Introduction
Hypertrophic growth of ventricular myocytes is a hallmark feature of numerous forms of cardiovascular disease1. The hypertrophic process is complex, involving a vast array of structural, signaling, transcriptional, electrophysiological, metabolic, and functional events within the growing cell2, 3. Other cellular elements within the ventricle – fibroblasts, vascular smooth muscle cells, endothelium – also manifest intricate stress responses, resulting in fibrosis, inflammatory cell infiltration, endothelial dysfunction, and vascular stiffness. Current thinking holds that these events, the heart’s reaction to a host of pathological stresses, provide short-term benefit. However, if disease-related stress remains unchecked, these remodeling events become maladaptive and predispose to cardiovascular morbidity and mortality.
Among the risks conferred by disease-related ventricular hypertrophy are ventricular tachyarrhythmia, predisposing to sudden cardiac death, and transition to heart failure. Ultimately, these events derive from wholesale reprogramming and relative dedifferentiation of the cardiac myocyte, coupled with similar events in other cell types.
Conventional thinking holds that hypertrophic growth of the myocardium is a “compensatory” response of the heart to increases in workload demand, serving to minimize wall stress and maintain contractile function. However, several lines of evidence – preclinical and epidemiological – highlight the maladaptive features of chronic ventricular hypertrophy. Indeed, in many instances suppression of load-induced growth is well tolerated4. Further, left ventricular hypertrophy is among the most robust markers of increased risk for developing chronic heart failure5. Therefore, we submit that suppression of load-induced ventricular hypertrophy warrants careful consideration as a therapeutic strategy.
Cardiomyocyte hypertrophy: Comprehensive reprogramming of the cell
Whereas evidence suggests that a small fraction of cells within the ventricle are capable of re-entering the cell cycle6-8, the vast majority of cardiomyocytes are post-mitotic and hence do not retain the ability to divide. Rather, they respond to stress by growing, shrinking, or dying. In the context of many disease-related stresses, cardiac myocytes undergo hypertrophic transformation which entails significant cellular growth. In the setting of uncontrolled hypertension, for example, cardiomyocytes hypertrophy. Similarly, following myocardial infarction, surviving cardiac myocytes in border and remote zones of tissue increase in size in response to increases in hemodynamic demand arising secondary to loss of ventricular tissue. In both cases, when the pressure overload state is persistent, the hypertrophic phenotype of the myocardium inexorably progresses to a state of decompensation and clinical heart failure. Mechanisms governing this transition from adaptive hypertrophy to maladaptive failure remain poorly understood.
Hypertrophic transformation of the cardiomyocyte involves much more than simple cell growth. Rather, it entails a near-comprehensive retooling of multiple aspects of cellular architecture and machinery. One element of this process is relative dedifferentiation of the cell and reactivation of numerous transcriptional, signaling, electrical, and metabolic events that characterized the cell during development. As part of this, a wide range of transcriptional and post-translational events occurs, including activation of a pattern of gene expression reminiscent of that observed during fetal development (“fetal gene program”). Indeed, the fetal gene program, which was extinguished shortly after birth, re-ignites rapidly in the setting of disease.
Based on the pattern of sarcomere reorganization, it is possible to distinguish two broad patterns of ventricular hypertrophy9. Pressure stress provokes concentric hypertrophy, which is characterized by recruitment of sarcomeres laid down in parallel; on the contrary, excess volume elicits eccentric hypertrophy in which cardiomyocytes respond with addition of sarcomeres in series9. In both cases, increases in wall thickness occur. [Concentric hypertrophy is marked by increases in wall thickness with relatively little change in ventricular volume; eccentric hypertrophy is marked by increases in both wall thickness and ventricular cavity size.] According to Grossman’s pioneering stress-adaptation hypothesis, these increases in wall thickness are an adaptive response10; based on Laplace’s law, ventricular wall stress is proportional to both ventricular pressure and cavity radius and inversely proportional to ventricular wall thickness11. Thus, increases in wall thickness tend to lessen wall stress and thereby diminish oxygen demand.
Myocyte growth is dictated by a delicate balance between protein synthesis and protein degradation. In the setting of elevated afterload, protein synthesis predominates, culminating in hypertrophic growth. However, it is important to recognize that hypertrophic remodeling is not a simple process of addition of new sarcomeres. Rather, this highly dynamic cellular response involves intricate coordination of de novo protein synthesis and organelle biogenesis, sarcomere remodeling, protein degradation, organelle breakdown, transcriptional reprogramming, and metabolic shifts. In many ways, the entire cellular architecture of the myocyte – frame, chassis, drive train, and engine – is retooled.
In contrast to disease-related triggers, physiological stresses, such as endurance exercise and pregnancy, induce a hypertrophic response characterized by normal or enhanced contractile function coupled with normal architecture and organization of cardiac structure12. Beyond differences in growth triggers, biological phenotypes, and clinical outcomes, pathological and physiological hypertrophy differ in the signaling cascades which drive the process. Some evidence suggests that the distinct phenotypes do not derive simply from differences in the duration of the stimulus, highlighting significant gaps in our understanding of these two remodeling responses13. Other data suggest that cell size is regulated by shared signaling pathways, but cell shape and sarcomeric organization are regulated by distinct pathways14. Current understanding does not allow us to parse the effects of the myriad genes and pathways involved, but it is thought that some confer benefit whereas others are maladaptive. Substantial evidence points to alterations in transmembrane Ca2+ fluxes – another central feature of pathological remodeling – as a proximal trigger contributing to the pathogenesis of hypertrophy and failure15. These alterations perturb excitation-contraction coupling, alter mitochondrial metabolism, and abnormally activate Ca2+-responsive signaling pathways. Recent evidence indicates that β-MHC protein is induced by pressure overload in rodents in only a minor subpopulation of smaller cardiac myocytes16. The myocytes that hypertrophied after surgical constriction of the thoracic aorta express α-MHC only. Thus, hypertrophic transformation may manifest yet another layer of complexity in that it manifests significant heterogeneity among myocytes.
Cardiac myocyte death
Cell death within the myocardium is characteristic of a number of cardiac diseases, and it can occur to some extent in cardiac hypertrophy. The major types of cardiomyocyte death are necrosis and apoptosis, with the former occurring to a greater extent. An emerging literature has demonstrated that necrosis can occur as a result of a series of programmed events, not just as simple catastrophic dismantling of the cell. Indeed, programmed necrosis and apoptosis share a number of features and may represent different manifestations of a common mechanism termed necroptosis17, 18.
Both dying and hypertrophying cells often harbor signs of activated autophagy, an evolutionarily ancient process of ordered recycling of intracellular contents19. Whether activation of the autophagic cascade reflects a cellular response to stress, serving to promote cell survival, or is a process contributing to cell death and disease progression, is context-dependent20.
Fibrosis
Another hallmark feature of pathological hypertrophic remodeling is accumulation and deposition of excessive extracellular matrix (ECM)21. This surplus ECM, which constitutes tissue “scar” or fibrosis, perturbs electrical conduction, thereby predisposing to rhythm disturbances. It also promotes dysfunction of both mechanical contraction and relaxation. As a result, cardiac fibrosis contributes importantly to morbidity and mortality in cardiac hypertrophy. Indeed, the amount of fibrotic scar in the myocardium correlates directly with the incidence of arrhythmias and sudden cardiac death22-24.
