Abstract
Introduction
Ischemic postconditioning has been recognized as effective in the prevention of reperfusion injury in situations of ischemia and reperfusion in various organs and tissues. However, it remains unclear what would be the best way to accomplish it, since studies show great variation in the method of their application.
Objective
To assess the protective effect of ischemic postconditioning on ischemia and reperfusion in rats undergoing five alternating cycles of reperfusion and ischemia of 30 seconds each one.
Methods
We studied 25 Wistar rats distributed in three groups: group A (10 rats), which underwent mesenteric ischemia (30 minutes) and reperfusion (60 minutes); Group B (10 rats), undergoing ischemia (30 minutes) and reperfusion (60 minutes), intercalated by postconditioning (5 alternating cycles of reperfusion and ischemia of 30 seconds each one); and group C - SHAM (5 rats), undergoing only laparotomy and manipulation of mesenteric artery. All animals underwent resection of an ileum segment for histological analysis.
Results
The mean lesions degree according to Chiu et al. were: group A, 2.77, group B, 2.67 and group C, 0.12. There was no difference between groups A and B (P>0.05).
Conclusion
Ischemic postconditioning was not able to minimize or prevent the intestinal tissue injury in rats undergoing ischemia and reperfusion process when used five cycles lasting 30 seconds each one.
Keywords: Ischemia, Reperfusion Injury, Ischemic Postconditioning, Mesenteric Vascular Occlusion, Rats
Abstract
Introdução
O pós-condicionamento isquêmico tem sido reconhecido como eficaz na prevenção das lesões de reperfusão em situações de isquemia e reperfusão em vários órgãos e tecidos. Entretanto, não está ainda claro qual seria a melhor maneira de realizá-lo, já que as publicações mostram grande variação de método no seu emprego.
Objetivo
Avaliar o efeito protetor do pós-condicionamento isquêmico na isquemia e reperfusão intestinal em ratos, através de cinco ciclos alternados de 30 segundos de isquemia e 30 segundos de reperfusão.
Métodos
Foram estudados 25 ratos Wistar, distribuídos em três grupos: grupo A (10 ratos), em que se realizou isquemia (30 minutos) e reperfusão (60 minutos) mesentérica; grupo B (10 ratos), isquemia e reperfusão, seguidos de pós-condicionamento isquêmico com 5 ciclos alternados de reperfusão e reoclusão, de 30 segundos cada; e grupo C (5 ratos), controle (SHAM). Ao final, ressecou-se um segmento do intestino delgado para análise histológica. Avaliaram-se os resultados pela classificação de Chiu et al. e procedeu-se ao tratamento estatístico.
Resultados
As médias dos graus de lesão tecidual segundo a classificação de Chiu et al. foram: no grupo A, 2,77; no grupo B, 2,67; e no grupo C, 0,12. A diferença entre o resultado do grupo A com o resultado do grupo B não teve significância estatística (P>0,05).
Conclusão
O pós-condicionamento isquêmico não foi capaz de minimizar ou prevenir a lesão tecidual intestinal de ratos submetidos ao processo de isquemia e reperfusão mesentérica quando utilizados cinco ciclos com duração de 30 segundos cada.
| Abbreviations, acronyms & symbols | |
|---|---|
| IPo | Ischemic postconditioning |
| IPr | Ischemic preconditioning |
| ROS | Reactive oxygen species |
INTRODUCTION
Since 1986, when Parks & Granger[1] demonstrated the harmful effects of toxic reactive oxygen species (ROS) produced during reperfusion, much research has been developed in search of an experimental model that could minimize this process in order to reduce cell and organ ischemia and reperfusion damage[2,3].
With the acquired knowledge on the pathophysiology of this process seems to be the way to complement reperfusion techniques already developed, such as ischemic preconditioning (IPr) - consisting of short and repeated episodes of ischemia before the ischemic event itself, and ischemic postconditioning (IPo) - consisting of short and repeated episodes of reperfusion, post-ischemia established, and prior to reperfusion period. But unfortunately they did not alter significantly the mortality of mesenteric ischemia.
The process of ischemia has been studied for many years and the knowledge on pathophysiology still faces some dilemmas. It is known that in any situation of ischemia, reperfusion also occurs, which is an important factor in the deterioration of the clinical picture, leading to local and systemic damage due to predisposition to the formation of oxygen free radicals and other substances responsible for the direct tissue damage, proven by Parks & Granger[1].
