Abstract
Studies investigating the impact of cannabis use on bipolar clinical characteristics and neurocognition are limited. The objective of the present study was to compare clinical and neurocognitive measures in individuals with bipolar disorder with a history of cannabis use disorder (CUD) versus those without a history of CUD. We conducted a retrospective analysis of a large cohort (N=200) of bipolar I subjects, either with (CUD+; N=50) or without (CUD−; N=150) a history of CUD. We compared the groups on clinical and demographic variables, as well as on performance on neurocognitive tests. Patient groups did not differ regarding age, age of onset or global assessment of functioning. Compared to the CUD− group, the CUD+ group had a higher proportion of men and a higher proportion of patients with a history of psychosis. CUD+ subjects demonstrated significantly better performance on measures of attention, processing speed, and working memory. The history of CUD is associated with history of psychosis, suggestive of poorer clinical prognosis. Interestingly, bipolar patients with history of CUD had better neurocognitive performance as compared to patients with no history of CUD.
Keywords: Bipolar Disorder, Cannabis, Neurocognition, Prognosis
1. Introduction
Cannabis is the most widely used illicit substance in western countries, with lifetime prevalence of 8-12% in United States (Conway et al., 2006; Stinson et al., 2006). Cannabis use has been associated with a number of clinical and functional impairments, such as impaired educational attainment (Lynskey et al., 2000) and reduced workplace productivity (Lehman et al., 1992). Cannabis has also been associated with cognitive impairments, particularly in heavy users (Pope, Jr. et al., 1996; Schweinsburg et al., 2008).
The impact of cannabis use disorders (CUD) is particularly pertinent among subjects with major psychiatric disorders. Compared to the general population, patients with schizophrenia or bipolar disorder have a 2 to 3 fold increase in CUD rates (Stinson et al., 2006; Koskinen et al., 2010). Moreover, studies have suggested that cannabis increases risk of psychotic presentations (Moore et al., 2007), but the topic is still controversial (Arseneault et al., 2004), and evidence for affective outcomes is even less clear (Strakowski et al., 2007). Among subjects with bipolar disorder, most studies suggest that cannabis use is associated with deleterious effects, such as greater treatment non-compliance and impaired psychosocial functioning (Goldberg et al., 1999; van Rossum et al., 2009). In addition, patients with bipolar disorder who use cannabis are more likely to have psychotic symptoms (van Rossum et al., 2009), make more frequent suicide attempts (Dalton et al., 2003; Weiss et al., 2005), and show a poorer response to lithium (Goldberg et al., 1999), when compared with bipolar patients who do not use cannabis. In contrast, at least one study has reported that cannabis use is associated with a more complete affective symptom remission in bipolar patients (Strakowski et al., 2007).
Several studies of CUD in schizophrenia have reported a somewhat counterintuitive finding with regard to the effects of comorbid CUD on neurocognitive functioning. Specifically, patients with schizophrenia who use cannabis outperform patients with schizophrenia without comorbid CUD on neurocognitive functions, such as tasks of motor speed, attention, memory and verbal fluency (Yucel et al., 2010; DeRosse et al., 2010). However, the direction of this relationship is unclear and causality has not yet been established. There is a paucity of data on the cognitive effects of comorbid CUD in patients with other severe psychiatric illnesses such as bipolar disorder. The only study that evaluated the relationship between cannabis use and cognition in bipolar disorder patients reported superior performance on a single measure of verbal fluency in 133 bipolar patients with a history of cannabis use when compared to patients with no CUD history (Ringen et al., 2010).
Therefore, the goal of the current study was to further explore the relationship between CUD history in bipolar disorder and neurocognitive functioning. In addition, we compared patient groups on several clinical features to determine the effects of CUD on course of illness.
