Abstract
Habitual aerobic exercise prevents age-related impairments in endothelium-dependent dilation (EDD). We hypothesized that the pro-inflammatory transcription factor nuclear factor κB (NF-κB) impairs EDD with sedentary aging and habitual aerobic exercise prevents this age-related suppression of EDD by NF-κB. To test this hypothesis, we inhibited NF-κB signaling via oral salsalate administration in healthy older aerobic exercise-trained adults (OT, n=14, 58±2 years), older non-exercising adults (ON, n=16, 61±1 years) and young non-exercising controls (YN, n=8, 23±1 years). Salsalate reduced endothelial cell expression of NF-κB p65 by ~25% in ON (P<0.05), but did not significantly change expression in OT or YN (P>0.05). EDD, assessed by brachial artery flow-mediated dilation (FMD), was improved by salsalate in ON (4.0±0.7% vs. 6.8±0.7%, placebo vs. salsalate, P<0.001), but did not change with salsalate in OT or YN (OT: 7.2±0.7% vs. 7.7±0.6%; YN: 7.6±0.9% vs. 8.1±0.8%; placebo vs. salsalate, P>0.05). Endothelium-independent dilation was not affected by salsalate in any group (P>0.05). In ON, vitamin C infusion improved FMD by ~30% during placebo (P<0.001), but had no affect during salsalate (P>0.05). In OT and YN, vitamin C infusion did not affect FMD during either placebo or salsalate (P>0.05). Salsalate reduced endothelial cell nitrotyrosine content by ~25% and NADPH oxidase p47phox expression by ~30% in ON (P<0.05), but had no effect in OT or YN (P>0.05). Our results suggest that endothelial NF-κB signaling is associated with oxidative stress-related impairment of EDD in healthy non-exercising, but not aerobically exercising older adults. This may be a key mechanism by which regular aerobic exercise preserves endothelial function and reduces cardiovascular risk with aging.
Keywords: Aging, exercise, endothelium-dependent dilation, flow-mediated dilation, NF-κB, oxidative stress
Vascular endothelial dysfunction, characterized by impaired endothelium-dependent dilation (EDD), is a predictor of future cardiovascular events [1–3]. EDD declines with age in healthy sedentary adults [4, 5], but is preserved with age in individuals who regularly participate in aerobic exercise [5–7]. Reduced pro-inflammatory signaling is thought to contribute to the cardiovascular benefits of regular aerobic exercise [8, 9]. However, the role of reduced inflammatory signaling by regular aerobic exercise in the preservation of EDD with advancing age has not been directly assessed.
A key pro-inflammatory transcription factor in vascular endothelial cells is nuclear factor κB (NF-κB) [10]. We have shown that endothelial cell expression of NF-κB increases with age [11, 12], but that this age-related increase does not occur in aerobic exercise-trained individuals [13]. We also have established that short-term (4-day), high-dose oral administration of salsalate, a non-acetylated salicylate that inhibits NF-κB translocation to the nucleus [14, 15], suppresses NF-κB signaling and improves EDD in overweight and obese adults with characteristics of the metabolic syndrome [16]. Moreover, in a preclinical study we found that increased vascular expression of NF-κB with primary aging has functional consequences, as inhibiting NF-κB activity with salicylate restores EDD in old mice, but has no effect in young mice [17]. Thus in a rodent model, it appears that augmented NF-κB signaling mediates the age-related impairments in EDD. However, the role of NF-κB signaling in endothelial dysfunction with primary aging in adult humans and the possibility that regular aerobic exercise preserves endothelial function with aging by inhibiting this adverse effect of NF-κB are unknown.
Augmented NF-κB signaling is associated with increased oxidative stress, particularly by activating transcription of the pro-oxidant enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, leading to increased superoxide production [18, 19]. Endothelial cell nitrotyrosine staining, a marker of oxidative stress, and NADPH oxidase expression increase with age [12], but these age-related increases are not present in older aerobic exercise-trained individuals [13]. Inhibiting NF-κB activity reduces endothelial cell nitrotyrosine and NADPH oxidase subunit p47phox expression in overweight/obese humans [16]. Furthermore, healthy sedentary middle-aged and older adults demonstrate tonic oxidative-stress mediated suppression of EDD, as indicated by improvements in EDD with acute infusion of the antioxidant vitamin C, an effect that is absent in their peers who habitually perform aerobic exercise [5]. However, it is unknown if the potential anti-inflammatory effects of habitual aerobic exercise on EDD with aging are mediated by reduced oxidative stress.
The goal of the present study was to determine the role of NF-κB signaling in impaired EDD with aging in healthy sedentary adults, and if NF-κB–mediated suppression of endothelial function is absent in older aerobic exercise-trained individuals. We hypothesized that inhibiting NF-κB activity with salsalate [14–16] would improve EDD, measured by flow-mediated dilation (FMD), in healthy non-exercising older adults, but have no effect on aerobic exercise-trained older adults or young non-exercising adults. We also hypothesized that salsalate would improve FMD in healthy non-exercising older adults by reducing oxidative stress, and that this effect would be associated with reduced endothelial expression of NADPH oxidase.