Probably, the best results previously published in controlling the production of ROS were obtained with the IPr, as numerous publications that followed Murry et al.[4], including ischemia and reperfusion. However, little practical and clinically applicable to situations in IPr, for example, acute abdomen, mesenteric vascular ischemia, since the time of diagnosis, there is already ischemia. It is for this reason that the IPo has increased interest in this aspect, since, if proven its effectiveness there were many clinical situations with the possibility of applying this method.
Some intestinal surgeries, especially resection and transplantation are usually held by temporary occlusion of mesenteric vessels to prevent bleeding.
This knowledge has led many researchers to develop a method to minimize the damage caused by reperfusion.
In 2003, Zhao et al.[2] presented the concept of ischemic postconditioning (IPo), which consists in performing one or more short cycles of reperfusion followed by one or more short cycles of ischemia, immediately after the ischemic phase and before establishment of permanent reperfusion.
In an experimental model, there is already evidence of a protective effect of IPo on the intestinal mucosa of rats undergoing mesenteric ischemia and reperfusion[5]. And recently, IPo was able to minimize the severity of liver injury in rats undergoing ischemia and reperfusion through 3 cycles of ischemia and reperfusion of two minutes[6].
Several published experiments analyzed the effects of IPo in other organs and tissues, among them we can mention Darling et al.[7] in which the IPo was able to minimize the area of myocardial infarction in rabbits; Tang et al.[8] demonstrated the effectiveness of IPo in the prevention of coronary lesions resulting from the ischemia and reperfusion in rats, since the ischemia time did not exceed 45 minutes; Huang et al.[3] have shown that the IPo was preventing tissue damage in the spinal cord of rats subjected to ischemia and reperfusion; Santos et al.[9] showed that the ICRP and IPo was able to minimize tissue injury in the intestinal mucosa of rats undergoing ischemia and reperfusion process.
However, Bretz et al.[10] in 2010, published a study in rabbits, showing that postconditioning performed with four cycles of 30 seconds reperfusion and 30 seconds of reocclusion during the initial four minutes of reperfusion, showed no statistical significance on degree of necrosis of the intestinal mucosa.
Thus, although there is much evidence of the effectiveness of IPo, it is not yet determined what would be the best method of developing it, how many cycles, the duration of each cycle, if there are differences when used for bowel or other organs, etc.
Thus, considering the current evidence of the value of IPo to minimize tissue damage resulting from ischemia and reperfusion, it becomes of paramount importance, and the aim of this study was to assess the effectiveness of IPo accomplished through five cycles of ischemia and reperfusion with five short cycles of ischemia and reperfusion.
Objective
To assess the protective effect of IPo on ischemia and reperfusion in rats by five alternating cycles of 30 seconds of reperfusion and 30 seconds of ischemia.
METHODS
The study was approved by the Ethics Committee on Animal Experimentation of the Federal University of Mato Grosso do Sul and was based on ethical principles defended by the Brazilian College of Animal Experimentation.
Animals Studied
Twenty-five rats (Rattus norvegicus) of Wistar lineage, adults, males, weighing 270-350 grams, with an average of 305 grams, from the vivarium of the Federal University of Mato Grosso do Sul. The rats were housed individually in cages where temperatures were maintained between 21ºC and 24ºC, with automatic alternation of light and dark periods of 12 hours, and received diet and water ad libitum.
Constituted groups
The animals were distributed into three groups: Ischemia-reperfusion (IR) group, with 10 animals, undergoing 30 minutes of ischemia and 60 minutes of reperfusion; group Ischemic postconditioning (IPo), with 10 animals, in which were performed five cycles of 30 seconds of reperfusion inserted by five cycles of 30 seconds of ischemia, immediately after ischemia period (30 minutes) and before reperfusion (60 minutes); and control group (SHAM), with five animals. They had undergone only laparotomy and manipulation of mesenteric cranial artery.
Anesthesia
The animals were weighed on an electronic precision balance and anesthetized with an intraperitoneal injection of 2:1 solution of Hydrochloride of Ketamine (Cetamin®), 50 mg/ml, and Hydrochloride of Xylazine (Xilazin®) 20mg/ml, respectively, at a dose of 0.1ml/100g.
Surgical procedure
After anesthetization, it was performed the trichotomy and placement of the animal on the operating table in the supine position, with all four members in abduction. The rats underwent midline longitudinal laparotomy of approximately four centimeters, exteriorization of the small intestine, identification and dissection of the cranial mesenteric artery. In the IR group, the cranial mesenteric artery was occluded by atraumatic vascular clamp that remained for 30 minutes (ischemic phase). After placement of the clamp, the small intestine was repositioned in the abdominal cavity and the wound closed with continuous skin suture with 4-0 monofilament nylon suture (mononylon®).
