Abstract
Objective
To perform a comparative assessment of two surgical techniques that are used creating an acute myocardial infarc by occluding the left anterior descending coronary artery in order to generate rats with a left ventricular ejection fraction of less than 40%.
Methods
The study was completely randomized and comprised 89 halothane-anaesthetised rats, which were divided into three groups. The control group (SHAM) comprised fourteen rats, whose left anterior descending coronary artery was not occluded. Group 1 (G1): comprised by 35 endotracheally intubated and mechanically ventilated rats, whose left anterior descending coronary artery was occluded. Group 2 (G2): comprised 40 rats being manually ventilated using a nasal respirator whose left anterior descending coronary artery was occluded. Other differences between the two techniques include the method of performing the thoracotomy and removing the pericardium in order to expose the heart, and the use of different methods and suture types for closing the thorax. Seven days after surgery, the cardiac function of all surviving rats was determined by echocardiography.
Results
No rats SHAM group had progressed to death or had left ventricular ejection fraction less than 40%. Nine of the 16 surviving G1 rats (56.3%) and six of the 20 surviving G2 rats (30%) had a left ventricular ejection fraction of less than 40%.
Conclusion
The results indicate a tendency of the technique used in G1 to be better than in G2. This improvement is probably due to the greater duration of the open thorax, which reduces the pressure over time from the surgeon, allowing occlusion of left anterior descending coronary artery with higher accuracy.
Keywords: Myocardial infarction; Echocardiography; Models, Cardiovascular
Abstract
Objetivo
Realizar uma avaliação comparativa de duas técnicas cirúrgicas que são usadas para criar um infarto agudo do miocárdio pela oclusão da artéria coronária descendente anterior esquerda, a fim de gerar ratos com uma fração de ejeção ventricular esquerda inferior a 40%.
Métodos
O estudo foi completamente randomizado e composto por 89 ratos anestesiados com halotano, que foram divididos dentro de três grupos. O grupo controle (SHAM) composto por 14 ratos, cuja artéria coronária descendente anterior esquerda não foi ocluída. Grupo 1 (G1): composto por 35 ratos intubados endotraquealmente e ventilados mecanicamente, cuja artéria coronária descendente anterior esquerda foi ocluída. Grupo 2 (G2): constituído por 40 ratos sendo ventilados manualmente utilizando um respirador nasal, cuja artéria coronária descendente anterior esquerda foi ocluída. Outras diferenças entre as duas técnicas incluem o método de realizar a toracotomia e remover o pericárdio, a fim de expor o coração, e o uso de diferentes métodos e tipos de sutura para fechar o tórax. Sete dias após a cirurgia, a função cardíaca de todos os ratos sobreviventes foi determinada por ecocardiografia.
Resultados
Nenhum rato do grupo SHAM foi a óbito ou teve fração de ejeção ventricular esquerda menor que 40%. Nove dos 16 ratos sobreviventes do G1 (56,3%) e seis dos 20 ratos sobreviventes do G2 (30%) tiveram uma fração de ejeção ventricular esquerda inferior a 40%.
Conclusão
Os resultados indicam uma tendência da técnica utilizada no G1 ser melhor do que a do G2. Esta melhora deve-se provavelmente à maior duração do tórax aberto, o que reduz a pressão de tempo sobre o cirurgião, permitindo uma oclusão da artéria coronária descendente anterior esquerda com maior acurácia.
| Abbreviations, acronyms & symbols | |
|---|---|
| AMI | Acute myocardial infarction |
| EDA | End diastolic area |
| EDV | End diastolic volume |
| ESA | End systolic area |
| ESV | End systolic volume |
| G1 | Group 1 |
| G2 | Group 2 |
| HR | Heart rate |
| IM | Intramuscular |
| IP | Intraperitoneal |
| LADCA | Left anterior descending coronary artery |
| LVEF | Left ventricular ejection fraction |
| SD | Standard deviation |
| SHAM | Control group |
INTRODUCTION
Cell therapy has been proposed as a future therapy for myocardial diseases, and the efficacy of the different types of cell therapy for cardiomyopathies has been investigated in rats with experimental acute myocardial infarction (AMI)[1]. Two different surgical techniques for creating an AMI in rats have been described in the medical science literature. Both techniques have four common steps: a lateral thoracotomy through the left fourth intercostal space, removal of the pericardium, permanent occlusion of the left anterior descending coronary artery (LADCA), and closure of the thorax. However, the techniques differ in terms of (a) the need for endotracheal intubation, (b) the type of ventilatory support, (c) the duration of the open-chest surgery, and (d) visualisation of the myocardial infarction after occlusion of the LADCA artery. The first technique, which was initially described by Johns & Olson[2], is simple, does not require sophisticated equipment, and is still widely used by other investigators[3-6]. In this technique, the animal can be nasally ventilated using a respirator because of the duration of open thorax is short, thereby eliminating the need for endotracheal intubation. In the second technique, which is also commonly used by investigators[1,7], the animals are obligatorily intubated and are mechanically ventilated in the positive end-expiratory pressure mode because the duration of open thorax is longer than that of the first technique. Other differences between the two techniques include the method of performing the thoracotomy and removing the pericardium in order to expose the heart, and the use of different methods and suture types for closing the open thorax.