Methods
Subjects
A total of 38 men and women were enrolled in the study: 16 non-exercising and 14 aerobic exercise-trained older adults (50–75 years) and 8 non-exercising young controls (20–25 years). “Non-exercising” was defined as no regular exercise (<30 min/day, <2 days/week) during the previous 2 years. “Aerobic exercise-trained” was defined as ≥4 sessions/week of vigorous aerobic-endurance exercise for ≥ 45 minutes/session. All of the subjects had total cholesterol <240 mg/dl, LDL-cholesterol <160 mg/dl, resting blood pressure <140/90 mmHg, fasting blood glucose <126 mg/dl, body mass index (BMI) <32 kg/m2 and were nonsmokers and free of clinical diseases as assessed by medical history, physical examination, blood chemistry, and resting and exercise ECG. Subjects were not taking medications and had refrained from antioxidants (e.g., vitamins C and E) and aspirin within 2 weeks of the study. Women were postmenopausal for at least 1 year and had not taken hormone replacement therapy for at least the previous 6 months. All procedures were approved by the Institutional Review Board of the University of Colorado at Boulder. The nature, benefits and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Procedures
All measurements were performed at the University of Colorado Boulder Clinical and Translational Research Center after a 12-hour fast and 24-hour abstention from alcohol and physical activity.
Subject characteristics and blood analyses
Waist and hip circumferences and BMI were measured by anthropometry. Percent body fat was measured by dual-energy X-ray absorptiometry (DXA-GE; Lunar Corporation; software version 5.60.003, Madison, Wis). Maximal oxygen consumption was measured during incremental treadmill exercise using open-circuit spirometry as previously described [20]. Arterial blood pressure was measured over the brachial artery during supine rest using a semi-automated device (Dinamap Pro 100, GE Health Care, Waukesha, WI). Fasting plasma metabolic factors were determined by the Clinical and Translational Research Center core laboratory using standard assays. ELISA was used to measure serum interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α, R&D Systems, Minneapolis, MN). C-reactive protein (CRP) was measured using a high-sensitivity Chemistry Immuno Analyzer (AU400e, Olympus America, Center Valley, PA).
EDD and endothelium-independent dilation
Duplex ultrasonography (Power Vision 6000, Toshiba) with a linear array transducer was used to assess brachial FMD, endothelium-independent dilation (dilation to glyceryl trinitrate, GTN, 0.4 mg) and shear rate in the supine position, as previously described by our laboratory [5, 21]. Pulsed Doppler signals were recorded at an angle of insonation of 68 degrees with a sample volume the entire width of the artery as previously described [16]. Time-averaged peak velocity was obtained from recording the first 10 velocity envelopes. Brachial artery peak hyperemic shear rate was calculated as 8 times (due to wide sample volume) the peak velocity immediately following 5 minutes of forearm occlusion, divided by occlusion diameter. FMD and GTN responses were expressed as relative (%) and absolute (mm) change from baseline diameter, per published guidelines [22]. During salsalate and placebo conditions (see below), FMD was measured first during saline infusion (control) and then during supraphysiological intravenous infusion of vitamin C as previously described [5]. 0.06 g/kg fat-free mass of vitamin C was infused for 20 minutes, immediately followed by FMD measurements during a 0.02 g/kg fat-free mass vitamin C maintenance drip infusion. GTN was only administered to subjects with resting blood pressure sufficient to safely tolerate vasodilation and no history of migraine headache, thus only 11 (9M/2F) non-exercising older, 8 (7M/1F) aerobic exercise-trained older and 6 (6M/0F) non-exercising young subjects received GTN under both conditions.
Endothelial cell protein expression
Endothelial cells were collected from peripheral veins and analyzed for protein expression as described in detail previously by our laboratory [16, 23, 24]. Briefly, cells were collected on J-wires advanced into an antecubital vein (~4 cm beyond the tip of the catheter) and recovered by washing and centrifugation. Formaldehyde fixed cells were incubated with primary antibodies for NF-κB p65 (Santa Cruz, Dallas, TX), nitrotyrosine (Abcam, Cambridge, MA), NADPH oxidase p47phox (Abcam, Cambridge, MA), AMP-activated protein kinase (AMPK)(Cell Signaling Technology, Inc., Danvers, MA) or phosphorylated AMPK (Thr172)(Cell Signaling Technology, Inc., Danvers, MA) and with Alexaflour 555 fluorescent secondary antibody (Invitrogen Corp, Carlsbad, Calif). Cells were viewed with a fluorescence microscope (Eclipse Ni-U, Nikon, Melville, NY) and analyzed with Metamorph Software (Universal Imaging Corp, Downingtown, Pa). Endothelial cells were identified by staining for vascular endothelial-cadherin (VE-cadherin) (Abcam, Cambridge, Mass) and nuclear integrity was confirmed with DAPI (4′,6′-diamidino-2-phenylindole hydrochloride). The expression of each primary protein of interest was quantified for each subject at each condition by calculating the average pixel intensity in the Texas Red channel in 30 cells that had been identified by positive VE-cadherin staining. Any intensity values found to be greater or less than 2 standard deviations from the mean of these 30 cells were excluded and the remaining values were used to calculate the average pixel intensity. To minimize day-to-day variability resulting from differences in intensity of staining between different staining sessions, each batch of staining included a slide of human umbilical vein endothelial cells (HUVECs). For each protein, HUVECs were grown in the same batch for the same number of passages and were washed, fixed and stored similar to cells collected from subjects. Values for each protein are reported as a ratio of endothelial cell to HUVEC average pixel intensity. AMPK activity was expressed as the ratio of p-AMPK to total AMPK. Endothelial cells were not successfully collected for all subjects during each condition; therefore, subject numbers are presented in the figure legend for each protein.