After the ischemic phase, the abdominal wall was opened again by removing the suture and the vascular clamp was removed too, initiating reperfusion phase, lasting 60 minutes. The abdomen was closed again by continuous skin suture until the end of the experiment (Figure 1). In IPo group, there was a phase of ischemia (30 minutes) and reperfusion (60 minutes). Preceding the reperfusion, ischemic postconditioning was then performed by five cycles of reperfusion (removal of atraumatic vascular clamp of the cranial mesenteric artery) lasting 30 seconds each one, interspersed by with five cycles of ischemia (re-occlusion of the cranial mesenteric artery by atraumatic vascular clamp), also lasting 30 seconds each one (Figure 1).
Fig. 1.

Schematic figure showing the times of ischemia and reperfusion in both groups.
After completing the reperfusion in both groups, the abdominal wall was opened again by removing the suture and a segment of 1cm of ileum was resected, five centimeters proximal to ileum-cecal valve, for subsequent histological analysis and classification according to Chiu et al.[11].
In the SHAM group, it was performed only incision of the abdominal wall, bowel exposure, followed by its closure by continuous skin suture with 4-0 nylon. Ninety minutes later it was resected a segment of ileum, as explained above.
All the animals were euthanized by anesthetic depth.
Histopathological study
The resected bowel segments, after fixation in formaldehyde solution 10%, were submitted to histological processing (hematoxylin-eosin) and examined under light microscopy by a pathologist without prior knowledge about this group within each rat, and were classified according to the degree of tissue injury second Chiu et al.[11].
Grade 0: no mucosal changes.
Grade 1: well-formed villi without cell lysis or inflammatory process, however, with formation of Grünhagen subepithelial space.
Grade 2: presence of cell lysis, formation of Grünhagen subepithelial space and increased spacing between the villi.
Grade 3: destruction of the free portion of the villi, presence of dilated capillaries and inflammatory cells.
Grade 4: structural destruction of the villi, with only some outline, formed by inflammatory cells and necrotic material, with hemorrhage and basal glandular ulceration.
Grade 5: destruction of the entire mucosa, no longer any glandular structure was observed, but only amorphous material deposited on the submucosa.
Statistical Analysis
The results were analyzed statistically by applying the non-parametric Kruskal-Wallis test, considering significant level of P<0.05. The BioStat 5.4 software was used.
RESULTS
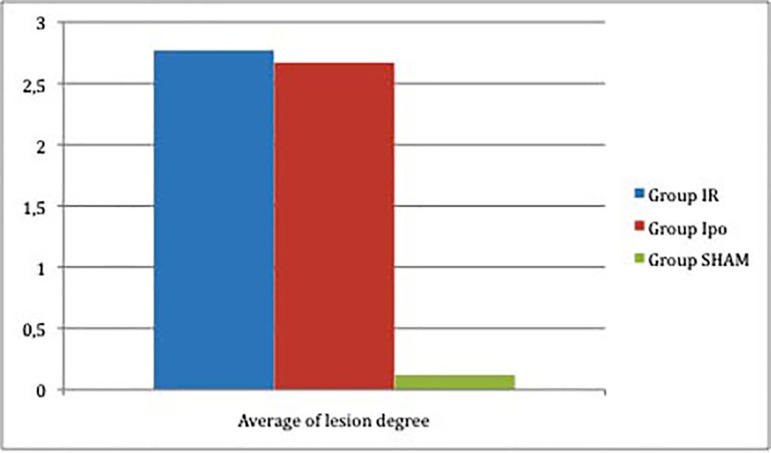
After histological analysis of the injury degree of the intestinal mucosa according Chiu et al.[11], we have found the following results (Table 1 and Figure 2).
Table 1.
Results of the histological analysis of the injury degree of the intestinal mucosa of rats according to Chiu et al.11.
| Rats | Mucosa lesion degree | ||
|---|---|---|---|
| Group IR | Group IPo | Group SHAM | |
| 1 | 2 | 1 | 1 |
| 2 | 4 | 4 | 2 |
| 3 | 3 | 3 | 1 |
| 4 | 2 | 3 | 1 |
| 5 | 2 | 3 | 1 |
| 6 | 3 | 2 | - |
| 7 | 3 | 3 | - |
| 8 | 3 | 2 | - |
| 9 | 2 | 3 | - |
| 10 | 4 | 3 | - |
| Average | 2.77 | 2.67 | 0.12 |
Obs: The P value between IR and IPo was >0.05; between IR and SHAM was <0.05, and between IPo and SHAM was <0.05
Fig. 2.
