Abstract
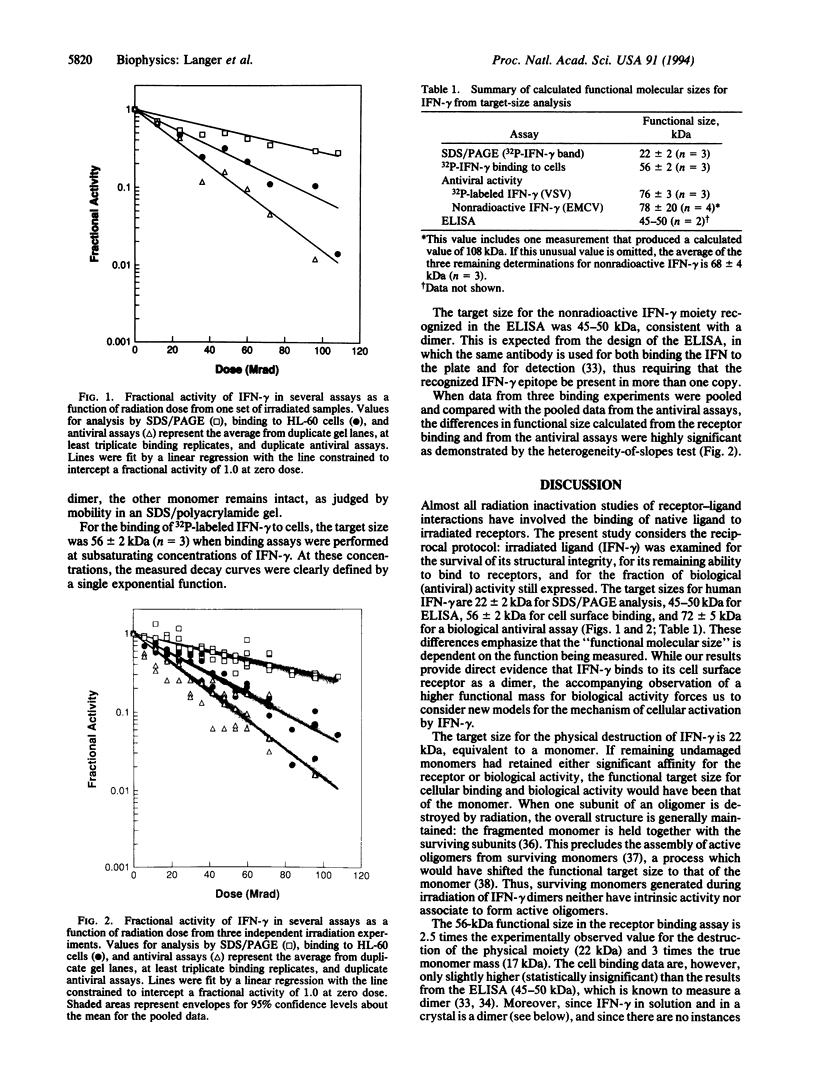
gamma-Interferon (IFN-gamma) is a 17-kDa broad-spectrum cytokine which exerts its effects on a variety of target cells through its interaction with the IFN-gamma receptor. Although physicochemical studies of Escherichia coli-derived IFN-gamma, as well as its crystal structure, demonstrate that it is a homodimer in solution (M(r) 34,000), previous radiation inactivation studies yielded a functional size for IFN-gamma of 63-73 kDa in an antiviral assay. To understand the relationship between the solution form of IFN-gamma and the moiety that actually binds to the cellular receptor and activates cells, we examined irradiated nonradioactive and 32P-labeled IFN-gamma for its migration in SDS/polyacrylamide gels (to determine its physical integrity), its binding to cells, its reactivity in an ELISA, and its antiviral activity. The functional size of IFN-gamma differed in the assays, being 22 +/- 2 kDa for the physical destruction of IFN-gamma, 56 +/- 2 kDa for the cellular binding assay, 45-50 kDa for reactivity in the ELISA, and 72 +/- 6 kDa for antiviral activity. The results from the binding assays constitute direct evidence that IFN-gamma binds to its cellular receptor as a dimer. However, for antiviral activity, the functional mass is equivalent to a tetramer. This is consistent with models involving ligand-induced receptor dimerization, whereby two dimers acting in concert (equivalent to the target size of a tetramer) are required to activate cells in the antiviral assay.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Dembić Z., Merlin G. Molecular cloning and expression of the human interferon-gamma receptor. Cell. 1988 Oct 21;55(2):273–280. doi: 10.1016/0092-8674(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee S., Majumdar S., Khire J., Das M. Ligand-induced dimerization of the platelet-derived growth factor receptor. Monomer-dimer interconversion occurs independent of receptor phosphorylation. J Biol Chem. 1989 Jul 15;264(20):11699–11705. [PubMed] [Google Scholar]
- Boulay J. L., Paul W. E. The interleukin-4-related lymphokines and their binding to hematopoietin receptors. J Biol Chem. 1992 Oct 15;267(29):20525–20528. [PubMed] [Google Scholar]
- Boyer T. D., Kempner E. S. Effect of subunit interactions on enzymatic activity of glutathione S-transferases: a radiation inactivation study. Anal Biochem. 1992 Nov 15;207(1):51–57. doi: 10.1016/0003-2697(92)90498-v. [DOI] [PubMed] [Google Scholar]
- Braude I. A. Purification of human gamma-interferon to essential homogeneity and its biochemical characterization. Biochemistry. 1984 Nov 6;23(23):5603–5609. doi: 10.1021/bi00318a034. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Ultsch M., De Vos A. M., Mulkerrin M. G., Clauser K. R., Wells J. A. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science. 1991 Nov 8;254(5033):821–825. doi: 10.1126/science.1948064. [DOI] [PubMed] [Google Scholar]