The aim of this study was to compare two different surgical methods for creating an AMI in order to generate rats with a left ventricular ejection fraction (LVEF) of less than 40%. The consequent on cardiac function in these rats was assessed using echocardiography.
METHODS
Animals
This animal study and the procedures detailed herein were reviewed and approved by the Local Ethics Committee on Animal Research (Identification numbers: PUCPR 180 and 540). The study comprised 89 male 100-day-old albino Wistar rats (Rattus norvegicus) (mean weight 348.6 grams ±24.3 (standard deviation (SD)]. The rats were obtained from the central animal facility of the Pontifícia Universidade Católica do Paraná, Curitiba, Brazil, which has an in-house breeding programme. The rats were housed in open-top polypropylene cages (41cm x 34cm x 16cm (height)) in groups of three or four rats/cage in a temperature- (18-21Cº) and humidity-controlled (55-65% relative humidity) environment with a 12-hour light-dark cycle, and had ad libitum access to a standard rodent chow (NUVITAL®, Colombo, Paraná, Brazil) and water. The bedding (pine wood shavings, Inbrasfama, São José dos Pinhais, Paraná, Brazil) in each cage was changed daily.
A comparative experimental study was performed. After a 2-day acclimatization period, the rats were randomly divided by lot of cages into three groups according to the surgical procedure that they underwent. An AMI was created in 35 rats of the Group 1 (G1) and 40 rats of the Group 2 (G2) by two different surgical methods (see later). Group 3 (SHAM) comprised fourteen rats, used as control of the experiment, in which ones the AMI was not created. The methods of anaesthesia, thoracotomy, and exposure of the heart and LADCA in these fourteen SHAM rats were identical to those that were done in the G2 rats (see later). In the SHAM rats, the LADCA was not occluded after placing 4-0 silk thread around the exposed vessel.
Anaesthesia
Full details of the anaesthetic protocols for the two groups of rats are presented in Table 1. For G1 rats, the rats were first pre-medicated by intraperitoneal (IP) injections of 1.25mg/kg diazepam (Valium®, 5 mg/ml, Teuto, Goiás, Brazil) and 12.5 mg/kg ketamine (Vetanarcol®, 50 mg/ml, Laboratórios König S.A., Avellaneda, Argentina), and an intramuscular (IM) injection of 5 mg/kg meperidine (Dolosal®, 50 mg/ml, Cristália, São Paulo, Brazil). Five minutes after the injections, anaesthesia was induced by ~4% halothane (Tanohalo®, Cristália, São Paulo, Brazil) in 100% oxygen in a glass induction chamber (Chiarorn, Brazil). Each rat was then endotracheally intubated, and their anaesthesia was maintained by ~2% halothane vaporized in 100% oxygen (~150 ml/minute) in a semi-closed breathing circuit. Halothane delivery to the anaesthetised rats was not continuous: it was stopped at the time of LADCA occlusion or when the rat was at the desired depth of anaesthesia. Each rat was mechanically ventilated using a ventilator (Harvard model 683 small animal ventilator, Harvard Apparatus, MA, USA), which was set 70-80 breaths/minute and a minute volume of 175-200 ml/min.
Table 1.