Results of the histological analysis of the injury degree of the intestinal mucosa of rats according to Chiu et al.11
DISCUSSION
The protective mechanism of IPo in ischemia and reperfusion process is still not entirely clear, but there is evidence that IPo may be related to a significant decrease in the levels of malondialdehyde and products related to lipid peroxidation. These observations suggest a reduction in the production of ROS and less injury mediated by oxidants with IPo[1-9].
The peak production of ROS occurs between the first and seventh minutes after the beginning of reperfusion, although these substances are detectable in later periods. An abundant production of ROS during this initial phase of reperfusion has been implicated as the primary factor in the pathogenesis of tissue injury[12]. The IPo acts at this stage, probably reducing the production of ROS by the gradual release of oxygen to tissue[13].
Santos et al.[14] proposed IPo evaluation using cycles of mesenteric ischemia and reperfusion (three alternate cycles of two minutes each one) after 30 minutes of ischemia and preceding 60 minutes of reperfusion. The results showed a protective effect of IPo.
However, in 2010, Bretz et al.[10] published a study which aim was to determine whether IPo could actually mitigate the injury caused by ischemia and reperfusion process. Six rabbits were distributed into control, IR and IPo groups. Ischemia was induced for 45 minutes of occlusion of the segment of jejunal artery, followed by two hours of reperfusion. The IPo was performed with four cycles of 30 seconds of reperfusion and 30 seconds of reocclusion during the initial four minutes of reperfusion. The histopathological evaluation was performed by a single observer and there was no significant difference in necrosis degree between the groups[10]. We have to keep in mind the small sample of this research. Despite this, it was the first publication demonstrating a bad result of IPo before our study.
Common among these publications, we can observe that the cycle duration of IPo is shorter than most of the articles[15]. It could be the reason of IPo doesn't work against reperfusion lesion, and we need more researches observing the cycles duration to prove this hypothesis.
However, recently Rosero et al.[16] had shown a different result. They had realized three different protocols of postconditioning in rats undergoing mesenteric ischemia and reperfusion, and had observed that shorter cycles offered better protection against reperfusion lesion. Despite this confrontable result, the ischemia period utilized was 60 minutes, different of our period, with 30 minutes. That's the biggest problem when we analyzed the literature: a great variation of methods of development of ischemia, reperfusion and postconditioning, hindering a confrontation of existing articles.
Sengui et al.[17] also demonstrated better result with short cycles of postconditioning. They had utilized three and six cycles of IPo in rats submitted to mesenteric ischemia and reperfusion and obtained better result with six cycles, but both were better than control group. Again we have to observe that they utilized different periods of ischemia and reperfusion when compared with our research (30 minutes of ischemia, 120 minutes of reperfusion).
Postconditioning had also shown effectiveness in other experimental models of ischemia and reperfusion, like spinal cord[18], kidney[19] and brain[20]. It was also analyzed in humans. Staat et al.[21] reported its beneficial effect by performing intermittent reperfusion during angioplasty in patients with acute myocardial infarction, having observed reduction in myocardial injury. Loukogeorgakis et al.[22] performed an experimental study in humans that caused transient upper limb ischemia followed by reperfusion, also observing protective effect of IPo.
CONCLUSION
Ischemic postconditioning was not able to minimize or prevent the intestinal tissue injury in rats undergoing ischemia and reperfusion process when used five cycles of reperfusion and ischemia lasting 30 seconds each one.