ECM deposition and fibrosis arise through the action of cardiac fibroblasts. These cells, the most abundant cell type in the myocardium, proliferate in response to pathological stress. Further, they differentiate into myofibroblasts, thereby gaining the capacity to contract and secrete collagen I, collagen III, and fibronectin25. Fibrosis can be categorized as reactive (perivascular or interstitial) or replacement, occurring at the site of an eliminated myocyte. Myofibroblasts derive from activated, resident fibroblasts, but may also originate from adult epicardial cells26 and circulating, collagen-secreting, bone marrow-derived cells27, 28. Both individual myofibroblasts and collagenous septa within the tissue facilitate and propagate the arrhythmic phenotype of the hypertrophied heart29-31
Cardiac fibrosis is an independent and predictive risk factor for heart failure development in the setting of ischemic or non-ischemic cardiomyopathy32-34. Importantly, strong evidence indicates that cardiac fibrosis, long held to be irreversible, may regress under certain conditions21, 35. However, whereas several signaling pathways have been implicated in fibrogenesis, tailored therapeutic approaches targeting cardiac fibrosis remain elusive21.
Electrical remodeling
Patients with left ventricular hypertrophy are at increased risk of malignant arrhythmia, which contributes significantly to morbidity and mortality. Indeed, arrhythmia, especially ventricular tachyarrhythmia, is a major cause of death in patients with cardiac hypertrophy or failure. Underlying mechanisms, collectively termed “electrical remodeling”, encompass alterations in multiple electrogenic transport and signaling processes within the cardiac myocyte. Whereas numerous insights have emerged in elucidating the molecular pathogenesis of cardiac hypertrophy3, 36, our understanding of mechanisms underlying the myriad facets of electrical remodeling remains limited. As a result, pharmacological treatment of hypertrophy-associated arrhythmias lacks efficacy, and device-based therapy has emerged as a widely used surrogate.
The action potential phenotype of ventricular myocyte hypertrophy is characterized by delayed repolarization leading to prolongation of action potential duration (APD). This derives, at least in part, from distorted transmembrane electrical currents2, 15, 37. Indeed, a wide range of alterations in myocyte ion channels and electrogenic ion transporters contribute to APD prolongation2, 15, 37. Delayed recovery of excitability, in turn, predisposes to early and late after-depolarizations. Hypertrophy is also associated with myocardial fibrosis (vide supra), altered electrotonic coupling among cells, slowed conduction, and dispersion of refractoriness, which together promote re-entrant arrhythmias.
This electrical remodeling response is heterogeneous within the ventricle. In the setting of excessive afterload, such as in severe transverse aortic constriction-induced heart failure, APD is prolonged more in subepicardial ventricular myocytes than in subendocardial myocytes38. Further evidence for heterogeneity of APD prolongation has been reported in a model of pacing-induced heart failure in dogs where APD prolongation in mid-myocardial cells was substantially greater than in subepicardial cells39.
Alterations in Ca2+ handling contribute to both hypertrophic signaling and electrical remodeling. For example, L-type Ca2+ current (ICa,L) is a major mechanism of Ca2+ influx in cardiac myocytes. As a general rule, ICa,L density correlates inversely with disease progression; in models of mild-to-moderate hypertrophy, ICa,L is often increased, whereas in severe hypertrophy and failure, ICa,L often manifests significant declines. Importantly, as membrane impedance is relatively high during phase 2 of the action potential, small changes in ICa,L can have significant effects on action potential morphology and duration. Further, entry of small amounts of extracellular Ca2+ triggers release of much larger amounts of Ca2+ from intracellular stores. As a consequence, modest changes in inward Ca2+ flux are amplified within the cell.
Electrical activity within the myocardium hinges critically on electrotonic cell-cell coupling, such that depolarization in one cell is transmitted seamlessly to neighboring cells. This coupling is mediated through gap junctions, such as connexin 43, which can become disorganized in the hypertrophied or failing heart, disrupting normal impulse conduction40. It is worth remembering that the atria are also touched by remodeling events in cardiac hypertrophy and failure. Reduced contractility, development of fibrosis, and chamber enlargement can occur, leading to heterogeneity of conduction velocity41 and propensity to atrial fibrillation42. This arrhythmia, in turn, eliminates the component of ventricular filling provided by atrial contraction. Further, it promotes decreases in atrial myocyte effective refractory period and shortened APD, which together promote sustained atrial fibrillation43.
Metabolic remodeling
The metabolic demands of the myocardium are exceptionally high. As a continuously working pump, the heart consumes robust quantities of adenosine triphosphate (ATP), more than any other organ in the body. And myocardial energy reserves are remarkably low, sufficient for fewer than 10 contractions. The cardiomyocyte derives ATP largely from fatty acid oxidation. That being said, the heart is a metabolic omnivore which can flexibly burn fuel derived from a wide range of sources. In the end, cardiomyocytes must generate energy continuously, consuming nutrients constantly and without interruption44.
Among the characteristic changes occurring with cardiac hypertrophy is a shift in energy substrate utilization45-47. This metabolic remodeling response entails up-regulation of glucose uptake and glycolysis, while β-oxidation of fatty acids is reduced. Approximate numbers are that glycolysis accounts for 10% of ATP production in the normal heart and 20% in the hypertrophied heart. Conversely, ATP production from fatty acid metabolism drops from 70% to 50%. These shifts are consistent with the over-arching process of cellular dedifferentiation in the pathologically stressed myocardium, as the shift in metabolism mimics the metabolic program in the fetal myocardium.
The impact of the partial switch to a fetal program of glucose metabolism from preferential utilization of lipid remains uncertain. Arguably the greatest impact derives from improvements in oxygen efficiency48. However, concerns have been raised due to evidence that cardiomyocytes cultured in high glucose media are dysfunctional49. To accomplish the synthesis of macromolecules and organelles required for cardiomyocyte hypertrophic growth, exogenous nutrients, such as glucose, cannot be metabolized exclusively for ATP production. Rather, metabolic intermediates must be channeled to support anabolic pathways. Here, interplay between the plasticity of metabolic pathways and protein quality control is critical to providing intermediate metabolites that feed into the tricarboxylic acid (TCA) cycle for ATP production, as well as serving to promote macromolecule synthesis50. Overall, currently available information implicates metabolic reprogramming in hypertrophied heart as an ultimately maladaptive adaptation51.
Inflammation
Activation of immune mechanisms participates in ventricular remodeling, contributing to long-term cardiac injury in certain contexts. In the case of heart failure, a variety of inflammatory molecules and pathways are activated52-55. In pre-clinical models, pressure overload triggers myocardial inflammation as demonstrated by increased expression of several proinflammatory cytokines and leukocyte infiltration within the myocardium56-58. This and other evidence, both in animals and patients, highlights the role of inflammation as an important component of pathological hypertrophy and points to the relationship between a pro-inflammatory state and load-induced ventricular remodeling59-62. Interestingly, myocardial cytokine levels, a surrogate marker of inflammation, are often higher in patients with elevated afterload and preserved left ventricular function compared with those patients with reduced ejection fraction63. These data challenge the concept that cytokine activity and an inflammatory response are necessarily detrimental in chronic pressure overload.
A crucial element within the inflammatory response observed in models of pressure overload involves macrophages that infiltrate ventricular tissue, triggering myocardial expression of NF-кB and inflammatory cytokines64. One of the mechanisms whereby macrophages are involved includes actions of macrophage-derived miR-155 which functions as a modulator of cardiomyocyte hypertrophy via paracrine mechanisms65.
These observations, coupled with the effects of anti-inflammatory therapies in animals, have prompted efforts to translate these insights into patients through clinical trials56, 66. However, to date, anti-inflammatory therapies for heart failure in humans have disappointed67-69. Looking to the future, the possible role of novel anti-inflammatory therapies using selective approaches targeting specific cellular and molecular elements of pathological cardiac remodeling and heart failure could be elucidated. Recently, a novel connection between autophagy, afterload stress, and inflammation was reported, where lack of autophagy-mediated removal of mitochondrial DNA promoted an inflammatory response contributing to depressed cardiac contractile performance70.