Details of the pre-medication and anaesthetic protocols and the peri-and post-operative medications of the two groups of rats in whom an acute myocardial infarction (AMI) was created.
| Anaesthesia Protocol | Group 1 (n =35) | Group 2 (n=40) |
|---|---|---|
| Inhalation Anaesthesia | Halothane (vaporizer) | Halothane (facial mask) |
| Dissociative Anaesthesia | Ketamine 12.5mg/kg IP | not given |
| Muscle Relaxant | Diazepam 1.25mg/kg IP | not given |
| Intraoperative Analgesic | Meperidine 5mg/kg IM | Meperidine 5mg/kg IM |
| Postoperative Analgesics | Morphine 1mg/kg SC, | Morphine 1mg/kg SC, |
| three times per day | three times per day | |
| for 48 hours | for 48 hours | |
| Flunixin 2.5mg/kg SC, | Flunixin 2.5mg/kg SC, | |
| once a day for 48 hours | once a day for 48 hours | |
| Antibiotic | Enrofloxacin 10mg/kg IM, | Enrofloxacin 10mg/kg IM, |
| once daily for 4 days | once daily for 4 days | |
| Anticholinergic* | Atropine 40μg/kg IM | Atropine 40μg/kg IM |
| Diuretic* | Furosemide 1-4mg/kg IM | Furosemide 1-4mg/kg IM |
| Positive Inotrope* | Adrenaline 0.04-0.2mg/kg IM | Adrenaline 0.04-0.2mg/kg IM |
IM=intramuscular route of administration; IP= intraperitoneal route of administration; SC=subcutaneous route of administration; n=sample size.
(*) Rats which developed adverse cardio-respiratory and renal effects following the creation of an AMI were treated with atropine, furosemide and adrenaline, if necessary
For G2 rats, different pre-medication and anaesthesia protocols were used because the duration of the surgery was shorter than that of the G1 rats. In this group, the rats were first pre-medicated by an IM injection of 5 mg/kg meperidine. Five minutes after the injection, anaesthesia was then induced by ~4% halothane in 100% oxygen in a glass induction chamber (Chiarorn. Brazil). The rats were not endotracheally intubated, and the anaesthesia was maintained by ~2% halothane in 100% oxygen (~350 ml/minute) using a facial mask. Halothane delivery to the anaesthetised rats was not continuous: it was stopped at the time of LADCA occlusion or when the rat was at the desired depth of anaesthesia. The rats were manually ventilated at ~70-80 breaths/minute and a minute volume of ~175-200 ml/min during the thoracotomy and in the immediate postoperative period using a nasal respirator that was adapted[7](Figure 1).
Fig. 1.
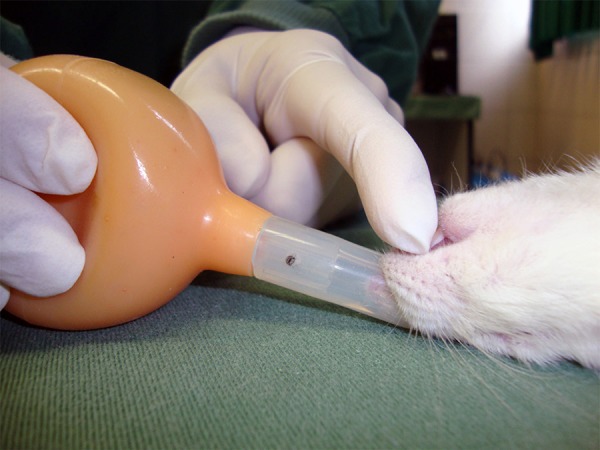
Nasal respirator for manual ventilation of the G2 rats during the thoracotomy and occlusion of the left anterior descending branch of the left coronary artery.
*Group 2 (G2)
The body temperature of the rats during the surgery and while they recovered from anaesthesia was maintained by keeping them on a heated table (MASTER DIGITAL SA-300®, Ch@mpion/Electronics, France). Post-operative pain was relieved for 48 hours after surgery using flunixin and morphine, whose doses and route and frequency of administration are given in Table 1. Rats which developed adverse cardio-respiratory and renal effects following creation of the AMI were treated with atropine, furosemide, adrenaline, if necessary, and the specific details of each drug's dose and route of administration are also listed in Table 1.
Surgical creation of an AMI
Before surgery, the surgical site was prepared by presurgical shaving and skin antisepsis using 95% alcohol and 2% chlorhexidine gluconate. In the G1 rats, the thoracotomy was performed through the left fourth intercostal space after surgically separating the latissimus dorsi and pectoral muscles. The intercostal space was kept open using a 7-cm Alm self-retaining retractor in order to visualize the beating heart. The pericardium was then removed using a sterile flexible cotton-tipped rod. After exposing the heart, which was not exteriorized, the LADCA was first identified, and then occluded 2 mm from its origin between the left atrial edge and the pulmonary artery sulcus using 7-0 polypropylene thread. The thorax was then closed in two layers with simple interrupted 4-0 monofilament nylon sutures.