| Authors’ roles & responsibilities | |
|---|---|
| RKN | Conception and design of the study; manuscript writing and review |
| CHMS | Final approval of the manuscript; conception and design of the study; manuscript writing and review |
| LNOM | Analysis and/or interpretation of data |
| MSA | Performing of operations and/or experiments |
| CMM | Performing of operations and/or experiments |
| MEF | Performing of operations and/or experiments |
| PCC | Performing of operations and/or experiments |
| ERJCP | Statistical analysis |
REFERENCES
- 1.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Pt 1Am J Physiol. 1986;250(6):G749–G753. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- 2.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 3.Huang H, Zhang L, Wang Y, Yao J, Weng H, Wu H, et al. Effect of ischemic post-conditioning on spinal cord ischemic-reperfusion injury in rabbits. Can J Anaesth. 2007;54(1):42–48. doi: 10.1007/BF03021898. [DOI] [PubMed] [Google Scholar]
- 4.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 5.Santos CHM, Gomes OM, Pontes JCDV, Miiji LNO, Bispo MAF. Post-conditioning: preliminary results of this new option in the treatment of mesenteric ischemia and reperfusion. Cardiovasc Sci Forum. 2007;2(2):13–24. [Google Scholar]
- 6.Santos CHM, Pontes JCDV, Miiji LNO, Nakamura DI, Galhardo CAV, Aguena SM. Postconditioning effect in the hepatic ischemia and reperfusion in rats. Acta Cir Bras. 2010;25(2):163–168. doi: 10.1590/s0102-86502010000200008. [DOI] [PubMed] [Google Scholar]
- 7.Darling CE, Jiang R, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol Heart Circ Physiol. 2005;289(4):H1618–H1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- 8.Tang XL, Sato H, Tiwari S, Dawn B, Bi Q, Li Q, et al. Cardioprotection by postconditioning in conscious rats is limited to coronary occlusions <45 min. Am J Physiol Heart Circ Physiol. 2006;291(5):H2308–H2317. doi: 10.1152/ajpheart.00479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dos Santos CH, Pontes JC, Gomes OM, Miiji LN, Bispo MA. Evaluation of ischemic postconditioning effect on mesenteric ischemia treatment: experimental study in rats. Rev Bras Cir Cardiovasc. 2009;24(2):150–156. doi: 10.1590/s0102-76382009000200010. [DOI] [PubMed] [Google Scholar]
- 10.Bretz B, Blaze C, Parry N, Kudej RK. Ischemic postconditioning does not attenuate ischemia-reperfusion injury of rabbit small intestine. Vet Surg. 2010;39(2):216–223. doi: 10.1111/j.1532-950X.2009.00619.x. [DOI] [PubMed] [Google Scholar]
- 11.Chiu CJ, Mcardle AH, Brown R, Scott HJ, Gurd FN. Intestinal Mucosal Lesion in Low-Flow States. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101(4):478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 12.Sun HY, Wang NP, Kerendi F, Halkos M, Kin H, Guyton RA, et al. Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol. 2005;288(4):1900–1908. doi: 10.1152/ajpheart.01244.2003. [DOI] [PubMed] [Google Scholar]
- 13.Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75(3):530–535. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos CH, Gomes OM, Pontes JCDV, Miiji LNO, Bispo MAF. Tratamento da isquemia mesentérica pelo pós-condicionamento isquêmico. Rev Bras Colo-proctol. 2008;28(2):187–192. [Google Scholar]
- 15.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 16.Rosero O, Onody P, Stangl R, Turoczi Z, Fulop A, Garbaisz D, et al. Postconditioning of the small intestine: which is the most effective algorithm in a rat model? J Surg Res. 2014;187(2):427–437. doi: 10.1016/j.jss.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 17.Sengul I, Sengul D, Guler O, Hasanoglu A, Urhan MK, Taner AS, et al. Postconditioning attenuates acute intestinal ischemia-reperfusion injury. Kaohsiung J Med Sci. 2013;29(3):119–127. doi: 10.1016/j.kjms.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Zhang L, Wang Y, Yao J, Weng H, Wu H, et al. Effect of ischemic postconditioning on spinal cord ischemic-reperfusion injury in rabbits. Can J Anaesth. 2007;54(1):42–48. doi: 10.1007/BF03021898. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Chen H, Zhan B, Xing B, Zhou J, Zhu H, et al. Attenuation of reperfusion injury by renal ischemic postconditioning: the role of NO. Biochem Biophys Res Commun. 2007;359(3):628–634. doi: 10.1016/j.bbrc.2007.05.129. [DOI] [PubMed] [Google Scholar]
- 20.Rehni AK, Singh N. Role of phosphoinositide 3-kinase in ischemic postconditioning-induced attenuation of cerebral ischemia-evoked behavioral deficits in mice. Pharmacol Rep. 2007;59(2):192–198. [PubMed] [Google Scholar]
- 21.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, et al. Postconditioning the human heart. Circulation. 2005;112(14):2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 22.Loukogeorgakis SP, Panagiotidou AT, Yellon DM, Deanfield JE, MacAllister RJ. Postconditioning protects against endothelial ischemia-reperfusion injury in the human forearm. Circulation. 2006;113(7):1015–1019. doi: 10.1161/CIRCULATIONAHA.105.590398. [DOI] [PubMed] [Google Scholar]