Salsalate administration
As previously described by our laboratory [16], in a double-blind randomized crossover design, subjects were assigned oral doses of salsalate or placebo for 3 days prior and on the morning of experimental testing. Serum salicylate was measured on the morning of days 2 and 3 and the day of experimental testing. Because of individual differences in pharmacokinetics due to sex, size, absorption, metabolism and other factors, subjects received a total of 2500 mg to 4500 mg salsalate each day (divided into a morning and evening dose) to result in a steady-state serum salicylate in the therapeutic range of 10 to 30 mg/dL. Doses were adjusted each day based on serum salicylate concentration to maintain salicylate in the therapeutic range without reaching toxicity (>30 mg/dl). Experimental testing days for placebo and salsalate conditions were separated by at least 1 week. For the 3 days prior to each experimental testing day, subjects received a standardized research diet prepared by the Clinical and Translational Research Center bionutritionist.
Data analysis
Statistical analyses were performed with IBM SPSS (version 20, Armonk, NY). Differences in subject characteristics between groups were assessed by one-way ANOVA, using a Student-Newman-Keuls post-hoc test. A 3×2 repeated measures ANOVA was performed to identify a group (older non-exercising, older aerobic exercise-trained, young non-exercising) x condition (placebo, salsalate) interaction for FMD, subject characteristics and brachial artery characteristics. In the case of a significant interaction, a paired t-test for within-group contrast and independent t-test for between-group contrasts (placebo condition only) were performed with a Bonferonni correction. The differences in FMD within subjects during saline vs. vitamin C with salsalate and placebo conditions were calculated by repeated measures ANOVA and in cases of a significant F, post-hoc analysis was performed by paired t-tests with a Bonferonni correction. The differences in endothelial cell protein expression within subjects between the placebo and salsalate conditions were assessed with a paired t-test for each group. Pearson correlation analysis was used to assess bivariate relations between the difference in %FMD and the difference in factors that could influence FMD between placebo and salsalate. Each of these variables (difference between placebo and salsalate) were normally distributed. The influence on FMD of clinical characteristics that changed with salsalate administration in any group was assessed by repeated measures ANCOVA. Significance was set at P<0.05. Values are mean±SE.
Results
Baseline subject characteristics
The groups of older aerobic exercise-trained, older non-exercising and young non-exercising men and women did not differ in systolic blood pressure, HDL-cholesterol, fasting glucose or waist:hip ratio (P>0.05, Table 1,2). The older aerobic exercise-trained and older non-exercising subjects had a similar age, diastolic blood pressure, total cholesterol, LDL-cholesterol and triglycerides (P>0.05), and all of these variables were greater in the older groups compared with the young non-exercising group (P<0.05, Table 1,2). The older aerobic exercise-trained subjects had lower BMI and percent body fat, and higher VO2max than the older non-exercising subjects (P<0.05), but not different from the young non-exercising subjects (P>0.05, Table 1). Older aerobic exercise-trained subjects had a lower resting HR than both the older non-exercising and young non-exercising subjects (P<0.05, Table 1).
Table 1.
Subject characteristics
| Variable | Young Non-exercising | Older Non-exercising | Older Exercise-Trained |
|---|---|---|---|
| n (male/female) | 8 (8/0) | 16 (11/5) | 14 (10/4) |
| Age (years) | 23 ± 1 | 61 ± 1 * | 58 ± 2 * |
| Body mass index (kg/m2) | 24 ± 1 | 27 ± 1 * | 23 ± 1 † |
| % Body fat | 19 ± 4 | 33 ± 2 * | 20 ± 2 † |
| Waist:Hip ratio | 0.80 ± 0.01 | 0.89 ± 0.03 | 0.84 ± 0.02 |
| VO2max (ml/kg/min) | 48 ± 2 | 29 ± 1 * | 44 ± 2 † |
| Resting heart rate (beats/min) | 61 ± 3 | 64 ± 3 | 53 ± 2 *† |
Data are mean ± SE. VO2max, maximal oxygen consumption.
P<0.05 vs. young non-exercising,
P<0.05 vs. old non-exercising
Table 2.
Blood pressure and circulating factors during placebo and salsalate
| Variable | Young Non-exercising
|
Older Non-exercising
|
Older Exercise-Trained
|
|||
|---|---|---|---|---|---|---|
| Placebo | Salsalate | Placebo | Salsalate | Placebo | Salsalate | |
| SBP (mm Hg) | 110 ± 4 | 109 ± 3 | 122 ± 4 | 117 ± 4 | 111 ± 4 | 111 ± 4 |
| DBP (mm Hg) | 61 ± 3 | 58 ± 2 | 73 ± 3 * | 71 ± 3 | 69 ± 2 * | 70 ± 2 |
| Total cholesterol (mg/dL) | 142 ± 7 | 126 ± 6 * | 182 ± 7 * | 163 ± 5 † | 186 ± 9 * | 167 ± 9 ‡ |
| LDL-cholesterol (mg/dL) | 79 ± 7 | 73 ± 5 | 114 ± 5 * | 102 ± 5 † | 113 ± 7 * | 107 ± 8 |
| HDL-cholesterol (mg/dL) | 47 ± 3 | 43 ± 3 | 47 ± 3 | 49 ± 3 | 49 ± 4 | 48 ± 3 |
| Triglycerides (mg/dL) | 58 ± 2 | 50 ± 4 | 104 ± 13 * | 59 ± 4 † | 106 ± 12 * | 64 ± 6 ‡ |
| Glucose (mg/dL) | 87 ± 2 | 83 ± 2 | 89 ± 2 | 84 ± 2 | 86 ± 1 | 82 ± 2 |
| C-reactive protein (mg/L) | 0.5 ± 0.1 | 0.7 ± 0.2 | 1.0 ± 0.2 | 1.2 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.1 |
| Interleukin-6 (pg/mL) | 0.8 ± 0.1 | 1.0 ± 0.2 | 1.4 ± 0.3 | 1.2 ± 0.2 | 0.8 ± 0.1 | 0.6 ± 0.1 |
| TNFα (pg/ml) | 0.8 ± 0.1 | 0.8 ± 0.0 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
Data are mean ± SE. SBP, systolic blood pressure; DBP, diastolic blood pressure; TNFα, tumor necrosis factor alpha.