Vascular remodeling
The vasculature that supplies the ventricle itself remodels in both physiological and pathological hypertrophy. Hypertrophic growth of the heart involves parallel increases in myocardial mass and vascular supply, the latter occurring through proliferation of endothelium, smooth muscle cells, and blood vessels71, 72. Indeed, it is possible to distinguish two interdependent phenomena involving heart vessels: 1) remodeling of pre-existing vasculature with modifications of endothelium, smooth muscle cells, and interstitial matrix, and 2) de novo myocardial angiogenesis involving an intricate network of molecules secreted from cardiomyocytes, leukocytes and fibroblasts (reviewed73, 74). Paracrine signaling through mediators released by endothelial cells may modify vascular smooth muscle cell function and extracellular matrix components75, 76. Also, in the setting of hypertension, vascular smooth muscle proliferates and hypertrophies, culminating in vascular wall thickening. In some settings, flow reserve is compromised.
Dysfunction of vascular endothelial cells occurring in pressure overload-induced heart failure may represent a crucial node in vascular remodeling occurring in pathological hypertrophy77, 78. Indeed, one model holds that capillary growth in pathological hypertrophy does not keep up with myocyte growth, leading to inadequate oxygen diffusion capacity. Along those lines, it has also been postulated that an imbalance in the capillary-to-cardiomyocyte ratio contributes to the transition from compensatory hypertrophy to decompensated heart failure74. Consistent with this notion, enhancing angiogenesis in a model of afterload stress can be protective79. In fact, therapeutic strategies aimed to restore the capillary network in heart failure have been evaluated in both preclinical models and in humans80-83. Despite some favorable findings, therapeutic myocardial angiogenesis has failed to achieve clinical significance, at least partly because these approaches typically involve delivery of genetic material or growth factors with attendant complications.
Functional role of pathological hypertrophy
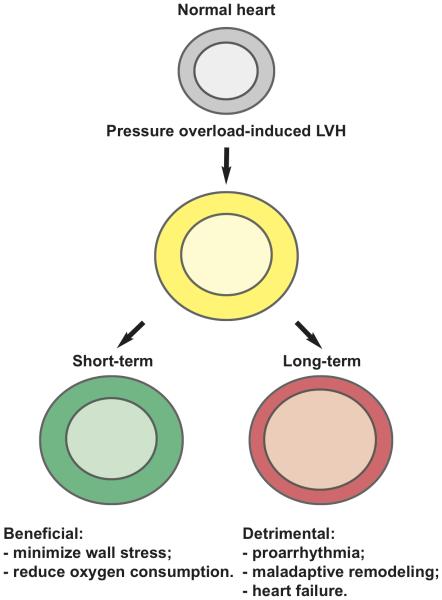
Three stages of hypertrophic transformation of the heart were initially proposed by Meerson and coworkers84. This model emphasizes that the duration of pressure overload dictates the progression of events, including hypertrophic growth and ultimately ventricular systolic function. In this model, “short-term” hypertrophy represents a beneficial event which serves to normalize wall stress, whereas prolonged hypertrophy is detrimental, provoking increased oxygen consumption and cardiomyocyte death (Figure 1).
Figure 1.
Canonical “three stages” model of hypertrophic transformation of the heart. This model posits “short-term” hypertrophy as a beneficial event and “long-term” hypertrophy as detrimental.
Considerable evidence, from both preclinical and clinical contexts, indicates that chronic, unremitting stress, as occurs in hypertension or valvular heart disease, leads inevitably to systolic dysfunction85-89. Moreover, the Framingham Heart Study established an association between ventricular hypertrophy and increased cardiac mortality5. However, the inevitability of the transition from hypertrophy to failure has been questioned. Drazner and colleagues90, 91 have identified three challenge to this point of view: 1) in animal models of pressure overload, hypertrophy can be blocked without development of heart failure92-94; 2) in humans, concentric hypertrophy does not uniformly progress to failure in the absence of myocardial infarction95-97; 3) some hypertensive subjects develop dilated cardiomyopathy apparently without antecedent concentric hypertrophy and without clinical evidence of myocardial infarction98. These important caveats are based on the notion that the time course of transition to heart failure in patients, if it occurs, is relatively uniform and can be captured within the time span of epidemiological studies.
In an effort to address these issues, magnetic resonance imaging data from the Dallas Heart Study have been used to propose a 4-tiered classification of left ventricular hypertrophy99. This scheme seeks to overcome the limitations of the concentric versus eccentric hypertrophy model by incorporating left ventricle end-diastolic volume as a categorical variable. Future work will determine whether this 4-tiered classification conveys prognostic information regarding prognosis or therapy.
Despite these caveats, the presence of left ventricular hypertrophy per se is unequivocally associated with adverse cardiovascular outcomes independent of the underlying cause5. As noted earlier, whereas ventricular hypertrophy may be beneficial in the short-term to minimize wall stress, in the long term it promotes progression to heart failure and other cardiovascular disorders. Numerous epidemiological studies have clearly demonstrated that left ventricular hypertrophy is not benign but rather represents a major risk factor for cardiovascular morbidity and mortality, more robust than other conventional risk factors5, 100-103. Studies from the Framingham Heart Study have demonstrated marked increases in coronary heart disease, heart failure, and sudden cardiac death associated with left ventricular hypertrophy, revealed by either electrocardiographic and echocardiographic approaches104,105, 106.
Together these data clearly identify left ventricular hypertrophy as an important risk factor of cardiovascular disease. However, it is important to highlight that these observations are strictly correlative, and no mechanism(s) of hypertrophy-dependent increase in risk can be inferred. Our knowledge of potential mechanism(s) by which ventricular hypertrophy confers increased cardiovascular risk is limited by the fact that transition from the early, compensatory stage of hypertrophy to the maladaptive phase is poorly characterized.
Is load-induced hypertrophy ever truly “compensatory”?
Traditionally, ventricular hypertrophy has been viewed as an obligatory initial response to pathological stress; only with time does it transition to a disease-promoting event. Consistent with this view, the term “compensatory” connotes an adaptive role of hypertrophy as a consequence of pressure stress based on the premise that it helps normalize ventricular wall stress and myocardial oxygen demand. However, evidence from genetic and pharmacological studies in mouse models of pressure overload have demonstrated that hypertrophic growth is not universally required to preserve cardiac function92, 94.
Shortly after the report107 that calcineurin is capable of triggering a robust cardiac growth response, we set out to determine whether calcineurin signaling is required for afterload-induced growth of the heart. Mice were exposed to thoracic aortic constriction and injected daily with cyclosporine or vehicle, delivered in an investigator-blinded manner. After three weeks, hearts were evaluated by echocardiography and by post mortem gravimetric analyses. In the animals exposed to cyclosporine, the load-induced growth response was abolished, leading us to conclude that calcineurin activation was, in fact, required for load-induced cardiac hypertrophy in this context. Very surprising to us, however, was the fact that ventricular size and function by echo was normal in the cyclosporine-treated animals. This was true despite the imposition of 70-80mmHg of afterload stress which was persistently present until the end of the entire study; ventricular volumes and contractile parameters were normal, and the animals behaved normally. This astonishing finding was published92, and we estimate that this observation, which often goes unnoticed in the reporting of studies, has been replicated independently at least 100 times since then.
These findings, demonstrating that load-induced hypertrophy is not always required to maintain ventricular size and performance, raise the prospect that the hypertrophic growth response may be a relevant target for therapeutic targeting4. It is important, however, to recognize that these observations derive largely from genetically engineered mice and, therefore, are based on models with important limitations. Heart rate in humans is substantially slower than that in mice, and left ventricular ejection fraction is lower. In addition, surgical banding of the aorta triggers an acute increase in pressure stress on the left ventricle and therefore does not mimic conditions of chronic, progressively increasing hemodynamic load as occurs in humans. Most preclinical studies in mice were conducted over a 3-4 week period, which amounts to approximately 3 years in humans; perhaps blocking hypertrophy for a longer period of time would be harmful. Unfortunately, the critical study in large animals has not been performed to date.
How can all this be?