In the G2 rats, the latissimus dorsi and pectoral muscles were first separated prior to thoracotomy, which was performed through either the left fourth intercostal space. A continuous cotton thread was then placed around the surgical incision before opening the chest and exposing the heart in order to facilitate rapid closure of the open thorax immediately following occlusion of the LADCA (Figure 2). The thorax was opened using 16-cm thoracic Crile forcepsand in the sequence it was applied a lateral compression to the right thorax to physically tear the pericardium exteriorizing the heart. After exteriorizing the heart, the LADCA was occluded 2 mm from its origin by ligating the artery between the pulmonary artery and the left atrial auricle with 4-0 silk thread. Silk thread was used to occlude the artery because it has a higher tensile strength than 7-0 polypropylene thread, thereby making it less likely to break when occluding the artery. The entire procedure was rapidly performed in order to ensure a high survival rate of the rats. The thorax was closed immediately after occlusion of the LADCA. Each rat was returned to its home cage when it was fully recovered from the anaesthesia and surgery, and kept under the identical conditions that were described in the "Animals" subsection.
Fig. 2.
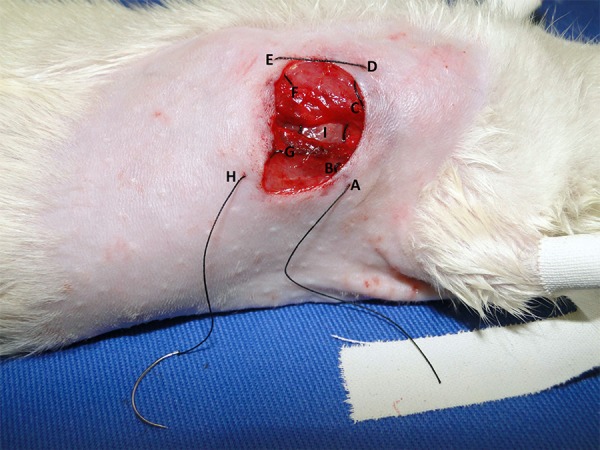
The route of the continuous cotton thread that is placed around the surgical incision before opening the chest in the G2 rats.
* Group 2 (G2), Skin (A), latissimus dorsal muscle (B), pectoral muscle (C), skin (D), skin (E) of the other incision edge, pectoral muscle (F), latissimus dorsal muscle (G), and skin (H), 4th intercostal space (I)
Echocardiography
Echocardiographic examination was performed by an experienced professional, without the knowledge of the groups formed. An echocardiographic examination of the fourteen SHAM rats and all surviving G1 and G2 rats was performed seven days after surgery. For this purpose, the rats were sedated by an IM injection of 50 mg/kg ketamine and 5mg/kg xylazine (Rompun®, 20 mg/ml, Bayer S.A., São Paulo, Brazil). When sedated, their thoracic hair was removed by shaving, and they were placed in the dorsal decubitus position with the body slightly inclined to the left. Two-dimensional transthoracic echocardiography was performed using a multi-frequency linear-array ultrasound transducer (15L6, bandwidth 15 MHz, Philips Ultrasound, USA) whose output was recorded on a Hewlett Packard Sonos 5500 Ultrasound System. Ejection fraction (LVEF), end systolic volume (ESV), end diastolic volume (EDV), end systolic area (ESA), and end diastolic area (EDA) of the left ventricle were determined from the images using Simpson's method[8]. The heart rate (HR) of these rats was simultaneously measured by an electrocardiograph that was incorporated into the ultrasound system. All echocardiographic measurements were performed using the same equipment and were repeated three times by the same examiner. The results are represented as the mean of three independent measurements.
After the echocardiographic examination, the rats were then returned to their home cages, where they were kept for thirty days under the identical conditions that were described in the "Animals" subsection. The rats were also followed-up daily for clinical signs of illness and behavioural problems, such as aggression or stereotypic behaviours. After 30 days, the rats were humanely killed without the presence of other rats by an overdose of halothane after being placed in the glass induction chamber that was used to induce anaesthesia. After confirmation of death, each rat was necropsied.