P<0.05 vs. young non-exercising placebo,
P<0.05 vs. older non-exercising placebo,
P<0.05 vs. older trained placebo
Salsalate administration
Salsalate administration resulted in serum salicylate concentrations in the therapeutic range for all subjects (>10 mg/dl) and concentrations were not different between groups (young non-exercising: 17.1±0.9 mg/dl, older non-exercising: 20.3±1.4 mg/dl, older exercise-trained: 19.2±0.8 mg/dl, P>0.05). Serum salicylate was undetectable in all subjects during the placebo condition. Salsalate administration did not affect systolic or diastolic blood pressure, HDL-cholesterol, or fasting glucose for any group (P>0.05, Table 2). Salsalate administration reduced total cholesterol in all groups, reduced triglycerides in both older groups, and reduced LDL-cholesterol in only the older non-exercising group (P<0.05, Table 2). The circulating inflammatory markers CRP, IL-6 and TNF-α did not change in any group with salsalate administration (P>0.05, Table 2).
Endothelial cell NF-κB
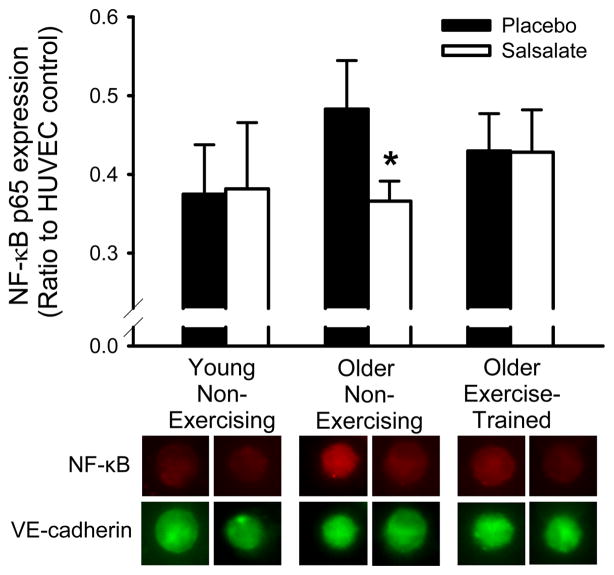
Salsalate administration did not affect endothelial cell NF-κB p65 expression in older aerobic exercise-trained or young non-exercising subjects (P>0.05, Figure 1). In older non-exercising subjects, salsalate administration reduced endothelial cell NF-κB p65 expression by ~25% compared with the placebo condition (P=0.03, Figure 1).
Figure 1.
Endothelial protein expression of nuclear factor-κB (NF-κB) p65 in young non-exercising (n=9: 9M,0F), older non-exercising (n=15: 11M,4F) and older aerobic exercise-trained (n=13: 10M,3F) adults during placebo and salsalate conditions. Values are fluorescent intensity relative to human umbilical vein endothelial cell (HUVEC) control intensity. Representative images are shown below summary graph for NF-κB p65 and VE-cadherin (to identify endothelial cells). Values are mean ± SE. *P<0.05 vs. placebo condition within group.
Endothelial Function
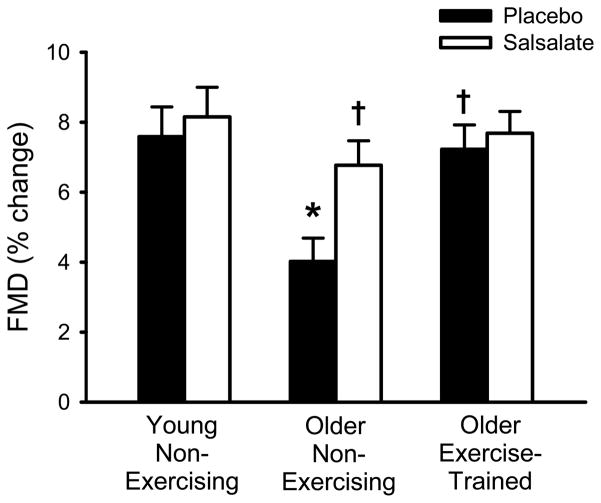
During the placebo condition, older aerobic exercise-trained subjects had a higher brachial FMD compared with the older non-exercising subjects (P<0.005) and not different from the young non-exercising subjects (P>0.05, Figure 2, Table 3). Brachial FMD was less in older non-exercising subjects than young non-exercising subjects during placebo (P<0.005, Figure 2, Table 3). Brachial FMD did not change in either the older aerobic exercise-trained or young non-exercising subjects with salsalate administration (P>0.05), but improved by ~70% in the older non-exercising subjects (P<0.001 vs. placebo, Figure 2, Table 3). Baseline brachial artery diameter, peak shear rate and endothelium-independent dilation were not different between groups and did not change with salsalate administration in any group (P>0.05, Table 3).
Figure 2.
Brachial flow-mediated dilation (FMD, percent change in diameter) in young non-exercising, older non-exercising and aerobic exercise-trained adults during placebo and salsalate conditions. Values are mean ± SE. *P<0.05 vs. young placebo condition. †P<0.05 vs. older non-exercising placebo condition.
Table 3.