- Devos R., Cheroutre H., Taya Y., Degrave W., Van Heuverswyn H., Fiers W. Molecular cloning of human immune interferon cDNA and its expression in eukaryotic cells. Nucleic Acids Res. 1982 Apr 24;10(8):2487–2501. doi: 10.1093/nar/10.8.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos R., Opsomer C., Scahill S. J., Van der Heyden J., Fiers W. Purification of recombinant glycosylated human gamma interferon expressed in transformed Chinese hamster ovary cells. J Interferon Res. 1984 Fall;4(4):461–468. doi: 10.1089/jir.1984.4.461. [DOI] [PubMed] [Google Scholar]
- Ealick S. E., Cook W. J., Vijay-Kumar S., Carson M., Nagabhushan T. L., Trotta P. P., Bugg C. E. Three-dimensional structure of recombinant human interferon-gamma. Science. 1991 May 3;252(5006):698–702. doi: 10.1126/science.1902591. [DOI] [PubMed] [Google Scholar]
- Falcoff R. Some properties of virus and immune-induced human lymphocyte interferons. J Gen Virol. 1972 Aug;16(2):251–253. doi: 10.1099/0022-1317-16-2-251. [DOI] [PubMed] [Google Scholar]
- Familletti P. C., Rubinstein S., Pestka S. A convenient and rapid cytopathic effect inhibition assay for interferon. Methods Enzymol. 1981;78(Pt A):387–394. doi: 10.1016/0076-6879(81)78146-1. [DOI] [PubMed] [Google Scholar]
- Farrar M. A., Schreiber R. D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Fountoulakis M., Juranville J. F., Maris A., Ozmen L., Garotta G. One interferon gamma receptor binds one interferon gamma dimer. J Biol Chem. 1990 Nov 15;265(32):19758–19767. [PubMed] [Google Scholar]
- Fountoulakis M., Zulauf M., Lustig A., Garotta G. Stoichiometry of interaction between interferon gamma and its receptor. Eur J Biochem. 1992 Sep 15;208(3):781–787. doi: 10.1111/j.1432-1033.1992.tb17248.x. [DOI] [PubMed] [Google Scholar]
- Gallati H., Pracht I., Schmidt J., Häring P., Garotta G. A simple, rapid and large capacity ELISA for biologically active native and recombinant human IFN gamma. J Biol Regul Homeost Agents. 1987 Jul-Sep;1(3):109–118. [PubMed] [Google Scholar]
- Gentz R., Hayes A., Grau N., Fountoulakis M., Lahm H. W., Ozmen L., Garotta G. Analysis of soluble human and mouse interferon-gamma receptors expressed in eukaryotic cells. Eur J Biochem. 1992 Dec 1;210(2):545–554. doi: 10.1111/j.1432-1033.1992.tb17453.x. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Greenlund A. C., Schreiber R. D., Goeddel D. V., Pennica D. Interferon-gamma induces receptor dimerization in solution and on cells. J Biol Chem. 1993 Aug 25;268(24):18103–18110. [PubMed] [Google Scholar]
- Harmon J. T., Nielsen T. B., Kempner E. S. Molecular weight determinations from radiation inactivation. Methods Enzymol. 1985;117:65–94. doi: 10.1016/s0076-6879(85)17008-4. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Ernlund A., Rorsman C., Rönnstrand L. Dimerization of B-type platelet-derived growth factor receptors occurs after ligand binding and is closely associated with receptor kinase activation. J Biol Chem. 1989 May 25;264(15):8905–8912. [PubMed] [Google Scholar]
- Hemmi S., Böhni R., Stark G., Di Marco F., Aguet M. A novel member of the interferon receptor family complements functionality of the murine interferon gamma receptor in human cells. Cell. 1994 Mar 11;76(5):803–810. doi: 10.1016/0092-8674(94)90355-7. [DOI] [PubMed] [Google Scholar]