Histopathology of the hearts
After euthanasia, the heart of each rat from the G1, G2, and SHAM groups was removed for histopathological analysis. For this purpose, the hearts were fixed in a 10% neutral buffered formalin solution (Biotec, Labmaster, Pinhais-PR) for 24 hours. Histological sections of the heart were prepared for haematoxylin and eosin (H&E) and Masson trichrome staining by standard procedures using a commercial kit (Easy Path®, Bio-Optica Milano S.P.A., Milan, Italy). Briefly, the formalin-maintained samples were washed in tap water, dehydrated using an ascending alcohol series, and then embedded in paraffin blocks. Sections (5-µm thick) were cut, mounted on glass slides, hydrated using distilled water, and then stained. H&E staining was performed in order to locate the infarction and Masson trichrome staining was used to assess collagen deposition in the infarct.
Statistical analysis
The echocardiographic measurements of all surviving G1 and G2 rats, whose LVEF was less than 40% and the rats SHAM group were compared and statistically analysed using a computerized statistical software programme (Prism version 5.0 for Windows, GraphPad Software Inc., CA, USA).
The normal distribution of the samples was assessed by the Kolmogorov-Smirnov test. Means of echocardiographic parameters as well as the duration of anesthesia and surgery were compared using the test of ONE-WAY (ANOVA) followed by Bonferroni test.
The chi-square test for proportions was used to determine whether the mortality rates in the SHAM, G1 and G2 groups were different from each other. The Fisher exact test was used to determine differences in proportions between groups SHAM, G1 and G2 for the occurrence of LVEF less than 40%.
Data are presented as mean±SD, the level of statistical significance was set at 5% (α=0.05).
RESULTS
The entire duration of anaesthesia and surgery was significantly longer (P<0.0001) in the G1 rats than in the G2 rats (Table 2). None of the fourteen SHAM rats died after surgery, and each SHAM rats had a LVEF that was greater than 40% (Table 2). Specifically, the values of the five echocardiographic measurements in these rats were 61.67±7.23% for LVEF, 0.59±0.07 ml for EDV, 0.23±0.05 ml for ESV, 1.09±0.08 cm2 for EDA, and 0.59±0.08 cm2 for ESA, statistically different in the G1 and G2 (Table 3).
Table 2.
Comparison of the duration of the anesthetic-surgical procedure and the percentage of rats with ejection fraction of the left ventricle (LVEF) less than 40% between G1, G2 and SHAM groups.
| Number of Operated Rats | Duration in minutes of the anaesthesia and surgery (mean ± standard deviation) | Number of surviving rats 24 hours after surgery | Mortality Rate (%) | Number (%) of surviving rats whose LVEF <40% |
|---|---|---|---|---|
| G1: n=35 | 14.6±0.1a | 16 | 54.3a | 56.3a |
| G2: n=40 | 5.5±0.2b | 20 | 50.0a | 30ab |
| SHAM: n=14 | - | 14 | 0b | 0b |
n=sample size; different letters between rows = P<0.05, and is the significance of the difference between the groups
Table 3.
Comparison of the means of echocardiographic measurements between the groups SHAM and G2, G1.
| Variables | LVEF (%) | ESV (mL) | EDV (mL) | ESA (cm2) | EDA (cm2) | HR (bpm) |
|---|---|---|---|---|---|---|
| G1 (n=9) | 28,0±9,2a | 0,54±0,12a | 0,74±0,09a | 1,12±0,35a | 1,23±0,1a | 258,4±41,6a |
| G2 (n=6) | 26,5±6,1a | 0,55±0,12a | 0,74±0,13a | 1,01±0,12a | 1,23±0,11a | 255,2±40,2a |
| SHAM (n=14) | 61.6±7.2b | 0.23 ± 0.05b | 0.59±0.07b | 0.59 ± 0.08b | 1.0±0.08b | 227,3 ± 34,8a |
| P value | 0,0001 | 0,0001 | 0,0007 | 0,0001 | 0,0022 | >0,05 |
LVEF=ejection fraction of the left ventricle; ESV=end systolic volume; EDV=end diastolic volume; ESA=end systolic area; EDA=end diastolic area; HR=heart rates; bpm=beats per minute. Different letters between rows: significant difference (P<0.05)
Nineteen G1 rats (54.3%) and 20 G2 rats (50%) died during the first 24 hours after surgery, and the mortality rates in the two groups were not significantly different (Table 2).
