Abstract
Introdution
Allogeneic blood is an exhaustible therapeutic resource. New evidence indicates that blood consumption is excessive and that donations have decreased, resulting in reduced blood supplies worldwide. Blood transfusions are associated with increased morbidity and mortality, as well as higher hospital costs. This makes it necessary to seek out new treatment options. Such options exist but are still virtually unknown and are rarely utilized.
Objective
To gather and describe in a systematic, objective, and practical way all clinical and surgical strategies as effective therapeutic options to minimize or avoid allogeneic blood transfusions and their adverse effects in surgical cardiac patients.
Methods
A bibliographic search was conducted using the MeSH term “Blood Transfusion” and the terms “Cardiac Surgery” and “Blood Management.” Studies with titles not directly related to this research or that did not contain information related to it in their abstracts as well as older studies reporting on the same strategies were not included.
Results
Treating anemia and thrombocytopenia, suspending anticoagulants and antiplatelet agents, reducing routine phlebotomies, utilizing less traumatic surgical techniques with moderate hypothermia and hypotension, meticulous hemostasis, use of topical and systemic hemostatic agents, acute normovolemic hemodilution, cell salvage, anemia tolerance (supplementary oxygen and normothermia), as well as various other therapeutic options have proved to be effective strategies for reducing allogeneic blood transfusions.
Conclusion
There are a number of clinical and surgical strategies that can be used to optimize erythrocyte mass and coagulation status, minimize blood loss, and improve anemia tolerance. In order to decrease the consumption of blood components, diminish morbidity and mortality, and reduce hospital costs, these treatment strategies should be incorporated into medical practice worldwide.
Keywords: Blood Transfusion, Bloodless Medical and Surgical Procedures, Blood Preservation, Operative Blood Salvage, Cardiac Surgical Procedures
Abstract
Introdução
O sangue alogênico é um recurso terapêutico esgotável. Novas evidências demonstram um consumo excessivo de sangue e uma diminuição das doações, resultando em estoques de sangue reduzidos em todo o mundo. As transfusões de sangue estão relacionadas a aumento na morbimortalidade e maiores custos hospitalares. Deste modo, torna-se necessário procurar outras opções de tratamento. Estas alternativas existem, porém são pouco conhecidas e raramente utilizadas.
Objetivo
Reunir e descrever de maneira sistemática, objetiva e prática todas as estratégias clínicas e cirúrgicas, como opções terapêuticas eficazes para minimizar ou evitar transfusões de sangue alogênico e seus efeitos adversos nos pacientes submetidos à cirurgia cardíaca.
Métodos
Foi efetuada uma pesquisa bibliográfica com busca ao descritor “Blood transfusion” (MeSH) e aos termos “Cardiac surgery” e “Blood management”. Estudos com títulos não relacionados diretamente ao tema da pesquisa, estudos que não continham nos resumos dados relacionados à pesquisa, estudos mais antigos que relataram estratégias repetidas foram excluídos.
Resultados
Tratar anemia e plaquetopenia, suspender anticoagulantes e antiplaquetários, reduzir flebotomias rotineiras, técnica cirúrgica menos traumática com hipotermia e hipotensão moderada, hemostasia meticulosa, uso de agentes hemostáticos sistêmicos e tópicos, hemodiluição normovolêmica aguda, recuperação sanguínea intraoperatória, tolerância à anemia (oxigênio suplementar e normotermia), bem como várias outras opções terapêuticas mostram ser estratégias eficazes em reduzir transfusões de sangue alogênico.
Conclusão
Existem múltiplas estratégias clínicas e cirúrgicas para otimizar a massa eritrocitária e o estado de coagulação, minimizar a perda de sangue e melhorar tolerância à anemia. Estes recursos terapêuticos deveriam ser incorporados à prática médica mundial, visando diminuir o consumo de hemocomponentes, reduzir a morbimortalidade e custos hospitalares.