Increases in afterload elicit early changes in myocyte biology across a wide range of mechanisms. Frank-Starling-related adaptations ensue, triggered by stretch. In addition, contractility continues to increase for over 10-15 minutes following the initial stretch, a response initially described over 100 years ago by Gleb Von Anrep and now termed the Anrep effect108. Initially attributed to increases in circulating catecholamines released by the adrenal gland, the Anrep effect was later demonstrated in isolated ventricular myofilaments and was attributed to increases in myofilament calcium sensitivity109. Thus, the Anrep effect, a response which remains poorly characterized, is a rapid, pro-contractile response that occurs in the setting of abrupt increases in afterload. It is possible that this response, coupled with hypertrophic growth, represents a load adaptation intrinsic to cardiac muscle.
Initial, rapid mechanisms whereby cardiac muscle responds to increased load are, however, insufficient to maintain cardiac contractility for an extended period if the inciting stress is not eliminated. Therefore, increases in cardiac mass ensue but which ultimately lead to untoward consequences. Indeed, with persistent pressure stress, the heart undergoes apparently irreversible decompensation, resulting in chamber dilatation and reduced systolic function. This “maladaptive” hypertrophy, as emerges when the inciting stress is not abated, represents a common feature in virtually all forms of heart failure. Therefore, the concept of transition from a short-term compensatory response to maladaptation points to cardiac hypertrophy as a novel therapeutic target.
Translational studies in large animals
Our understanding of mechanisms that govern the transition between compensated ventricular hypertrophy to heart failure remains incomplete. This stems partly from lack of longitudinal analyses of ventricular structure and function in patients with increased afterload (e.g. aortic stenosis, arterial hypertension). To date, most evidence that provides insight into the effects of suppressing cardiac hypertrophy on cardiac function derives from studies in rodents110. However, due to intrinsic differences in contractile performance, Ca2+ handling, myosin isoform distributions, heart rate and lifespan between rodents and humans, these models do not recapitulate faithfully human disease. In this regard, large animal models could be more informative.
Pressure overload-induced cardiac hypertrophy has been studied in non-human primates, where left ventricular hypertrophy and myocardial fibrosis comparable to that seen in patients with aortic stenosis are seen111. Studies to evaluate molecular mechanisms and pathophysiological adaptations in pressure overload-induced heart failure have been conducted primarily in dogs112, 113,114. In view of the considerable remaining gaps in our understanding of this biology, additional exploration of the role of compensatory hypertrophy in large animal models is warranted.
How might hypertrophy be targeted?
Clinical management of pathological left ventricular hypertrophy currently focuses on the underlying growth cues (e.g. hypertension, valve disease) and typically involves a wide spectrum of pharmacologic agents that have shown safety and efficacy in reducing hypertrophy. These pharmacological agents are mostly directed against the crucial neurohormonal axes activated in response to stress. Adrenergic and renin-angiotensin-aldosterone systems (RAAS) represent two mechanisms recruited to increase contractility in the early phases of stress, becoming deleterious in the chronic context. β-adrenergic receptor blockers (β-blockers), ACE inhibitors, and angiotensin receptor blockers are effective in reducing left ventricular mass and are associated with a favorable clinical outcome in clinical settings 115, 116.
Pharmacotherapy to reduce blood pressure can lead to regression of ventricular hypertrophy36. Although all antihypertensive drugs promote hypertrophy regression to some extent, current evidence suggests the RAAS-targeting drugs are the most efficacious at regressing ventricular hypertrophy. Based on robust evidence that regression of hypertrophy is independently associated with improved cardiovascular outcome, numerous clinical trials have documented the favorable impact of antihypertensive drug therapy. For example, the Losartan Intervention for Endpoint Reduction in a Hypertension (LIFE) study reported greater reduction in left ventricular mass index in the losartan-treated cohort compared with an atenolol-based regimen117. Several other trials reached a similar conclusion, pointing to a class effect of RAAS-targeting antihypertensive agents at promoting hypertrophy regression118-121. Together, these data highlight the role of current therapies targeting chronic neurohormonal activation in the prevention of heart failure and limiting progression of ventricular hypertrophy, lending further support to the notion that inhibiting ventricular hypertrophy is an attractive therapeutic option in patients.
All of these trials were conducted using compounds targeting cardiomyocyte cell-surface receptors. More recently, efforts have focused on anti-hypertrophic effects of molecules that act inside the cardiomyocyte, targeting crucial signaling cascades that alter gene expression and protein function. These agents include histone deacetylase (HDAC) inhibitors, a wide spectrum of pro-hypertrophic microRNAs (miRs) (reviewed122), and several other small molecules (reviewed123 [Table]).
Table 1.
Novel compounds and targets with confirmed or potential anti-hypertrophic activity.
| Target(s)/Mechanism(s) of action | Refs. (PMID) | |
|---|---|---|
| HDAC inhibitors | ||
| Apicidin | Class I HDACs | 18697792 |
| Vorinostat | Class I, II HDACs | 20139990-17211407 |
| Romidepsin | Class I, II HDACs | 20139990-21699444 |
| Trichostatin A | Pan-HDACs | 16380549-16735673- 12975471 |
| Valproic Acid | Class I HDACs (weak) | 16380549 |
| Scriptaid | Pan-HDACs | 16735673 |
| SK-7041 | Pan-HDACs | 16380549 |
| Pro-hypertrophic microRNAs as therapeutic targets | ||
| miR-199a | Hif1α/Sirtuin 1 | 20458739 |
| miR-199b | Dyrk1a | 21102440 |
| miR-208a | THRAP1 and myostatin | 19726871-21900086 |
| miR-23a | MuRF1 | 19574461 |
| miR-499 | DRP1 | 22752967 |
| miR-21 | Sprouty2 and Spry1 | 17234972-19043405 |
| miR-24 | E2F2 | 23307820-19748357 |
| miR-27b | PPAR-γ | 21844895 |
| miR-350 | MAPK11/14 and MAPK8/9 | 23000971 |
| miR-195 | HMGA1 | 17108080-25100012 |
| miR-221 | p27 | 22275134 |
| miR-212/132 | Fox03 | 23011132 |
| miR-25 | SERCA2a | 24670661 |
| miR-155 | Socs1 | 23956210 |
| Other compounds | ||
| CPG-ODN c274 | TLR9 agonist | 23638055 |
| Curcumin | P300 inhibitor | 18292809 |
| FTY720 | Sphingolipid/NFAT inhibitor | 23753531 |
| BAY94-8862 | MR antagonist | 22791416 |
| Cinaciguat | sGC stimulator | 22778174 |
| LCI699 | Aldosterone synthase inhibitor | 21986283 |
| Resveratrol | Antioxidant | 23784505 |
| MTP-131 | Antioxidant | 21620606 |
| TRV120027 | AT1 receptor | 22891045 |
| LCZ696 | Angiotensin/neprilysin inhibitor | 23731190 |
| HA-1077(Fasudil) | ROCK | 14500337-15840407-15096457 |
| Y-27632 | ROCK | 12623300-12176125 |
| Sildenafil | PDE5 | 25139994 |
| Ruboxistaurin | PKCb | 15878171 |
Abbreviations: HDAC: Histone deacetylase; Hif1α: Hypoxia inducible factor1α; DyrkIA: Dual specificity tyrosine-phosphorylation-regulated 1A; THRAP1: Thyroid Hormone Receptor Associated Protein 1; MuRF1: Muscle RING-finger protein-1; THRAP1: Dynamin-related proteini; E2F2: E2F transcription factor 2; PPAR: Peroxisome proliferator-activated receptor; MAPK: Mitogen-activated protein kinase; HMGA1: High mobility group A1; Fox03: Forkhead box 03; SERCA2A: Sarcoplasmic reticulum Ca2+ ATPase; Socs1: Suppressor of cytokine signaling 1; TLR9: Toll-like receptor 9; NFAT: Nuclear factor of activated T-cells; MR: Mineralocorticoid receptor; AT1: Angiotensin II receptor type 1; ROCK: Rho-associated protein kinase; PDE5: Phosphodiesterase type 5; PKCβ: Protein kinase C β.