The propensity for complications during surgery, namely pulmonary atelectasis and haemorrhage, was higher in the G2 rats than in the G1 rats. In contrast, the propensity for post-operative complications, namely respiratory depression, heart failure, and pulmonary oedema, was higher in the G1 rats than in the G2. The main causes of death in the G1 and G2 rats were cardiac dilation, heart failure, and pulmonary oedema. Nine of the 16 surviving G1 rats (56.3%) and six of the 20 surviving G2 rats (30%) had a LVEF of less than 40%. The proportion of rats with a LVEF of less than 40% in the groups G1 and G2 was not significantly different from each other (P=0.176). The proportion of rats with LVEF less than 40% was significantly higher in G1 compared to SHAM group (P=0.014), but there was no difference in this rate between the G2 and SHAM group (P=0.0743), (Table 2). Overall, 25.7% of all G1 rats and 15% of all G2 rats had a LVEF of less than 40%. Table 3 shows the comparison of the means of echocardiographic measurements between the groups SHAM and G2, G1. The mean values of each echocardiographic measurement in the nine G1 rats were not significantly different from those in the six G2 rats.
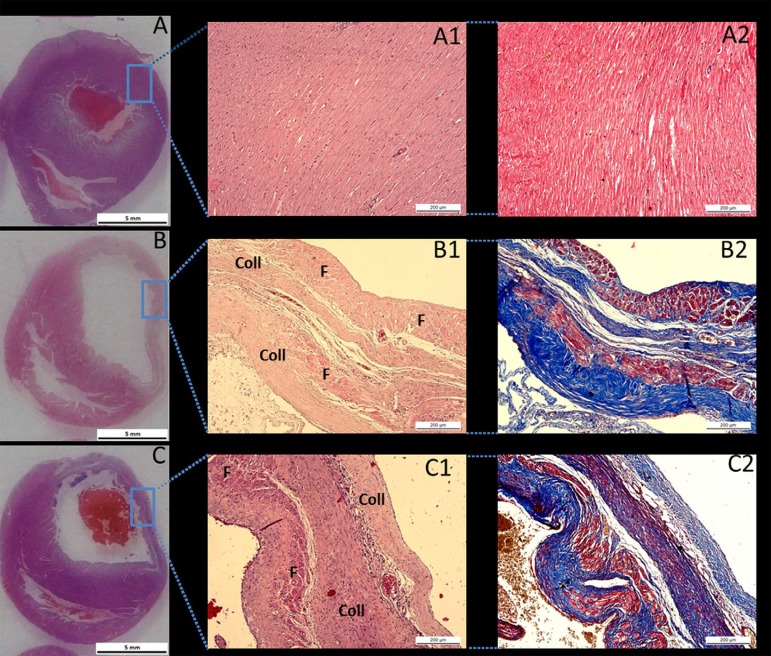
Histopathological analysis of hearts from the ten SHAM rats revealed preserved cardiac muscle tissue (Figure 3). Histological analysis of hearts from both the G1 and G2 rats revealed the presence of an organized transmural infarct with fibrosis and intense collagen deposits in the free wall of the left ventricle (Figure 3).
Fig. 3.
Panoramic photomicrographs of the cross-sections of the heart from a SHAM rat (A), a G1 rat (B), and a G2 rat (C).
*In the G1 and G2 rats, a transmural infarct is observed on the free wall of the left ventricle. Haematoxylin and eosin staining of cardiac tissue from a SHAM rat (A1) in which no myocardial infarct was observed, the myocardium is preserved and no histological alterations in cardiac microarchitecture were found. Haematoxylin and eosin staining of cardiac tissue in a G1 rat (B1) and a G2 rat (C1) shows an organized area of cicatricial collagen (Coll) in the transmural infarct of remanescent muscle fibres (F). Masson trichrome staining of cardiac tissue from a SHAM rat (A2), a G1 rat (B2), and a G2 rat (C2) in which the muscle fibres are stained red and the collagen is stained blue
DISCUSSION
The mortality rate in the G1 and G2 during the first 24 hours following surgery in this study was between 50-55%, and this rate is similar to that reported (40-65%) in other studies[3,9]. Zomoff et al.[10] reported that the cardiac remodelling that occurs after AMI is associated with a high prevalence of cardiac rupture, arrhythmias, and the formation of aneurysms. Other investigators have reported that arrhythmias, such as sustained ventricular tachycardia and ventricular fibrillation, are the principal cause of death in rats with an experimentally-induced AMI[9,11].