- Kelder B., Rashidbaigi A., Pestka S. A sandwich radioimmunoassay for human IFN-gamma. Methods Enzymol. 1986;119:582–587. doi: 10.1016/0076-6879(86)19079-3. [DOI] [PubMed] [Google Scholar]
- Kempner E. S. Damage to proteins due to the direct action of ionizing radiation. Q Rev Biophys. 1993 Feb;26(1):27–48. doi: 10.1017/s0033583500003954. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Miller J. H. Effect of environmental conditions on radiation target size analyses. Anal Biochem. 1994 Feb 1;216(2):451–455. doi: 10.1006/abio.1994.1067. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Pestka S. Radiation inactivation and target size analysis of interferons. Methods Enzymol. 1986;119:255–260. doi: 10.1016/0076-6879(86)19037-9. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Bekesi E. Phosphorylation of human immune interferon (IFN-gamma). Methods Enzymol. 1986;119:296–301. doi: 10.1016/0076-6879(86)19045-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langer J. A., Pestka S. Procedures for studying binding of interferon to human cells in suspension cultures. Methods Enzymol. 1986;119:305–311. doi: 10.1016/0076-6879(86)19046-x. [DOI] [PubMed] [Google Scholar]
- Langford M. P., Georgiades J. A., Stanton G. J., Dianzani F., Johnson H. M. Large-scale production and physicochemical characterization of human immune interferon. Infect Immun. 1979 Oct;26(1):36–41. doi: 10.1128/iai.26.1.36-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Li W., Stanley E. R. Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J. 1991 Feb;10(2):277–288. doi: 10.1002/j.1460-2075.1991.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn C. A., Davies L., Dalgarno D., Narula S. K., Zavodny P. J., Lundell D. An active covalently linked dimer of human interferon-gamma. Subunit orientation in the native protein. J Biol Chem. 1992 Sep 5;267(25):17920–17924. [PubMed] [Google Scholar]
- Miyata K., Yamamoto Y., Ueda M., Kawade Y., Matsumoto K., Kubota I. Purification of natural human interferon-gamma by antibody affinity chromatography: analysis of constituent protein species in the dimers. J Biochem. 1986 Jun;99(6):1681–1688. doi: 10.1093/oxfordjournals.jbchem.a135643. [DOI] [PubMed] [Google Scholar]
- Nathan I., Groopman J. E., Quan S. G., Bersch N., Golde D. W. Immune (gamma) interferon produced by a human T-lymphoblast cell line. Nature. 1981 Aug 27;292(5826):842–844. doi: 10.1038/292842a0. [DOI] [PubMed] [Google Scholar]
- Pellegrini S., Schindler C. Early events in signalling by interferons. Trends Biochem Sci. 1993 Sep;18(9):338–342. doi: 10.1016/0968-0004(93)90070-4. [DOI] [PubMed] [Google Scholar]
- Pestka S., Kelder B., Familletti P. C., Moschera J. A., Crowl R., Kempner E. S. Molecular weight of the functional unit of human leukocyte, fibroblast, and immune interferons. J Biol Chem. 1983 Aug 25;258(16):9706–9709. [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Potier M., Thauvette L., Michaud L., Giroux S., Beauregard G. Inactivation mechanism of tetrameric beta-galactosidase by gamma-rays involves both fragmentation and temperature-dependent denaturation of protomers. Biochemistry. 1991 Aug 20;30(33):8151–8157. doi: 10.1021/bi00247a009. [DOI] [PubMed] [Google Scholar]