Our group has focused on reversible protein acetylation as a tractable means of governing cardiomyocyte growth124-127, autophagy126, and disease-responsiveness128. Among those studies, we have noted that inhibition of HDACs is capable of blunting load-induced growth127. Beyond that, HDAC inhibitors can promote regression of ventricular hypertrophy despite the persistent presence of afterload stress126 (and with preservation – even improvement – in contractile performance.) HDAC inhibitors, three of which are FDA approved as third-line therapy for Sézary syndrome, may emerge as a novel means of targeting ventricular hypertrophy. Indeed, we have speculated that it might be possible to employ HDAC inhibitors to “sculpt” the hypertrophied ventricle in patients with heart failure with preserved ejection fraction (HFpEF), trimming left ventricular wall thickness progressively, to afford clinical benefit.
Comprehensive understanding of molecular events involved in maladaptive cardiac hypertrophy and the remodeling that culminates in decompensated heart failure is the first step toward developing novel treatments with clinical potential. This is especially true for patients with HFpEF, most of whom harbor ventricular hypertrophy which contributes to symptoms and clinical outcomes129 and for which evidence-based therapy is lacking. Although these pathways manifest redundancy in their effects, hypertrophy often remains present in models in which one pathway is suppressed, suggesting that serial pharmacological brakes may be required for clinical gain.
A note of caution
Considerable evidence points to a progression of remodeling events in which stress elicits a 1) hypertrophic growth response which has beneficial features; 2) maladaptive hypertrophy, which is a clear-cut marker for untoward events; and 3) the clinical syndrome of heart failure. However, assuming this model has validity, little is known regarding specific markers of these different phases of disease pathogenesis or transition points separating them. A large number of preclinical studies have demonstrated that it is possible to blunt load-induced hypertrophy, even in the setting of persistent afterload stress, without affecting contractile function4, 92, 94. These studies, then, have delineated a strategy wherein one might target maladaptive hypertrophy and thus obviate the untoward consequences of continued progression of this process. Consistently, in both preclinical studies and clinical trials, inhibition of cardiac hypertrophic growth usually results in the amelioration of left ventricular dysfunction5, 9, 130.
Caution is warranted. Not all forms of pathological cardiac hypertrophy can be blocked without provoking ventricular dysfunction131-134. Whereas the great majority of studies – again, exclusively in rodents to date – demonstrate that the load-induced growth response can be inhibited without untoward effects, there are examples where hypertrophy elicited by a specific signaling cascade appears to be required135-137. Also, ventricular mass alone may not provide the full picture of the myriad remodeling events involved in heart growth138. Therefore, further work is required to determine whether accompanying alterations (e.g. contractile function, coronary hemodynamics) associated with ventricular hypertrophy can be reversed by treatment and in which category of patients.
We wish to highlight that whereas cardiac hypertrophy in response to pathological stimuli manifests common characteristics irrespective of the triggering stress, it is likely that several subtypes of pathological hypertrophy exist. Thus, whereas efforts to block the inciting stimuli are warranted, we do not know whether all forms of maladaptive hypertrophy should be prevented.
Stress-induced cardiac growth: A model
How is it that hypertrophy can be both beneficial and deleterious? We propose a model in which hypertrophic transformation of the heart under conditions of disease-related stress is similar to many other processes in biology. There has been significant evolutionary pressure to promote organismal survival until procreation can occur and young can be fostered to independence. However, our species did not evolve to live 9 decades! In the settings of life-threatening stress, activation of both the β-adrenergic cascade and the RAAS axis occurs, and the end-result is enhanced cardiac performance and improved survival. These pathways allow for survival in the setting of sublethal injury, for example. However, chronic activation of these processes, life-saving in the short term, clearly conveys markedly enhanced risk in the long term. Indeed, chronic suppression of those evolutionarily ancient neurohumoral responses is fundamental to present day, evidence-based therapy for heart failure. Again, a large number of studies have demonstrated robust benefits from therapeutic strategies in which evolutionary adaptations to stress are blocked.
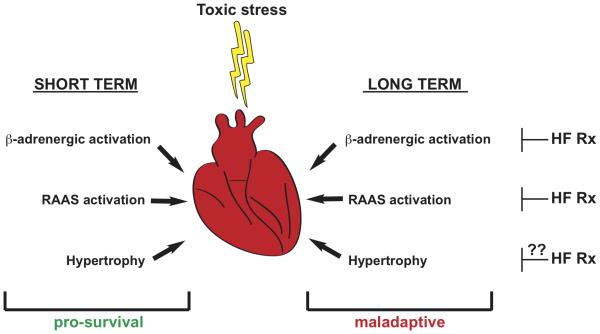
We suggest that ventricular hypertrophy is the same. It provides short-term benefit and long-term harm (Figure 2). If this model holds true, then suppression of ventricular hypertrophy – a therapeutic target currently not under widespread consideration – emerges as a novel and potentially important target for consideration of drug development going forward.
Figure 2.
Model of the hypertrophic response to toxic stress. We propose that hypertrophic transformation of the heart under stress is similar to other biological processes which evolved to provide short-term benefit. These include activation of the β-adrenergic and renin-angiotensin-aldosterone axes. Cornerstone therapy for heart failure involves interruption of these responses, and we suggest that judicious suppression of hypertrophy may provide similar benefit.
Summary and perspective
Heart failure is defined as a syndrome in which cardiac output is unable to meet the metabolic needs of peripheral tissues. In this setting, when cardiac output is depressed, the myocardium has a limited repertoire of responses, and, as a result, so does the treating physician. The heart is capable of responding in 4 ways: increases in heart rate, ventricular filling, contractility, and mass. Clinically, we have a full spectrum of means to regulate heart rate and optimize ventricular filling pressures. Contractility is a mechanism we target routinely in the acute setting, but no safe and effective therapies are available which promote contractility chronically. This leaves ventricular hypertrophy as the “final frontier” of heart failure therapy, a target which has never been developed and which, in fact, seems counterintuitive on initial consideration.
That said, it is critical to recognize that hypertrophic transformation of the ventricle is just that – a transformation involving cellular dedifferentiation and comprehensive reprogramming of the cardiac myocyte and other cellular elements within the ventricle. Whereas increases in myocyte size, and consequent increases in ventricular mass, are hallmark features, a wide range of additional events occurs in these stressed cells. Here, we present an overview of the large body of robust epidemiological and preclinical data pointing to the untoward consequences of hypertrophic transformation of the myocardium. These data, at the very least, raise the prospect of targeting hypertrophy therapeutically.
In recent years, significant strides have been achieved in our understanding of, and therapeutic targeting of, pathological hypertrophic remodeling. We view hypertrophic transformation as a fundamental, potentially indispensable step in the myocardial response to pressure overload which is coupled with significant detrimental consequences. This response may be beneficial in the short term, but when maintained chronically it becomes significantly detrimental. It is analogous to the responses of β-adrenergic and RAAS activation, which we now go to great lengths to block and whose suppression seemed counter-intuitive at first. We argue that hypertrophy is comparable, and patients may benefit from judicious attenuation of this chronic, long-term hypertrophic response.
Acknowledgments
We thank members of the Hill lab for constructive comments.
Funding Sources: This work was supported by grants from the NIH (HL-120732; HL-100401), AHA (14SFRN20740000), CPRIT (RP110486P3), Leducq Foundation (11CVD04) and the STAR program (UNINA).
Footnotes
Disclosures: None.