Brachial artery parameters during placebo and salsalate
| Variable | Young Non-exercising
|
Older Non-exercising
|
Older Exercise-Trained
|
|||
|---|---|---|---|---|---|---|
| Placebo | Salsalate | Placebo | Salsalate | Placebo | Salsalate | |
| Brachial artery diameter (mm) | 4.0 ± 0.1 | 4.1 ± 0.1 | 3.9 ± 0.1 | 3.8 ± 0.2 | 3.7 ± 0.2 | 3.7 ± 0.2 |
| FMD (mm change) | 0.30 ± 0.03 | 0.33 ± 0.03 | 0.15 ± 0.02 * | 0.25 ± 0.02 † | 0.26 ± 0.02 † | 0.27 ± 0.01 |
| Peak shear rate (1/s) | 808 ± 67 | 855 ± 70 | 840 ± 65 | 890 ± 68 | 941 ± 68 | 906 ± 53 |
| GTN dilation (% change) | 25 ± 1 | 24 ± 1 | 21 ± 2 | 22 ± 2 | 26 ± 2 | 24 ± 1 |
| GTN dilation (mm change) | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.0 |
Data are mean ± SE. FMD, flow-mediated dilation; GTN, glyceryl trinitrate.
P<0.05 vs. young non-exercising placebo,
P<0.05 vs. older non-exercising placebo
In the overall group or within specific age/training groups, the change in FMD with salsalate was not related to changes in clinical characteristics including blood pressure, lipids, fasting glucose or circulating inflammatory markers. When accounting for clinical factors that changed in response to salsalate (total cholesterol, LDL-cholesterol, triglycerides), the improvement in FMD with salsalate in the older non-exercising group remained significant (ANCOVA, P<0.05). The change in FMD with salsalate for either older group did not differ between males and females (P>0.05) and the FMD improvement in the older non-exercising group remained significant when accounting for sex (ANCOVA, P<0.05).
Oxidative stress
During the placebo condition, vitamin C infusion had no effect in the older aerobic exercise-trained or young non-exercising subjects (P>0.05, Figure 3A,C, Supplemental Table 1), but improved brachial FMD by ~30% in the older non-exercising subjects (P<0.001, Figure 3B, Supplemental Table 1). During the salsalate condition, vitamin C infusion did not affect FMD in any subject group (P>0.05, Figure 3, Supplemental Table 1).
Figure 3.
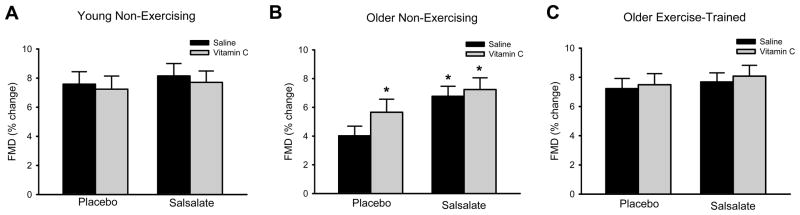
Brachial flow-mediated dilation (FMD, percent change in diameter) during intravenous saline and vitamin C infusion for placebo and salsalate conditions of (A) young non-exercising, (B) older non-exercising and (C) older aerobic exercise-trained adults. Values are mean ± SE. *P<0.05 vs. placebo saline condition within group.
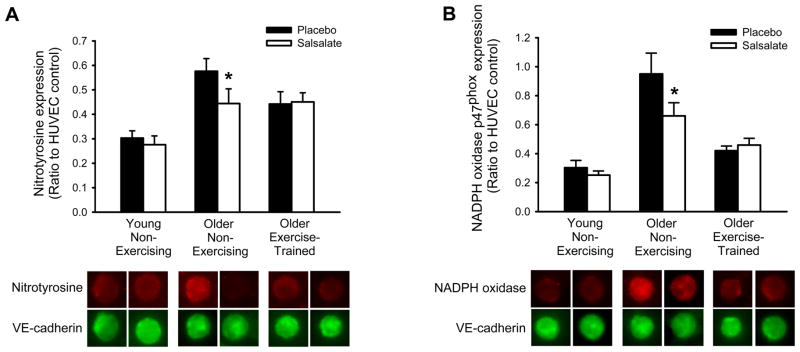
Endothelial cell content of nitrotyrosine and expression of NADPH oxidase p47phox did not change in either the older aerobic exercise-trained or young subjects with salsalate administration (P>0.05, Figure 4). In the older non-exercising subjects, salsalate administration resulted in a 25% reduction in nitrotyrosine content (P=0.02) and a 30% reduction in NADPH oxidase p47 expression (P<0.001, Figure 4).
Figure 4.
Endothelial protein expression of (A) nitrotyrosine and (B) nicotinamide adenine dinucleotide phosphate (NADPH) oxidase p47phox in young non-exercising (n=7–8: 7–8M, 0F), older non-exercising (n=13–14: 10–11M, 3F) and older aerobic exercise-trained (n=14: 11M, 3F) adults during placebo and salsalate conditions. Values are fluorescent intensity relative to human umbilical vein endothelial cell (HUVEC) control intensity. Representative images are shown below summary graphs for nitrotyrosine or NADPH oxidase p47phox and VE-cadherin (to identify endothelial cells). Values are mean ± SE. *P<0.05 vs. placebo condition within group.
AMPK
Recent studies indicate that salsalate activates AMPK in cultured cells [25, 26]. Because salsalate administration improved brachial FMD in the older non-exercising subjects in the present study, we assessed AMPK activation in endothelial cells obtained from that group (n=8, 5M/3F). AMPK activation did not change with salsalate administration (p-AMPK/total AMPK ratio; placebo: 1.26±0.18; salsalate: 0.84±0.22; P>0.05).