2. Methods
2.1 Sample
The study cohort was recruited from The Zucker Hillside Hospital (ZHH)-North Shore LIJ Health System (NSLIJHS), in Glen Oaks, N.Y. Subjects included in these analyses were selected from all patients with a diagnosis of bipolar I disorder for whom we had complete or nearly complete data on measures of interest. Data were collected over a 9 year period (2000- 2009) as a part of a larger genetics study including multiple DSM-IV diagnoses. All subjects provided written informed consent to a protocol approved by the Institutional Review Board of the NSLIJHS. Subjects were between the ages of 18 and 65, with no history of neurological disorders, and no major CNS trauma. Subjects had an estimated premorbid IQ (based on the Wide Range Achievement Test-3rd edition (WRAT-3) Reading subtest) of greater than 70.
2.2 Clinical assessment
Each participant completed the Structured Clinical Interview for the DSM-IV (SCID-IV) administered by trained and reliable raters. Information obtained from the SCID was supplemented by a review of medical records and interviews with family informants when possible, and compiled into a narrative case summary. Primary and secondary diagnoses were then determined by a consensus among a minimum of three expert diagnosticians from the ZHH faculty. All subjects included in the current analyses met full DSM-IV criteria for bipolar I disorder; bipolar II patients were excluded. Cannabis use history was also documented by the SCID interview and patients who met criteria for a history of cannabis abuse or dependence were included in the bipolar disorder with a cannabis use disorder (CUD+) and were compared with bipolar disorder patients who had never met criteria for abuse or dependence (CUD−). Subjects were included with current, early partial, and sustained full remission (data below).
An index of the severity of mood symptoms at the time of assessment was derived from SCID data by summing the scores for each symptom item used to determine diagnostic criteria for current episodes of mania and depression. Ratings on each of the items were recorded based on the subject's report during the interview, observation, as well as medical records. Symptoms at the time of assessment were rated on a scale such that 1 = absent, 2 = subthreshold, and 3 = present at threshold level. Summation of each symptom contributing toward the determination of episode severity captured the overall symptom severity of each polarity at the time of SCID interview.
2.3 Neurocognitive assessment
Participants were administered a battery of standardized cognitive measures comprised of the California Verbal Learning Test (CVLT)-Abridged; Controlled Oral Word Association Test (COWAT); Animal Naming; Wechsler Adult Intelligence Test-Revised (WAIS-R)-Digit Span; and Trail Making Parts A and B. Following common practice in the psychiatric literature (Keefe et al., 2005), we estimated premorbid IQ using the Wide Range Achievement Test-Third Edition-Reading (WRAT-3). The WRAT-3 is a test that assesses single word reading skill which, like command of general knowledge and vocabulary, is particularly resistant to the effects of deterioration associated with brain disease and is considered an estimate of premorbid IQ in patient populations. All participants completed cognitive testing in a single session within one week of the SCID interview.
2.4 Statistical analyses
Analyses compared bipolar patients without a history of comorbid CUD (CUD−) to bipolar patients with CUD (CUD+). Initially, groups were compared on demographic variables including sex, race, parental socioeconomic status (PSES) (Hollingshead, 1975) and family history of psychotic illness using Chi square analyses. Group comparisons of current age, age at onset of bipolar disorder, global assessment of functioning (GAF) score, illness duration, premorbid IQ (as measured by WRAT-3), and education level were carried out using independent t-tests. Any demographic variable that was shown to differentiate groups was used as a covariate in the analysis comparing CUD+ and CUD− groups on symptom and neurocognitive measures using multivariate analyses of covariance (MANCOVAs). Neurocognitive data are presented in raw format (mean and standard deviation) as well as in a z-score scale for graphic presentation and easy interpretation. Z-scores were calculated using a demographically-matched healthy control sample (n=245) for illustrative purposes only.