Although there was no statistical difference between groups, we found that the number of G1 rats with a LVEF of less than 40% was greater than found in the group G2. We attribute this increased to the duration of the surgical procedure that was used to create the AMI. The resultant number of rats with a LVEF of less than 40% is also determined by several intrinsic and extrinsic factors. The intrinsic determinants include the rat's age and lineage, individual variations in the anatomy of the coronary artery, and individual physiological factors. On the age of the rat, spontaneous improvement in systolic function and ventricular volume can occur within 30 days after creation of an AMI in young rats[12]. Hence, adult rats whose ages of the approximately 150 days and have a body weight of approximately 350 grams are preferred for creating the experimental AMI[12]. The extrinsic determinants include the site of the occlusion, which is dependent of the surgeon's experience and skill in occluding the LADCA. If the LADCA is occluded close to its origin, the size of the infarct will be large and the mortality rate can be as high as 100%[13].
Kissin et al.[14] has also reported that the prolonged duration (three hours) of halothane-induced anaesthesia can influence the onset of postoperative deleterious effects and could cause postoperative death in rats with an experimentally-induced AMI. Specifically, they showed that increasing the duration of halothane anaesthesia after creation of the AMI causes prolonged hypotension and increases the size of the myocardial infarction. Then, it is possible that, beyond the surgery, the duration of halothane-induced anaesthesia accounted for the higher propensity for a severe left ventricular dysfunction in the G1 rats than in the G2 rats. However, in a mild form, as in this study the difference in anaesthesia time between groups was only nine minutes.
In our study, histopathological examination of the hearts of all rats with a LVEF of less than 40% revealed that these hearts had histopathological characteristics that were similar to those that have been reported by others[3,4]. Specifically, we found that the infarcted hearts were dilated, and this dilation was associated with a reduced thickness of the free wall of the left ventricle with intense collagen deposits. According to Fishbein et al.[4], these cardiac alterations in the G1 and G2 rats probably occur about 21 days after creation of the experimental AMI. Consequently, cardiac muscle tissue loss results in a decrease in LVEF with an increase in end systolic and end diastolic volume of the left ventricle. This process results in ventricular dilatation and an increase in diastolic tension[10,12]. We found no significant differences in the echocardiographic measurements of the G1 and G2 rats whose LVEF were less than 40%.
However, when comparing the two surgical techniques for creating the AMI, the technique that was used in the G2 rats is more advantageous than that used in the G1 rats in terms of (a) the duration of the surgical procedure, (b) the need for specific rodent equipment or instrumentation, (c) the healing process, as measured by the low incidence of suture breakdown, and the reduced time of the complete closure of the surgical wound and removal of the sutures, and (d) the extent of acoustic shadowing in the echocardiographic evaluation. Nonetheless, the surgical technique that was used for creating the AMI in the G1 rats does have some advantages over that used for creating the AMI in the G2 rats. Firstly, the rate of operative complications, such as the frequency of pulmonary atelectasis and haemorrhage, is lower. Secondly, the use of a flexible cotton-tipped rod enables easier removal of the pericardium. Thirdly, the risk of cardiac rupture is lower because the heart is not exteriorized as in the G1. Finally, the duration of the open thorax is longer, which reduces the time pressure on the surgeon.
Our study has several limitations. The techniques showed are the most practiced, but the low rate of severely infarcted rats remains a limitation of this experimental model. Due to this limitation, the power of the statistical test used to analyze the proportion of LVEF less than 40% between the groups was low (0.35). To increase the power of the test, we would have a greater number of samples in each group, making the statistical results of this analysis more reliable, but for ethical reasons this was not done. A second limitation of our study was the necessity of sedate the rats with ketamine and xylazine for echocardiographic examination. This sedative mixture not only affects the HR, but also decreases cardiac contractility[15]. Although the doses of ketamine and xylazine were identical for all rats, we used minimal doses in order to immobilize the rats for the examination. Echocardiographic measurements were also performed in the fourteen SHAM rats, which were also sedated by the identical doses of ketamine and xylazine. We found that the values of these measurements were higher than those in the rats with an AMI. Accordingly, we concluded that the changes in the echocardiographic measurements that were found in the rats with an AMI are due to the presence of the AMI and not to the sedatives.