Discussion
In the present study, we found that salsalate reduces endothelial NF-κB expression and improves EDD in healthy non-exercising older adults. However, salsalate does not affect EDD in older exercising adults, consistent with an absence of tonic NF-κB –mediated suppression of EDD in this group. Furthermore, the improvement in EDD with salsalate in non-exercising older adults is mediated in part by reduced oxidative stress associated with decreased endothelial expression of NADPH oxidase. Taken together, these results are consistent with the concept that older adults who habitually perform aerobic exercise have preserved vascular endothelial function associated with less NF-κB signaling and oxidative stress.
Aging, habitual aerobic exercise and endothelial NF-κB
Sedentary aging is regarded as a state of chronic low-grade inflammation [9, 27]. Indeed, we have previously found that expression of the master pro-inflammatory mediator NF-κB is greater in endothelial cells collected from older adults compared with young adults [12]. Furthermore, we have shown that reducing NF-κB activity with salicylate treatment improves EDD in old mice [17], and recently it was reported that genetic inhibition of NF-κB specifically in endothelial cells leads to increased lifespan and diminishes features of vascular dysfunction with aging [28]. Here, using a previously established experimental approach [16], we extend these prior findings in mice to human aging by using administration of oral salsalate to reduce NF-κB expression in endothelial cells. We find that salsalate improves FMD in older non-exercising adults and this effect is independent of changes in circulating factors or clinical characteristics. In contrast, salsalate treatment had no effect on FMD in young controls or on endothelium-independent dilation in either group, suggesting that the effects were specific to the endothelium. Collectively, these observations support the hypothesis that endothelial function is suppressed by vascular NF-κB signaling in primary human aging, and that endothelial NF-κB signaling is an important modulator of vascular function and health with advancing age.
Habitual aerobic exercise is associated with numerous cardiovascular health benefits that include preventing or lessening the age-related declines in endothelial function [5, 29]. Reduced pro-inflammatory signaling has been proposed to contribute to the cardiovascular benefits of regular aerobic exercise [8, 9]. Previously, we have shown that older aerobic exercise-trained individuals have lower endothelial cell NF-κB expression compared with their non-exercising peers [13]. Using a randomized, cross-over experimental design, in the present study we extend these earlier observations by indicating that the enhanced vascular endothelial function of aerobic exercise-trained vs. non-exercising older adults is likely mediated by an absence of NF-κB-mediated suppression of EDD.
In agreement with our previous observations and those of others [16, 30, 31], in the present study salsalate administration reduced endothelial NF-κB expression, but did not affect circulating inflammatory markers. Collectively, these results suggest that the improvements in EDD are a result of reduced local endothelial rather than systemic inflammation.
Consistent with previous findings of reduced total cholesterol and/or LDL-cholesterol with salsalate administration [16, 32], in the present study we found modest reductions in total cholesterol in all groups and LDL-cholesterol in the older non-exercising group. However, we find that the improvement in FMD with salsalate in the older non-exercising adults remains significant when accounting for the reduction in LDL-cholesterol. Nevertheless, even modest elevations in LDL-cholesterol are associated with impaired FMD among healthy non-exercising older adults [33]. Given our small sample size, we cannot discount that reduced LDL-cholesterol, although it does not statistically account for improvement in FMD, may have a physiologic role in the benefits of salsalate among older non-exercising adults.
Oxidative stress
We originally reported that nitrotyrosine, a cellular marker of oxidative stress, is greater in endothelial cells obtained from healthy older compared with young healthy adults [12], and more recently demonstrated that habitual aerobic exercise prevents this age-related increase in endothelial oxidative stress in humans [13]. Consistent with the latter, we and others also have shown that aerobic exercise prevents oxidative stress-mediated suppression of endothelial function (i.e., EDD) with aging [5, 34]. Here, we extend these previous observations by showing that salsalate treatment reduces endothelial nitrotyrosine content, a marker of oxidative damage, in non-exercising, but not aerobic exercise-trained older adults, nor in young controls. We further show that vitamin C improves EDD in older non-exercising adults during placebo, but not salsalate, suggesting that NF-κB signaling contributes to the increased oxidative stress that suppresses EDD. However, the improvements in EDD seen with vitamin C alone (~30%) are not as great as the improvements with salsalate alone (~70%). This could be the result of incomplete neutralizing of excessive superoxide (oxidative stress) by vitamin C, the difference in treatment time of the 2 interventions (20-minute infusion vs. 3 days), or a contribution of additional mechanisms to the EDD improvement with salsalate, for example, an increase in phosphorylation of endothelial NO synthase [17]. Oxidative stress does not affect EDD in aerobic exercise-trained older individuals or young controls, as indicated by no effect of vitamin C in these groups during placebo or salsalate. Thus, the findings of the present study support the idea that NF-κB-mediated oxidative stress impairs EDD in older adults, but habitual aerobic exercise prevents this adverse effect.
One key source of reactive oxygen species in endothelial cells is NADPH oxidase [35]. We previously demonstrated that older otherwise healthy adults have greater endothelial cell NADPH oxidase p47phox expression compared with young controls [12]. We further showed that older aerobic exercise-trained adults have less NADPH oxidase p47phox expression than their non-exercising peers and similar levels as young adults [13]. In mice, we have demonstrated that NADPH oxidase contributes to carotid endothelial dysfunction in old non-exercising animals, but not in old animals given access to voluntary running wheels [36]. In the present study, we extend these findings by showing that salsalate reduces endothelial NADPH oxidase p47phox expression in non-exercising older adults, but has no effect in older aerobic exercise-trained adults. Thus, in sedentary older adults, it is likely that reducing endothelial NF-κB signaling leads to reduced NADPH oxidase transcription.
