FIGURE 1.
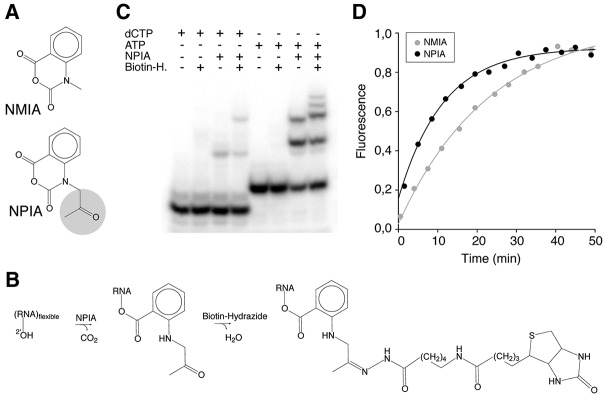
SHAPE Selection chemistry. (A) Chemical structures of N-methyl isatoic anhydride (NMIA) and N-propanone isatoic anhydride (NPIA). The N-methyl group of NMIA is exchanged to an N-propanone group in NPIA (marked in gray). (B) Reaction of NPIA with RNA and biotin (long arm) hydrazide. RNA in a flexible conformation is acylated by NPIA via the 2′-OH group, forming a stable 2′-O-adduct containing an N-propanone group. The N-propanone group is then biotinylated with biotin (long arm) hydrazide. (C) Polyacrylamide gel electrophoresis with products obtained by reacting radioactively labeled dCTP and ATP with NPIA and subsequently with biotin (long arm) hydrazide (Biotin-H). dCTP and ATP have reduced mobility in the polyacrylamide gel after reaction with NPIA, and the migration is further reduced upon reaction with biotin (long arm) hydrazide. (D) Comparative hydrolysis reactivity of NMIA (gray) and NPIA (black). The reaction with water was measured as an increase in fluorescence over time at 25°C, and the lines represent nonlinear regression to all data points.