CONCLUSION
Both surgical techniques can be used to create an AMI in order to generate rats with a LVEF of less than 40%. However, our results indicate a tendency of the technique used G1 to be better than G2. This improvement is probably due to the greater duration of the open thorax, which reduces the time pressure on the surgeon, allowing a LADCA occlusion with greater accuracy.
| Authors’ roles & responsibilities | |
|---|---|
| LGAC | Study design, anesthetic and surgical procedures, handling and care of animals, interpretation of results and writing |
| FB | Histopathological analysis, handling and care of animals, interpretation of results |
| GSO | Anesthetic and surgical procedures and interpretation of results |
| NIM | Echocardiographic examination |
| PHS | Preparation of images and discussion |
| CLKR | Histopathological analysis and interpretation of results |
| CTP | Statistical analysis |
| ACS | Study design, revision of the manuscript and final approval |
| PRB | Orientation for study design and final approval |
REFERENCES
- 1.Senegaglia AC, Barboza LA, Dallagiovanna B, Aita CA, Hansen P, Rebelatto CL, et al. Are purified or expanded cord blood-derived CD133+ cells better at improving cardiac function? Exp Biol Med (Mywood) 2010;235(1):119–129. doi: 10.1258/ebm.2009.009194. [DOI] [PubMed] [Google Scholar]
- 2.Johns TN, Olson BJ. Experimental myocardial infarction: I. A method of coronary occlusion in small animals. Ann Surg. 1954;140(5):675–682. doi: 10.1097/00000658-195411000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zornoff LA, Paiva SA, Minicucci MF, Spadaro J. Experimental myocardium infarction in rats: analysis of the model. Arq Bras Cardiol. 2009;93(4):434–440. doi: 10.1590/s0066-782x2009001000018. [DOI] [PubMed] [Google Scholar]
- 4.Fishbein MC, Maclean MB, Maroko PR. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol. 1978;90(1):57–70. [PMC free article] [PubMed] [Google Scholar]
- 5.Pimentel EB, de Moraes AC, Forechi L, Machado RC, Baldo MP, Mill JG. Kinetics of the electrocardiographic changes after permanent coronary occlusion in rats: Relationship with infarct size. Pathophysiology. 2012;19(4):277–281. doi: 10.1016/j.pathophys.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Louzada RA, Oliveira PF, Cavalcanti-de-Albuquerque JP, Cunha-Carvalho L, Baldanza MR, Kasai-Brunswick TH, et al. Granulocyte-colony stimulating factor treatment of chronic myocardial infarction. Cardiovasc Drugs Ther. 2010;24(2):121–130. doi: 10.1007/s10557-010-6215-2. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Zhan ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, et al. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res. 2007;101(3):304–312. doi: 10.1161/CIRCRESAHA.107.148940. [DOI] [PubMed] [Google Scholar]
- 8.Schiller NB, Foster E. Analysis of left ventricular systolic function. Heart. 1996;75(6) Suppl 2:17–26. doi: 10.1136/hrt.75.6_suppl_2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opitz CF, Mitchell GF, Pfeffer MA, Pfeffer JM. Arrhythmias and death after coronary artery occlusion in the rat: Continuous telemetric ECG monitoring in conscious, untethered rats. Circulation. 1995;92(2):253–261. doi: 10.1161/01.cir.92.2.253. [DOI] [PubMed] [Google Scholar]
- 10.Zornoff LA, Paiva SA, Duarte DR, Spadaro J. Ventricular remodeling after myocardial infarction: concepts and clinical implications. Arq Bras Cardiol. 2009;92(2):150–164. doi: 10.1590/s0066-782x2009000200013. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Kloner RA. Is there a gender difference in infarct size and arrhythmias following experimental coronary occlusion and reperfusion? J Thromb Thrombolysis. 1995;2(3):221–225. doi: 10.1007/BF01062713. [DOI] [PubMed] [Google Scholar]
- 12.Pabis FC, Miyague NI, Francisco JC, Woitowicz V, Carvalho KA, Faria-Neto JR, et al. Echocardiographic assessment of myocardial infarction evolution in young and adult rats. Arq Bras Cardiol. 2008;91(5):321–326. doi: 10.1590/s0066-782x2008001700007. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, et al. Myocardial infarct size and ventricular function in rats. Circ Res. 1979;44(4):503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- 14.Kissin I, Stanbridge R, Bishop SP, Reves JG. Effect of halothane on myocardial infarct size in rats. Can Anaesth Soc J. 1981;28(3):239–243. doi: 10.1007/BF03005507. [DOI] [PubMed] [Google Scholar]
- 15.Stein AB, Tiwari S, Thomas P, Hunt G, Levent C, Stoddard MF, et al. Effects of anesthesia on echocardiographic assessment of left ventricular structure and function in rats. Basic Res Cardiol. 2007;102(1):28–41. doi: 10.1007/s00395-006-0627-y. [DOI] [PubMed] [Google Scholar]