References
- 1.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–80. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Hill JA, Olson EN. Muscle: Fundamental Biology and Mechanisms of Disease. 2012:1528. [Google Scholar]
- 3.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–9. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinhauser ML, Lee RT. Regeneration of the heart. EMBO Mol Med. 2011;3:701–12. doi: 10.1002/emmm.201100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–9. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 10.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burch GE, Ray CT, Cronvich JA. Certain mechanical peculiarities of the human cardiac pump in normal and diseased states. Circulation. 1952;5:504–13. doi: 10.1161/01.cir.5.4.504. [DOI] [PubMed] [Google Scholar]
- 12.Weeks KL, McMullen JR. The athlete's heart vs. the failing heart: can signaling explain the two distinct outcomes? Physiology. 2011;26:97–105. doi: 10.1152/physiol.00043.2010. [DOI] [PubMed] [Google Scholar]
- 13.Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim HS, Smithies O, Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–60. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bass GT, Ryall KA, Katikapalli A, Taylor BE, Dang ST, Acton ST, Saucerman JJ. Automated image analysis identifies signaling pathways regulating distinct signatures of cardiac myocyte hypertrophy. J Mol Cell Cardiol. 2012;52:923–30. doi: 10.1016/j.yjmcc.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill JA. Electrical remodeling in cardiac hypertrophy. Trends Cardiovasc Med. 2003;13:316–22. doi: 10.1016/j.tcm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Lopez JE, Myagmar BE, Swigart PM, Montgomery MD, Haynam S, Bigos M, Rodrigo MC, Simpson PC. beta-myosin heavy chain is induced by pressure overload in a minor subpopulation of smaller mouse cardiac myocytes. Circ Res. 2011;109:629–38. doi: 10.1161/CIRCRESAHA.111.243410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 18.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Ann Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–22. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest. 2015;125:55–64. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockey DC, Bell DP, Hill JA. Fibrosis: A Common Pathways of Organ Injury and Failure. N Engl J Med. 2015 doi: 10.1056/NEJMra1300575. In Press. [DOI] [PubMed] [Google Scholar]
- 22.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–9. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 23.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 24.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marban E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–21. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 26.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 30.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98:801–10. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 31.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–8. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 32.Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–84. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 33.Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, Taylor AJ. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57:821–8. doi: 10.1016/j.jacc.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 34.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–9. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 35.Berry JM, Le V, Rotter D, Battiprolu PK, Grinsfelder B, Tannous P, Burchfield JS, Czubryt M, Backs J, Olson EN, Rothermel BA, Hill JA. Reversibility of adverse, calcineurin-dependent cardiac remodeling. Circ Res. 2011;109:407–17. doi: 10.1161/CIRCRESAHA.110.228452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie M, Burchfield JS, Hill JA. Pathological ventricular remodeling: therapies: part 2 of 2. Circulation. 2013;128:1021–30. doi: 10.1161/CIRCULATIONAHA.113.001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Hill JA. Electrophysiological remodeling in heart failure. J Mol Cell Cardiol. 2010;48:619–32. doi: 10.1016/j.yjmcc.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Cheng J, Joyner RW, Wagner MB, Hill JA. Remodeling of early-phase repolarization: a mechanism of abnormal impulse conduction in heart failure. Circulation. 2006;113:1849–56. doi: 10.1161/CIRCULATIONAHA.106.615682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003;93:638–45. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 40.Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol. 1991;139:801–21. [PMC free article] [PubMed] [Google Scholar]
- 41.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 42.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–8. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 43.Pang H, Ronderos R, Perez-Riera AR, Femenia F, Baranchuk A. Reverse atrial electrical remodeling: a systematic review. Cardiol J. 2011;18:625–31. doi: 10.5603/cj.2011.0025. [DOI] [PubMed] [Google Scholar]
- 44.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 45.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–73. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- 46.Allard MF. Energy substrate metabolism in cardiac hypertrophy. Curr Hypertens Rep. 2004;6:430–5. doi: 10.1007/s11906-004-0036-2. [DOI] [PubMed] [Google Scholar]
- 47.Wang ZV, Ferdous A, Hill JA. Cardiomyocyte autophagy: metabolic profit and loss. Heart Fail Rev. 2013;18:585–94. doi: 10.1007/s10741-012-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1345–51. doi: 10.1152/ajpheart.2000.278.4.H1345. [DOI] [PubMed] [Google Scholar]
- 49.Davidoff AJ, Davidson MB, Carmody MW, Davis ME, Ren J. Diabetic cardiomyocyte dysfunction and myocyte insulin resistance: role of glucose-induced PKC activity. Mol Cell Biochem. 2004;262:155–63. doi: 10.1023/b:mcbi.0000038231.68078.4b. [DOI] [PubMed] [Google Scholar]
- 50.Wang ZV, Hill JA. Protein Quality Control and Metabolism: Bidirectional Control in the Heart. Cell Metab. 2015;21:215–226. doi: 10.1016/j.cmet.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolwicz SC, Jr., Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. 2013;113:603–16. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aukrust P, Gullestad L, Lappegard KT, Ueland T, Aass H, Wikeby L, Simonsen S, Froland SS, Mollnes TE. Complement activation in patients with congestive heart failure: effect of high-dose intravenous immunoglobulin treatment. Circulation. 2001;104:1494–500. doi: 10.1161/hc3801.096353. [DOI] [PubMed] [Google Scholar]
- 53.Diwan A, Tran T, Misra A, Mann DL. Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med. 2003;3:161–82. doi: 10.2174/1566524033361537. [DOI] [PubMed] [Google Scholar]
- 54.Soejima H, Irie A, Fukunaga T, Oe Y, Kojima S, Kaikita K, Kawano H, Sugiyama S, Yoshimura M, Kishikawa H, Nishimura Y, Ogawa H. Osteopontin expression of circulating T cells and plasma osteopontin levels are increased in relation to severity of heart failure. Circ J. 2007;71:1879–84. doi: 10.1253/circj.71.1879. [DOI] [PubMed] [Google Scholar]
- 55.Caforio AL, Mahon NG, Baig MK, Tona F, Murphy RT, Elliott PM, McKenna WJ. Prospective familial assessment in dilated cardiomyopathy: cardiac autoantibodies predict disease development in asymptomatic relatives. Circulation. 2007;115:76–83. doi: 10.1161/CIRCULATIONAHA.106.641472. [DOI] [PubMed] [Google Scholar]
- 56.Gullestad L, Aukrust P. Review of trials in chronic heart failure showing broad-spectrum anti-inflammatory approaches. Am J Cardiol. 2005;95:17C–23C. doi: 10.1016/j.amjcard.2005.03.008. discussion 38C-40C. [DOI] [PubMed] [Google Scholar]
- 57.Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Card Res. 2009;81:474–81. doi: 10.1093/cvr/cvn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 59.Nicoletti A, Michel JB. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Card Res. 1999;41:532–43. doi: 10.1016/s0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 60.Ammarguellat FZ, Gannon PO, Amiri F, Schiffrin EL. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: role of ET(A) receptors. Hypertension. 2002;39:679–84. doi: 10.1161/hy0202.103481. [DOI] [PubMed] [Google Scholar]
