubMed] [Google Scholar]
- 28.Goncalves-Neto J, Witzel SS, Teodoro WR, et al. Changes in collagen matrix composition in human posterior tibial tendon dysfunction. Joint Bone Spine. 2002;69:189–194. doi: 10.1016/s1297-319x(02)00369-x. [DOI] [PubMed] [Google Scholar]
- 29.Wenstrup RJ, Florer JB, Brunskill EW, et al. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 30.Birch HL, Bailey AJ, Goodship AE. Macroscopic ’degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet J. 1998;30:534–539. doi: 10.1111/j.2042-3306.1998.tb04530.x. [DOI] [PubMed] [Google Scholar]
- 31.Ireland D, Harrall R, Curry V, et al. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–169. doi: 10.1016/s0945-053x(01)00128-7. [DOI] [PubMed] [Google Scholar]
- 32.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 33.Vailas AC, Deluna DM, Lewis LL, et al. Adaptation of bone and tendon to prolonged hindlimb suspension in rats. J Appl Physiol. 1988;65:373–376. doi: 10.1152/jappl.1988.65.1.373. [DOI] [PubMed] [Google Scholar]
- 34.Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Rest M, Aubert-Foucher E, Dublet B, et al. Structure and function of the fibril-associated collagens. Biochem Soc Trans. 1991;19:820–824. doi: 10.1042/bst0190820. [DOI] [PubMed] [Google Scholar]
- 36.Chiquet M, Gelman L, Lutz R, et al. From mechano-transduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Popova SN, Barczyk M, Tiger CF, et al. Alpha11 beta1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor. Mol Cell Biol. 2007;27:4306–4316. doi: 10.1128/MCB.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiger CF, Fougerousse F, Grundstrom G, et al. Alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev Biol. 2001;237:116–129. doi: 10.1006/dbio.2001.0363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.