Experimental model: short-term, high-dose salsalate administration as a mechanistic probe
In the present study, short-term, high-dose administration of salsalate was used as an experimental approach to “pharmaco-dissect” the role of NF-κB signaling in the differences in endothelial function in healthy exercising and non-exercising middle-aged/older adults. Our results do not address the potential therapeutic efficacy of longer-term, lower-dose salsalate treatment in this or other groups. In contrast to short-term salsalate administration in the present study and previous investigation in overweight/obese adults [16], recent work in patients with type 2 diabetes [30] and coronary artery disease [37] found no improvements in FMD with longer-term administration of salicylate-based compounds (salsalate and sulfasalazine, respectively). These differences may result from differences in compounds and dosing, concurrent use of medications such as statins, ACE inhibitors and/or metformin that improve endothelial dysfunction, or different etiologies of endothelial dysfunction among these groups.
Limitations and alternative explanations
This study was a cross-sectional comparison of young controls and groups of healthy older adults who did or did not perform regular vigorous aerobic exercise. The results of the present investigation provide the experimental basis for conducting intervention studies targeting endothelial NF-κB signaling to improve endothelial function in older non-exercising adults and/or assessing the effects of aerobic exercise. Whereas, the older subject groups are fairly well-matched by sex, we were not able to successfully complete the study on any young female participants as a result of difficulties in controlling for menstrual cycle phase in this complex intervention study design. However, we did not find that the FMD improvement with salsalate was affected by sex in the older non-exercising group. Furthermore, as all older women were post-menopausal, there is a minimal influence of estrogen/progesterone in all subject groups.
We were limited in the number and type of analyses we could perform on the collected endothelial cells and, therefore, could only assess specific inflammatory and oxidative stress pathways and were unable to measure enzyme activities. In addition, a limitation of analyzing protein expression with an epifluorescence microscope is that we cannot accurately determine cellular localization. We also utilized a qualitative assessment of VE-cadherin expression to identify endothelial cells that could potentially introduce bias towards of the exclusion of endothelial cells with low VE-cadherin expression. However, as the individual performing cell imaging and analysis was blinded to group/condition, we do not believe this potential bias affected our results. In addition, control experiments involving the analysis of NF-κB expression with use of a blocking peptide (data not shown) indicates that any leak of VE-cadherin fluorescence into the NF-κB channel, while potentially present, was not different between subjects. Moreover, we assessed only endothelial cells obtained from venous sampling because the noninvasive measurements of brachial FMD and short treatment period did not allow for repeated placement of arterial catheters in our young and older human subjects. However, we previously demonstrated that protein expression in endothelial cells obtained from venous sampling is strongly, positively related to that in cells obtained from arterial sampling [24].
We recognize that our results do not definitively prove cause-and-effect associations between NF-κB and endothelial function. However, the study uses a combination of functional assessments, pharmaco-dissection of NF-κB and oxidative stress signaling, and novel molecular analyses of biopsied ECs in humans to provide as much insight into these relations as is possible in a clinical research setting. Salicylates are known to modulate other pathways in addition to NF-κB, but we do not believe that these other targets affected our results and conclusions. Although aspirin can inhibit cyclooxygenase leading to decreased prostaglandin production, salsalate lacks the acetyl group necessary to produce this effect [16, 38, 39]. Additionally, we have previously demonstrated that the salsalate dosing regimen used in this study does not affect endothelial cell cyclooxygenase 1/2 protein expression or circulating 6-keto-prostaglandin F1α concentrations (Pierce, 2009). Recent studies indicate that salsalate activates AMPK in cultured embryonic kidney cells and cultured hepatocytes [26], however, this effect has not been demonstrated in endothelial cells. In our study, we found that short-term salsalate administration did not affect AMPK activation in endothelial cells from non-exercising older adults. We limited our analyses to the older non-exercising subjects because salsalate administration improved brachial FMD only in this group. It is possible that systemic activation of AMPK explains the reduction in circulating lipids with salsalate administration; however, we found that the improvements in FMD in older non-exercising adults are independent of changes in lipids. Thus, although salicylates/salsalate have potential effects on cyclooxygenase and AMPK, we do not believe that these effects explain our findings related to EDD.
Conclusions
In the present study, we demonstrate that salsalate reduces endothelial NF-κB expression and improves EDD, partially by reducing oxidative stress, in healthy older non-exercising adults, but has no effect in young non-exercising or older exercising adults. These results suggest that NF-κB signaling mediates the impairment of EDD with primary human aging in part by increasing oxidative signaling and this is prevented by habitual aerobic exercise. Identifying interventions to improve endothelial function in sedentary older adults is of great clinical importance as these interventions could reduce the risk of cardiovascular diseases and events. Lifestyle interventions, such as aerobic exercise, improve vascular endothelial function in many older adults, but not all individuals can or will participate in aerobic exercise. Thus, identifying the molecular pathways responsible for such improvements is important as future interventions can be designed to target these pathways. Here we demonstrate that NF-κB signaling is likely an important modulating influence on endothelial function with aging, and that the “healthy endothelial effects” of aerobic exercise with aging are explained, at least in part, by suppression of excessive NF-κB signaling.
Supplementary Material
Clinical Perspectives.
Habitual aerobic exercise prevents the age-related decline in endothelial function, however the underlying molecular mechanisms responsible are incompletely understood.