- 61.Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–91. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 62.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension. 2004;43:739–45. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 63.Vanderheyden M, Paulus WJ, Voss M, Knuefermann P, Sivasubramanian N, Mann D, Baumgarten G. Myocardial cytokine gene expression is higher in aortic stenosis than in idiopathic dilated cardiomyopathy. Heart. 2005;91:926–31. doi: 10.1136/hrt.2004.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Card Res. 2014;102:240–8. doi: 10.1093/cvr/cvu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heymans S, Corsten MF, Verhesen W, Carai P, van Leeuwen RE, Custers K, Peters T, Hazebroek M, Stoger L, Wijnands E, Janssen BJ, Creemers EE, Pinto YM, Grimm D, Schurmann N, Vigorito E, Thum T, Stassen F, Yin X, Mayr M, de Windt LJ, Lutgens E, Wouters K, de Winther MP, Zacchigna S, Giacca M, van Bilsen M, Papageorgiou AP, Schroen B. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation. 2013;128:1420–32. doi: 10.1161/CIRCULATIONAHA.112.001357. [DOI] [PubMed] [Google Scholar]
- 66.Mann DL. Targeted anticytokine therapy and the failing heart. Am J Cardiol. 2005;95:9C–16C. doi: 10.1016/j.amjcard.2005.03.007. discussion 38C-40C. [DOI] [PubMed] [Google Scholar]
- 67.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 68.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 69.Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker-Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss HP, Tschope C, Van Bilsen M, Zannad F, McMurray J, Shah AM. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:119–29. doi: 10.1093/eurjhf/hfn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–5. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anversa P, Levicky V, Beghi C, McDonald SL, Kikkawa Y. Morphometry of exercise-induced right ventricular hypertrophy in the rat. Circ Res. 1983;52:57–64. doi: 10.1161/01.res.52.1.57. [DOI] [PubMed] [Google Scholar]
- 72.Anversa P, Capasso JM, Ricci R, Sonnenblick EH, Olivetti G. Morphometric analysis of coronary capillaries during physiologic myocardial growth and induced cardiac hypertrophy: a review. Int J Microcirc Clin Exp. 1989;8:353–63. [PubMed] [Google Scholar]
- 73.Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–43. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114:565–71. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 75.Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988;62:757–65. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- 76.Perry GJ, Wei CC, Hankes GH, Dillon SR, Rynders P, Mukherjee R, Spinale FG, Dell'Italia LJ. Angiotensin II receptor blockade does not improve left ventricular function and remodeling in subacute mitral regurgitation in the dog. J Am Coll Cardiol. 2002;39:1374–9. doi: 10.1016/s0735-1097(02)01763-1. [DOI] [PubMed] [Google Scholar]
- 77.Bauersachs J, Widder JD. Endothelial dysfunction in heart failure. Pharmacol Rep. 2008;60:119–26. [PubMed] [Google Scholar]
- 78.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–69. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 79.Friehs I, Moran AM, Stamm C, Choi YH, Cowan DB, McGowan FX, del Nido PJ. Promoting angiogenesis protects severely hypertrophied hearts from ischemic injury. Ann Thorac Surg. 2004;77:2004–10. doi: 10.1016/j.athoracsur.2003.11.003. discussion 2011. [DOI] [PubMed] [Google Scholar]
- 80.Pearlman JD, Hibberd MG, Chuang ML, Harada K, Lopez JJ, Gladstone SR, Friedman M, Sellke FW, Simons M. Magnetic resonance mapping demonstrates benefits of VEGF-induced myocardial angiogenesis. Nat Med. 1995;1:1085–9. doi: 10.1038/nm1095-1085. [DOI] [PubMed] [Google Scholar]
- 81.Hariawala MD, Horowitz JR, Esakof D, Sheriff DD, Walter DH, Keyt B, Isner JM, Symes JF. VEGF improves myocardial blood flow but produces EDRF-mediated hypotension in porcine hearts. J Surg Res. 1996;63:77–82. doi: 10.1006/jsre.1996.0226. [DOI] [PubMed] [Google Scholar]
- 82.Mack CA, Patel SR, Schwarz EA, Zanzonico P, Hahn RT, Ilercil A, Devereux RB, Goldsmith SJ, Christian TF, Sanborn TA, Kovesdi I, Hackett N, Isom OW, Crystal RG, Rosengart TK. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor 121 improves myocardial perfusion and function in the ischemic porcine heart. J Thorac Cardiovasc Surg. 1998;115:168–76. doi: 10.1016/s0022-5223(98)70455-6. discussion 176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–4. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 84.Meerson FZ. Compensatory hyperfunction of the heart and cardiac insufficiency. Circ Res. 1962;10:250–8. doi: 10.1161/01.res.10.3.250. [DOI] [PubMed] [Google Scholar]
- 85.Gunther S, Grossman W. Determinants of ventricular function in pressure-overload hypertrophy in man. Circulation. 1979;59:679–88. doi: 10.1161/01.cir.59.4.679. [DOI] [PubMed] [Google Scholar]
- 86.Huber D, Grimm J, Koch R, Krayenbuehl HP. Determinants of ejection performance in aortic stenosis. Circulation. 1981;64:126–34. doi: 10.1161/01.cir.64.1.126. [DOI] [PubMed] [Google Scholar]
- 87.Krayenbuehl HP, Hess OM, Ritter M, Monrad ES, Hoppeler H. Left ventricular systolic function in aortic stenosis. Eur Heart J. 1988;9(Suppl E):19–23. doi: 10.1093/eurheartj/9.suppl_e.19. [DOI] [PubMed] [Google Scholar]
- 88.Milani RV, Lavie CJ, Mehra MR, Ventura HO, Kurtz JD, Messerli FH. Left ventricular geometry and survival in patients with normal left ventricular ejection fraction. Am J Cardiol. 2006;97:959–63. doi: 10.1016/j.amjcard.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 89.Milani RV, Drazner MH, Lavie CJ, Morin DP, Ventura HO. Progression from concentric left ventricular hypertrophy and normal ejection fraction to left ventricular dysfunction. Am J Cardiol. 2011;108:992–6. doi: 10.1016/j.amjcard.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 90.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–34. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 91.Krishnamoorthy A, Brown T, Ayers CR, Gupta S, Rame JE, Patel PC, Markham DW, Drazner MH. Progression from normal to reduced left ventricular ejection fraction in patients with concentric left ventricular hypertrophy after long-term follow-up. Am J Cardiol. 2011;108:997–1001. doi: 10.1016/j.amjcard.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 92.Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–9. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 93.Hill JA, Rothermel B, Yoo KD, Cabuay B, Demetroulis E, Weiss RM, Kutschke W, Bassel-Duby R, Williams RS. Targeted inhibition of calcineurin in pressure-overload cardiac hypertrophy. Preservation of systolic function. J Biol Chem. 2002;277:10251–5. doi: 10.1074/jbc.M110722200. [DOI] [PubMed] [Google Scholar]
- 94.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 95.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110:101–7. doi: 10.7326/0003-4819-110-2-101. [DOI] [PubMed] [Google Scholar]
- 96.Mehta SK, Rame JE, Khera A, Murphy SA, Canham RM, Peshock RM, de Lemos JA, Drazner MH. Left ventricular hypertrophy, subclinical atherosclerosis, and inflammation. Hypertension. 2007;49:1385–91. doi: 10.1161/HYPERTENSIONAHA.107.087890. [DOI] [PubMed] [Google Scholar]
- 97.Heidland UE, Strauer BE. Left ventricular muscle mass and elevated heart rate are associated with coronary plaque disruption. Circulation. 2001;104:1477–82. doi: 10.1161/hc3801.096325. [DOI] [PubMed] [Google Scholar]
- 98.Drazner MH. The transition from hypertrophy to failure: how certain are we? Circulation. 2005;112:936–8. doi: 10.1161/CIRCULATIONAHA.105.558734. [DOI] [PubMed] [Google Scholar]
- 99.Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging. 2010;3:164–71. doi: 10.1161/CIRCIMAGING.109.883652. [DOI] [PubMed] [Google Scholar]