3. Results
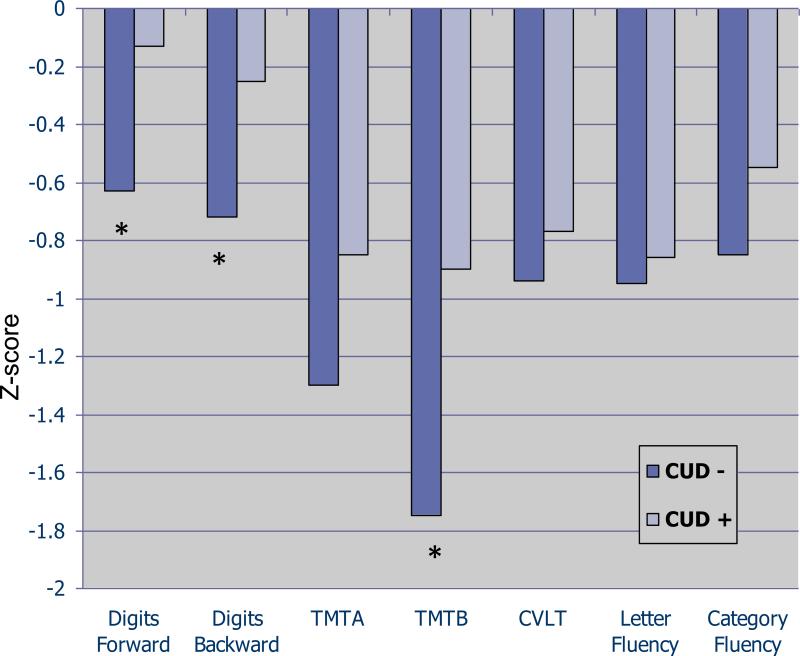
A total of 200 bipolar I patients were included in the analyses; 50 of whom met criteria for a past cannabis use disorder, while 150 never met CUD criteria. Of the 50 with CUD history, 9 subjects met criteria for current abuse/dependence; 5 met for early partial remission; 3 met for early full remission; 13 met for sustained full remission; and the remaining 20 subjects met for remote past CUD. Demographics: Patient groups did not differ regarding age, racial background, or highest education level achieved (Table 1). Sex distribution differed significantly among groups such that a larger proportion of patients with a history of CUD (CUD+) were male as compared with patients without CUD history (CUD−) (62% vs 43.3%, p=0.02). Course of illness: Bipolar patients with CUD had similar age at onset as patients without CUD (Table 1). CUD groups differed significantly with regard to history of psychosis dring mood episodes such that patients with a history of CUD were more likely to have experienced psychosis at some time during their illness course than patients who never met criteria for a CUD (82% vs 67.3%, x2= 3.91, p=0.048). Current functioning and clinical severity at the time of assessment: Global assessment of functioning (GAF score) was comparable between groups and the proportion of patients meeting SCID criteria for a current affective episode (symptomatic vs non-symptomatic) was similar (Table 1). Controlling for sex and psychosis history, subjects with or without history of CUD had similar scores on measures of current manic (12.1 +/− 6.3 vs 12.7 +/− 6.1, F=1.9; df=196; p=0.17) and current depressive psychopathology (11.8 +/− 5.5 vs 13.0 +/− 6.7, F=0.04; df= 196; p=0.83), as measured by SCID item severity. Neurocognitive functioning: All neurocognitive variables were normally distributed and Levene's Test for Equality of Variance revealed no significant group differences – thus raw data were used for subsequent analyses. The neurocognitive analyses indicated a relatively generalized pattern of superior performance in the CUD+ subjects in comparison with the CUD− subjects. After controlling for variables that differed significantly by cannabis group (sex distribution and psychosis history included as fixed factors), MANCOVAs revealed significant group differences for measures of attention (Digits forward; F=4.1; df= 1,103; p=0.04), processing speed/set-shifting (Trails B; F=4.6; df=1,147; p=0.03), and working memory (Digits Backward; F=4.7; df=1, 103; p=0.03). On all significant measures, CUD+ subjects performed better than CUD− subjects. Figure 1 presents these data on a z-score scale with a mean of zero and standard deviation of one. There were no significant main effects of sex or psychosis history and no significant 2-way or 3-way interaction effects for any of the neurocognitive measures.