| Abbreviations, acronyms and symbols | |
|---|---|
| AF | Atrial fibrillation |
| ANH | Acute normovolemic hemodilution |
| ASA | Acetylsalicylic acid |
| CABG | Coronary artery bypass graft |
| CPB | Cardiopulmonary bypass |
| DDAVP | 1-deamino-8-D-arginine vasopressin |
| EuroSCORE | European System for Cardiac Operative Risk Evaluation |
| HF | Heart failure |
| KF | Kidney failure |
| LILACS | Literatura Latino-Americana em Ciências da Saúde |
| MEDLINE | Medical Literature Analysis and Retrieval System Online |
| MeSH | Medical Subject Headings |
| MI | Myocardial infarction |
| PCC | Prothrombin complex concentrate |
| PRBC | Packed red blood cell |
| r-FVIIa | Recombinant activated factor VII |
| r-Hu-EPO | Recombinant human erythropoietin |
| r-Hu-TPO | Recombinant human thrombopoietin |
| SciELO | Scientific Electronic Library Online |
INTRODUCTION
Since the 19th century, allogeneic blood has been frequently utilized in increased numbers worldwide. The percentage of transfusions of hemocomponents in coronary artery bypass graft (CABG) has reached 92.8% for red cells, 97.5% for fresh frozen plasma, and 90.4% for platelet infusion[1]. Currently, reduction in blood donations worldwide has resulted in a shortage of blood supply in blood banks[2]. This situation is a real problem among us and it will likely get worse, as the blood demand in the country is not proportional to the donations, indicating a possibility in the near future a shortage of this therapeutic resource to be used to perform and/or complete surgical procedures[3]. Therefore, since 2008, medical professionals have been concerned over what to do with a patient who is bleeding and no blood is available for transfusion[4].
For over half a century, transfusion therapy had not been questioned since no significant evidence of adverse effects existed. However, since the 1980's, numerous studies have been analyzing the safety and efficacy of blood transfusions. Initially, a correlation has been found between transfusion of blood components in CABG and clinical complications, such as kidney failure (KF), infectious processes, prolonged ventilation, and neurological damages[5]. Most recently, it became evident that allogeneic blood transfusion in cardiac surgery is a therapy with other serious adverse effects such as atrial fibrillation (AF), stroke, respiratory infections, sepsis, myocardial infarction (MI)[6,7], including the risk of death[8-10]. Even after taking into account factors such as age, gender, weight, height, as well as various diseases such as diabetes mellitus, hypertension, chronic obstructive pulmonary disease, peripheral vascular disease, heart failure (HF), cerebrovascular disease, the use of blood transfusions can lead to an increase up to 70% in the mortality rate of cardiac surgery postoperatively[8]. In our research, we have found that transfusion of red cells was an independent predictor factor of death after CABG in a population of 1,888 patients. Even in a subgroup of low-risk patients (age < 60 years and EuroSCORE < 2%), there was a significant higher mortality rate in the group who had received blood transfusions[9].
Other studies have showed the mortality rate to be directly proportional to the number of packed red blood cell (PRBC) units transfused. Each unit of PRBC transfused can increase the mortality risk to 77% post CABG[5]. After analyzing 3,010 patients who had undergone CABG, Santos et al.[10] have also observed that the mortality risk is dose-dependent on the number of units of allogeneic PRBCs transfused. The higher the units of red blood cells transfused, the higher the mortality risk postoperatively.
In addition to the risks related to blood transfusions, the cost is also a factor that needs to be considered. Although it varies in other countries, when all activity-based costs of blood transfusion was taken into account, in 2010 the estimated cost for one unit of blood in the United States of America (USA) was about 1,200 dollars[11]. Transfusion of PRBC has also been associated with increased length of hospital stay resulting in higher hospital costs[6,7].
Given the evidence of increased incidence of infections, sepsis, stroke, AF, KF, HF, MI, increased risk of death[5-10], higher costs[6,7] and shortage of blood components[2,3], other nontransfusional treatment options are necessary. In the attempt to decrease allogeneic blood consumption during cardiac surgeries, numerous alternative treatment options have been proposed[4,12-16], however, due to the practicality of transfusional therapy, these options are virtually unknown and are rarely used.
The aim of this review is to gather and describe in a systematic, objective, and practical way all clinical and surgical strategies as therapeutic treatment options that could assist the surgeon, anesthesiologist, clinical and/or critical care physician, as well as other critical care professionals, to reduce or avoid allogeneic blood transfusions in cardiac surgeries, and consequently, their adverse effects.