- 100.Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis. 2009;52:153–67. doi: 10.1016/j.pcad.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–52. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 102.Aronow WS, Epstein S, Koenigsberg M, Schwartz KS. Usefulness of echocardiographic left ventricular hypertrophy, ventricular tachycardia and complex ventricular arrhythmias in predicting ventricular fibrillation or sudden cardiac death in elderly patients. Am J Cardiol. 1988;62:1124–5. doi: 10.1016/0002-9149(88)90562-0. [DOI] [PubMed] [Google Scholar]
- 103.Casale PN, Devereux RB, Milner M, Zullo G, Harshfield GA, Pickering TG, Laragh JH. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med. 1986;105:173–8. doi: 10.7326/0003-4819-105-2-173. [DOI] [PubMed] [Google Scholar]
- 104.Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med. 1970;72:813–22. doi: 10.7326/0003-4819-72-6-813. [DOI] [PubMed] [Google Scholar]
- 105.Lavie CJ, Ventura HO, Messerli FH. Left ventricular hypertrophy. Its relationship to obesity and hypertension. Postgrad Med. 1992;91:131–2. doi: 10.1080/00325481.1992.11701350. 135-8, 141-3. [DOI] [PubMed] [Google Scholar]
- 106.Lee M, Gardin JM, Lynch JC, Smith VE, Tracy RP, Savage PJ, Szklo M, Ward BJ. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health Study. Am Heart J. 1997;133:36–43. doi: 10.1016/s0002-8703(97)70245-x. [DOI] [PubMed] [Google Scholar]
- 107.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cingolani HE, Perez NG, Cingolani OH, Ennis IL. The Anrep effect: 100 years later. Am J Physiol Heart Circ Physiol. 2013;304:H175–82. doi: 10.1152/ajpheart.00508.2012. [DOI] [PubMed] [Google Scholar]
- 109.Kentish JC, Wrzosek A. Changes in force and cytosolic Ca2+ concentration after length changes in isolated rat ventricular trabeculae. J Physiol. 1998;506:431–44. doi: 10.1111/j.1469-7793.1998.431bw.x. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail. 2009;2:138–44. doi: 10.1161/CIRCHEARTFAILURE.108.839761. [DOI] [PubMed] [Google Scholar]
- 111.Weber KT, Janicki JS, Pick R, Abrahams C, Shroff SG, Bashey RI, Chen RM. Collagen in the hypertrophied, pressure-overloaded myocardium. Circulation. 1987;75:I40–7. [PubMed] [Google Scholar]
- 112.Nagatomo Y, Carabello BA, Coker ML, McDermott PJ, Nemoto S, Hamawaki M, Spinale FG. Differential effects of pressure or volume overload on myocardial MMP levels and inhibitory control. Am J Physiol Heart Circ Physiol. 2000;278:H151–61. doi: 10.1152/ajpheart.2000.278.1.H151. [DOI] [PubMed] [Google Scholar]
- 113.Alyono D, Anderson RW, Parrish DG, Dai XZ, Bache RJ. Alterations of myocardial blood flow associated with experimental canine left ventricular hypertrophy secondary to valvular aortic stenosis. Circ Res. 1986;58:47–57. doi: 10.1161/01.res.58.1.47. [DOI] [PubMed] [Google Scholar]
- 114.Nguyen TN, Chagas AC, Glantz SA. Left ventricular adaptation to gradual renovascular hypertension in dogs. Am J Physiol. 1993;265:H22–38. doi: 10.1152/ajpheart.1993.265.1.H22. [DOI] [PubMed] [Google Scholar]
- 115.Kjeldsen SE, Dahlof B, Devereux RB, Julius S, Aurup P, Edelman J, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn S, Wedel H. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA. 2002;288:1491–8. doi: 10.1001/jama.288.12.1491. [DOI] [PubMed] [Google Scholar]
- 116.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 117.Devereux RB, Dahlof B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110:1456–62. doi: 10.1161/01.CIR.0000141573.44737.5A. [DOI] [PubMed] [Google Scholar]
- 118.Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–21. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 119.Gosse P, Sheridan DJ, Zannad F, Dubourg O, Gueret P, Karpov Y, de Leeuw PW, Palma-Gamiz JL, Pessina A, Motz W, Degaute JP, Chastang C. Regression of left ventricular hypertrophy in hypertensive patients treated with indapamide SR 1.5 mg versus enalapril 20 mg: the LIVE study. J Hypertens. 2000;18:1465–75. doi: 10.1097/00004872-200018100-00015. [DOI] [PubMed] [Google Scholar]
- 120.Devereux RB, Palmieri V, Sharpe N, De Quattro V, Bella JN, de Simone G, Walker JF, Hahn RT, Dahlof B. Effects of once-daily angiotensin-converting enzyme inhibition and calcium channel blockade-based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension: the prospective randomized enalapril study evaluating regression of ventricular enlargement (preserve) trial. Circulation. 2001;104:1248–54. doi: 10.1161/hc3601.095927. [DOI] [PubMed] [Google Scholar]
- 121.Solomon SD, Appelbaum E, Manning WJ, Verma A, Berglund T, Lukashevich V, Cherif Papst C, Smith BA, Dahlof B. Effect of the direct Renin inhibitor aliskiren, the Angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119:530–7. doi: 10.1161/CIRCULATIONAHA.108.826214. [DOI] [PubMed] [Google Scholar]
- 122.Thum T, Condorelli G. Long Noncoding RNAs and MicroRNAs in Cardiovascular Pathophysiology. Circ Res. 2015;116:751–762. doi: 10.1161/CIRCRESAHA.116.303549. [DOI] [PubMed] [Google Scholar]
- 123.McKinsey TA, Kass DA. Small-molecule therapies for cardiac hypertrophy: moving beneath the cell surface. Nat Rev Drug Discov. 2007;6:617–35. doi: 10.1038/nrd2193. [DOI] [PubMed] [Google Scholar]
- 124.Berry JM, Cao DJ, Rothermel BA, Hill JA. Histone deacetylase inhibition in the treatment of heart disease. Expert Opin Drug Saf. 2008;7:53–67. doi: 10.1517/14740338.7.1.53. [DOI] [PubMed] [Google Scholar]
- 125.Xie M, Hill JA. HDAC-dependent ventricular remodeling. Trends Cardiovasc Med. 2013;23:229–35. doi: 10.1016/j.tcm.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, Gillette TG, Hill JA. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci U S A. 2011;108:4123–8. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, Hill JA. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–88. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation. 2014;129:1139–51. doi: 10.1161/CIRCULATIONAHA.113.002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown EJ, Jr., Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH, New York Heart Failure C Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–8. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 130.Berenji K, Drazner MH, Rothermel BA, Hill JA. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am J Physiol Heart Circ Physiol. 2005;289:H8–H16. doi: 10.1152/ajpheart.01303.2004. [DOI] [PubMed] [Google Scholar]
- 131.Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr., Muller W, Chien KR. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–98. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 132.Rogers JH, Tamirisa P, Kovacs A, Weinheimer C, Courtois M, Blumer KJ, Kelly DP, Muslin AJ. RGS4 causes increased mortality and reduced cardiac hypertrophy in response to pressure overload. J Clin Invest. 1999;104:567–76. doi: 10.1172/JCI6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Badorff C, Ruetten H, Mueller S, Stahmer M, Gehring D, Jung F, Ihling C, Zeiher AM, Dimmeler S. Fas receptor signaling inhibits glycogen synthase kinase 3 beta and induces cardiac hypertrophy following pressure overload. J Clin Invest. 2002;109:373–81. doi: 10.1172/JCI13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brancaccio M, Fratta L, Notte A, Hirsch E, Poulet R, Guazzone S, De Acetis M, Vecchione C, Marino G, Altruda F, Silengo L, Tarone G, Lembo G. Melusin, a muscle-specific integrin beta1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nature Med. 2003;9:68–75. doi: 10.1038/nm805. [DOI] [PubMed] [Google Scholar]
- 135.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–50. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oh H, Taffet GE, Youker KA, Entman ML, Overbeek PA, Michael LH, Schneider MD. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci U S A. 2001;98:10308–13. doi: 10.1073/pnas.191169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–60. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hill JA. Hypertrophic reprogramming of the left ventricle: translation to the ECG. J Electrocardiol. 2012;45:624–9. doi: 10.1016/j.jelectrocard.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]