Table 1.
Clinical and Demographic Characteristics of the Sample
| Characteristics | CUD + | CUD – | Statistic, df (p-value) |
|---|---|---|---|
| Age | 34.3(12.5) | 37.5(12.1) | t= 1.6, 198 (0.11) |
| Sex-Male (%) | 62% | 43.3% | Chi2= 5.24,1 (0.02) |
| Race - White (%) | 66% | Chi2= 0.03, 1 (0.86) | |
| Education (yrs) | 13.8 (2%) | 14.4 (2.3) | t= 1.34, 151 (0.18) |
| Parental Socioeconomic status (Hollingshead Index) | 2.6 (1.2) | 2.6(0.8) | t=0.55, 147 (0.58) |
| Estimated premorbid IQ | 98.02 (9.19) | 96.4(11.1) | t= 0.80, 137 (0.42) |
| GAF score | 46.2 (14.7) | 44.14 (14.6) | t= 0.6, 94 (0.55) |
| Duration of illness (yrs) | 13.0 (10.8) | 15.0 (11.3) | t= 1.11, 198 (0.27) |
| Age of onset | 21.3 (7.2) | 22.5 (8.4) | t= 1.02, 198 (0.32) |
| History of psychosis (%) | 82.0% | 67.3% | Chi2= 3.92, 1 (0.05) |
| Family history of Psychosis (%) | 22.8% | 25.0% | Chi2= 0.48, 1 (0.49) |
| Mean number of drugs | 3.54 (2.8) | 2.89 (2.5) | t= 1.54, 198 (0.13) |
| Antipsychotic use (%) | 93.8% | 73.6% | Chi2 = 2.14,1(0.14) |
| Anticonvulsants use (%) | 58.5% | 56.9% | Chi2= 0.03 (0.86) |
| Lithium use (%) | 36.6% | 33.1% | Chi2= 0.171, 1 (0.68) |
| Clinically symptomatic at time of assessment (%) | 46% | 59.9% | Chi2= 2.92, 1 (0.09) |
Figure 1. Neurocognitive performance by history of Cannabis Use.
Subjects with history of cannabis use demonstrated significantly better performance on measures of attention (Digits forward; F=4.1; df= 1,103; p=0.04), processing speed/setshifting (Trails B; F=4.6; df=1,147; p=0.03), and working memory (Digits Backward; F=4.7; df=1, 103; p=0.03).
In secondary analyses, we tested the hypothesis that perhaps a history of alcohol misuse might explain the somewhat counterintuitive results of the cognitive analyses. Specifically, it could be argued that cognitive deficits in the CUD− subjects when compared with the CUD+ subjects could result from alcohol misuse in the CUD− subjects. On testing this, we found the opposite pattern: CUD+ subjects were much more likely to have a history of alcohol misuse (29/50) as compared with CUD− subjects (36/150; Chi-square = 19.76; df=1; p<0.001). Thus, a higher rate of alcohol use in CUD− subjects cannot explain our neurocognitive results.
4. Discussion
Results from our analysis suggest that subjects with bipolar disorder and history of cannabis use disorders demonstrate significantly better neurocognitive performance, particularly on measures of attention, processing speed, and working memory. These findings are consistent with a previous study that demonstrated that bipolar subjects with history of cannabis use had superior verbal fluency performance as compared to bipolar patients without a history of cannabis use (Ringen et al., 2010). Similar results have also been found in schizophrenia in several studies (Rabin et al., 2011). These data could be interpreted to suggest that cannabis use may have a beneficial effect on cognitive functioning in patients with severe psychiatric disorders. However, it is also possible that these findings may be due to the requirement for a certain level of cognitive function and related social skills in the acquisition of illicit drugs. Therefore, more intact patients may be more likely to successfully acquire cannabis and develop substance use disorders than less cognitively intact patients. This would suggest that the group differences on cognitive performance noted in our study may have been present before the onset of either CUD or bipolar disorder. It should be noted, however, that in our sample individuals with or without history of cannabis use did not differ significantly on estimates of premorbid IQ.