Our results suggest that endothelial NF-κB signaling suppresses endothelial function in healthy non-exercising healthy older adults, and that this suppression is absent in exercising older adults.
This may be a key mechanism by which regular aerobic exercise preserves endothelial function and reduces cardiovascular risk with aging.
Inhibition of NF-κB signaling may have therapeutic potential in older adults who are unable or unwilling to perform regular aerobic exercise.
Summary statement.
Our results suggest that inflammatory regulator nuclear factor-κB is associated with impaired arterial function in healthy non-exercising, but not exercising older adults. This may be a mechanism by which regular aerobic exercise preserves function and reduces cardiovascular risk with aging.
Acknowledgments
We would like to thank Brooke Lawson, Eric Chung, Jessica Santos-Parker and Tsuzumi Kanaoka for technical assistance.
Funding. This work was supported by National Institutes of Health awards AG031617, AG006537, AG013038, AG022241, AG000279, and TR000154 and American Heart Association 0715735Z.
Footnotes
Author contributions. All experiments were carried out in the Department of Integrative Physiology at the University of Colorado Boulder. AEW, GLP and DRS contributed to the conception and design of experiments. AEW, REK, MJN and GLP contributed to the collection of data. AEW, REK and DRS drafted the manuscript. All authors contributed to the analysis and interpretation of data and revision of the manuscript. All authors approved of the final version of the manuscript.
References
- 1.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 2.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 3.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 5.Eskurza I, Monahan K, Robinson J, Seals D. Effect of acute and chronic ascorbic acid augmentation on flow-mediated dilation with physically active and sedentary aging. J Physiol. 2004;556:215–224. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol. 2000;88:761–766. doi: 10.1152/jappl.2000.88.2.761. [DOI] [PubMed] [Google Scholar]
- 7.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci. 2011;120:13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gielen S, Walther C, Schuler G, Hambrecht R. Anti-inflammatory effects of physical exercise. A new mechanism to explain the benefits of cardiac rehabilitation? J Cardiopulm Rehabil. 2005;25:339–342. doi: 10.1097/00008483-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bruunsgaard H, Skinhoj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Read MA, Whitley MZ, Williams AJ, Collins T. NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J Exp Med. 1994;179:503–512. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 13.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10:1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 15.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 16.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci. 2011;66:409–418. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manea A, Manea SA, Gafencu AV, Raicu M. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem. 2007;113:163–172. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- 19.Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 20.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 21.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol. 2008;51:1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 23.Donato AJ, Pierce GL, Lesniewski LA, Seals DR. Role of NFkappaB in age-related vascular endothelial dysfunction in humans. Aging (Albany NY) 2009;1:678–680. doi: 10.18632/aging.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res. 2010;47:1–8. doi: 10.1159/000231715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, Kemp BE, Sakamoto K, Steinberg GR, Hardie DG. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung TW, Choi HY, Lee SY, Hong HC, Yang SJ, Yoo HJ, Youn BS, Baik SH, Choi KM. Salsalate and Adiponectin Improve Palmitate-Induced Insulin Resistance via Inhibition of Selenoprotein P through the AMPK-FOXO1alpha Pathway. PLoS One. 2013;8:e66529. doi: 10.1371/journal.pone.0066529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa Y, Saito T, Ogihara T, Ishigaki Y, Yamada T, Imai J, Uno K, Gao J, Kaneko K, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Blockade of the nuclear factor-kappaB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012;125:1122–1133. doi: 10.1161/CIRCULATIONAHA.111.054346. [DOI] [PubMed] [Google Scholar]
- 29.Rywik TM, Blackman MR, Yataco AR, Vaitkevicius PV, Zink RC, Cottrell EH, Wright JG, Katzel LI, Fleg JL. Enhanced endothelial vasoreactivity in endurance-trained older men. J Appl Physiol. 1999;87:2136–2142. doi: 10.1152/jappl.1999.87.6.2136. [DOI] [PubMed] [Google Scholar]
- 30.Goldfine AB, Buck JS, Desouza C, Fonseca V, Chen YD, Shoelson SE, Jablonski KA, Creager MA for the TFMDAST. Targeting Inflammation Using Salsalate in Patients With Type 2 Diabetes (TINSAL): Effects on flow-mediated dilation. Diabetes Care. 2013;36:4132–4139. doi: 10.2337/dc13-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta SK, Johnson RM, Saha C, Mather KJ, Greenwald ML, Waltz JS, Rehman J, Dube MP. Improvement in HIV-related endothelial dysfunction using the anti-inflammatory agent salsalate: a pilot study. AIDS. 2008;22:653–655. doi: 10.1097/QAD.0b013e3282f470d2. [DOI] [PubMed] [Google Scholar]
- 32.Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, Shoelson SE Targeting Inflammation Using Salsalate in Type 2 Diabetes Study T. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159:1–12. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens. 2009;22:250–256. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 35.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabit CE, Holbrook M, Shenouda SM, Dohadwala MM, Widlansky ME, Frame AA, Kim BH, Duess MA, Kluge MA, Levit A, Keaney JF, Jr, Vita JA, Hamburg NM. Effect of sulfasalazine on inflammation and endothelial function in patients with established coronary artery disease. Vasc Med. 2012;17:101–107. doi: 10.1177/1358863X12440117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu KK. Aspirin and salicylate: An old remedy with a new twist. Circulation. 2000;102:2022–2023. doi: 10.1161/01.cir.102.17.2022. [DOI] [PubMed] [Google Scholar]
- 39.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101:1206–1218. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.