- Rashidbaigi A., Kung H. F., Pestka S. Characterization of receptors for immune interferon in U937 cells with 32P-labeled human recombinant immune interferon. J Biol Chem. 1985 Jul 15;260(14):8514–8519. [PubMed] [Google Scholar]
- Rinderknecht E., O'Connor B. H., Rodriguez H. Natural human interferon-gamma. Complete amino acid sequence and determination of sites of glycosylation. J Biol Chem. 1984 Jun 10;259(11):6790–6797. [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993 Sep 24;261(5129):1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Samudzi C. T., Burton L. E., Rubin J. R. Crystal structure of recombinant rabbit interferon-gamma at 2.7-A resolution. J Biol Chem. 1991 Nov 15;266(32):21791–21797. [PubMed] [Google Scholar]
- Silvennoinen O., Schindler C., Schlessinger J., Levy D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Soh J., Donnelly R. J., Kotenko S., Mariano T. M., Cook J. R., Wang N., Emanuel S., Schwartz B., Miki T., Pestka S. Identification and sequence of an accessory factor required for activation of the human interferon gamma receptor. Cell. 1994 Mar 11;76(5):793–802. doi: 10.1016/0092-8674(94)90354-9. [DOI] [PubMed] [Google Scholar]
- Soh J., Donnelly R. J., Mariano T. M., Cook J. R., Schwartz B., Pestka S. Identification of a yeast artificial chromosome clone encoding an accessory factor for the human interferon gamma receptor: evidence for multiple accessory factors. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8737–8741. doi: 10.1073/pnas.90.18.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto M., Tarutani T., Ogawa K., Higashi N. Effect of glycosidases on the properties of human interferon gamma. J Interferon Res. 1984;4(1):111–114. doi: 10.1089/jir.1984.4.111. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Skorecki K., Ausiello D. A. Radiation inactivation of oligomeric enzyme systems: theoretical considerations. Proc Natl Acad Sci U S A. 1984 Jan;81(1):150–154. doi: 10.1073/pnas.81.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip Y. K., Pang R. H., Urban C., Vilcek J. Partial purification and characterization of human gamma (immune) interferon. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1601–1605. doi: 10.1073/pnas.78.3.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yphantis D. A., Arakawa T. Sedimentation equilibrium measurements of recombinant DNA derived human interferon gamma. Biochemistry. 1987 Aug 25;26(17):5422–5427. doi: 10.1021/bi00391a031. [DOI] [PubMed] [Google Scholar]
- de Ley M., van Damme J., Claeys H., Weening H., Heine J. W., Billiau A., Vermylen C., de Somer P. Interferon induced in human leukocytes by mitogens: production, partial purification and characterization. Eur J Immunol. 1980 Nov;10(11):877–883. doi: 10.1002/eji.1830101113. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]