We also found that in our sample of patients with bipolar disorder, a history of cannabis use disorder was associated with an increased rate of psychosis during acute episodes. This observation suggests a more severe clinical presentation for subjects with bipolar disorder and comorbid cannabis use versus bipolar patients who do not use cannabis. These data are consistent with a large prospective study which indicated that bipolar cannabis users demonstrated less treatment compliance and more severe overall illness severity, mania, and psychosis symptoms during 1 year of treatment when compared to non-users. Interestingly, in that study social outcomes were only moderately affected by cannabis use, and in fact users engaged in more social activities than non users (van Rossum et al., 2009). The implications of these findings are difficult to determine. Certainly, results that indicate a worsened course of illness, as marked by more psychosis, contradict the findings of superior cognitive performance in CUD+ subjects. Treatment implications might include the potential development of pharmacologic agents with similar properties as cannabis, without its psychotomimetic effects, to be tested in cognitive enhancement trials in patients with bipolar disorder or schizophrenia.
Some limitations should be noted. The retrospective design of this analysis and the lack of a quantification variable for history of cannabis use prevent the determination of causality underlying the reported associations. Future prospective studies may help to elucidate the nature of these relationships. Symptom severity at the time of assessment was not characterized with commonly used scales (e.g. Hamilton Rating Scale for Depression). However, we were able to classify patients as symptomatic or non-symptomatic based on current levels of symptomatology from the SCID interview. Finally, it is possible that group differences regarding neurocognitive function might be explicable by differences in secondary clinical characteristics of the groups such as frequency of antipsychotic drug use or duration of illness. Nonetheless, the groups were similar in most relevant clinical features and results remained significant after correction for variables with significant between-group differences. Multiple testing is a concern in many studies making group comparisons across multiple cognitive and clinical measures, and this study is no exception. Nonetheless, we felt that the comprehensive analyses outweighed the reduced power due to multiple tests.
Despite potential limitations, these analyses indicate an interesting pattern suggesting superior neurocognitive performance among bipolar patient with comorbid CUD when compared to bipolar patients with history of cannabis use. Moreover, this cognitive advantage is noted in spite of evidence of a more severe clinical course. These results extend previous findings of similar studies reported in patients with schizophrenia and add significantly to the limited literature on cannabis use in bipolar illness. We hope that the results from our study will help guide and encourage future large studies and help further elucidate the multifaceted associations and possible impact of cannabis use in bipolar disorder.
Table 2.
Descriptive Raw Neurocognitive Data by Group
| Test | Cannabis History | N | Mean | Std. Deviation |
|---|---|---|---|---|
| Trails A (Time) | Absent | 107 | 46.22 | 39.35 |
| Present | 44 | 36.56 | 19.66 | |
| Trails B (Time) | Absent | 104 | 120.77 | 71.25 |
| Present | 44 | 93.32 | 61.70 | |
| Digits Forward | Absent | 75 | 6.25 | 1.40 |
| Present | 29 | 7.00 | 1.28 | |
| Digits Backward | Absent | 75 | 4.23 | 1.24 |
| Present | 29 | 4.93 | 1.58 | |
| CVLT Learning | Absent | 98 | 41.19 | 11.51 |
| Present | 42 | 43.00 | 12.83 | |
| Letter Fluency | Absent | 105 | 32.00 | 11.78 |
| Present | 44 | 33.07 | 12.40 | |
| Animal Fluency | Absent | 97 | 17.57 | 5.71 |
| Present | 43 | 19.14 | 6.39 |
Acknowledgements
Financial Support for this work included grants from the National Institute of Mental Health (NIMH) to KEB (K23MH077807) and to AKM (R01MH79800; P50MH080173)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. British Journal of Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 2.Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- 3.Dalton EJ, Cate-Carter TD, Mundo E, Parikh SV, Kennedy JL. Suicide risk in bipolar patients: the role of co-morbid substance use disorders. Bipolar.Disorders. 2003;5:58–61. doi: 10.1034/j.1399-5618.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 4.DeRosse P, Kaplan A, Burdick KE, Lencz T, Malhotra AK. Cannabis use disorders in schizophrenia: effects on cognition and symptoms. Schizophrenia Research. 2010;120:95–100. doi: 10.1016/j.schres.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg JF, Garno JL, Leon AC, Kocsis JH, Portera L. A history of substance abuse complicates remission from acute mania in bipolar disorder. Journal of Clinical Psychiatry. 1999;60:733–740. doi: 10.4088/jcp.v60n1103. [DOI] [PubMed] [Google Scholar]
- 6.Hollingshead AA. Four-Factor Index of Social Status. Yale University; New Haven, CT.: 1975. [Google Scholar]
- 7.Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biological Psychiatry. 2005;57:688–691. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophrenia Bulletin. 2010;36:1115–1130. doi: 10.1093/schbul/sbp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehman WE, Simpson DD. Employee substance use and on-the-job behaviors. Journal of Applied Psychology. 1992;77:309–321. doi: 10.1037/0021-9010.77.3.309. [DOI] [PubMed] [Google Scholar]
- 10.Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95:1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- 11.Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 12.Pope HG, Jr., Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–527. [PubMed] [Google Scholar]
- 13.Rabin RA, Zakzanis KK, George TP. The effects of cannabis use on neurocognition in schizophrenia: A meta-analysis. Schizophrenia Research. 2011 doi: 10.1016/j.schres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Ringen PA, Vaskinn A, Sundet K, Engh JA, Jonsdottir H, Simonsen C, Friis S, Opjordsmoen S, Melle I, Andreassen OA. Opposite relationships between cannabis use and neurocognitive functioning in bipolar disorder and schizophrenia. Psychological Medicine. 2010;40:1337–1347. doi: 10.1017/S0033291709991620. [DOI] [PubMed] [Google Scholar]
- 15.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Review. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychological Medicine. 2006;36:1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- 17.Strakowski SM, DelBello MP, Fleck DE, Adler CM, Anthenelli RM, Keck PE, Jr., Arnold LM, Amicone J. Effects of co-occurring cannabis use disorders on the course of bipolar disorder after a first hospitalization for mania. Archives of General Psychiatry. 2007;64:57–64. doi: 10.1001/archpsyc.64.1.57. [DOI] [PubMed] [Google Scholar]
- 18.van Rossum I, Boomsma M, Tenback D, Reed C, van Os J. Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. Journal of Nervous and Mental Disease. 2009;197:35–40. doi: 10.1097/NMD.0b013e31819292a6. [DOI] [PubMed] [Google Scholar]
- 19.Weiss RD, Ostacher MJ, Otto MW, Calabrese JR, Fossey M, Wisniewski SR, Bowden CL, Nierenberg AA, Pollack MH, Salloum IM, Simon NM, Thase ME, Sachs GS. Does recovery from substance use disorder matter in patients with bipolar disorder? Journal of Clinical Psychiatry. 2005;66:730–735. doi: 10.4088/jcp.v66n0609. [DOI] [PubMed] [Google Scholar]
- 20.Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, Conus P, Takagi MJ, Fornito A, Wood SJ, McGorry PD, Pantelis C. The Impact of Cannabis Use on Cognitive Functioning in Patients With Schizophrenia: A Meta-analysis of Existing Findings and New Data in a First-Episode Sample. Schizophrenia Bulletin. 2010;38:316–330. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]