METHODS
Search Strategy
For this review, a bibliographic search was performed in the month of February, 2014 using the online databases PUBMED/MEDLINE, LILACS, COCHRANE library and SciELO for articles published between January 1, 1980 and January 31, 2014. The search was limited to the MeSH (Medical Subject Headings) term "Blood transfusion" and the terms "Cardiac surgery" and "Blood management". Despite not being MeSH terms, "Blood management" and "Cardiac surgery" were included in the search because they are important keywords highly relevant to the subject being discussed. The combination of the terms "Blood management" and "Cardiac surgery", "Blood management" and "Blood transfusion", and finally, "Blood transfusion" and "Cardiac surgery" resulted in 9,018; 11,299 and 5,998 articles, respectively.
Inclusion Criteria
Presence of one or more alternative treatment options for blood transfusion in the article. In order to assemble all possible therapeutic options with evidence of reducing allogeneic blood consumption, recent articles were selected containing a systematic review process and meta-analysis, prospective multicenter randomized cohort studies, retrospective cohort studies, case studies and others, without restriction on the minimum number of participants for each study, in all languages from January 1, 1980 to January 31, 2014.
Exclusion Criteria
Studies excluded from this review were: studies with titles not directly related to this research; studies that did not contain in their abstract data relevant to this research; older studies reporting on the same strategies (intraoperative blood salvage, acute normovolemic hemodilution, use of erythropoiesis-stimulating agents, use of various systemic and topical hemostatic agents, strategies to restrict the use of blood transfusion and others).
Selection of Studies
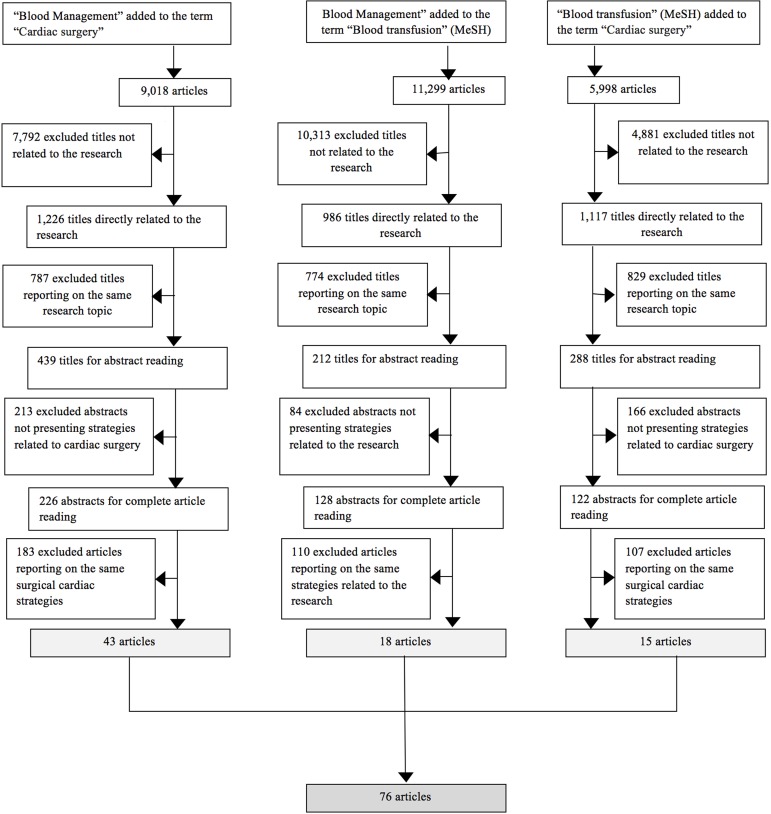
By means of an individual triage of the articles found after the database search was performed using specific terms, data containing the main and most frequent treatment alternatives to the use of blood components in cardiac surgery was extracted from the articles. Figure 1 shows an organogram of the methodology used for obtaining the 76 articles selected for this review. Initially, an analysis was performed of all the tittles found during the search combination of the specific terms used. Titles directly related to the study, with emphasis on strategies to minimize blood transfusions and their adverse effects during cardiac surgery, passed during this initial triage process. Then, we excluded similar repetitive titles, selecting the most up-to-date ones to read their respective abstracts. Abstracts that did not present strategies related to cardiac surgery or presented strategies that could not be applied to cardiovascular surgery, were also not selected for a complete analysis of the article. The final selection of the articles relevant to this research was performed through individual searches with data crossing done by two authors and supervised by a third.
Fig. 1.
Organogram of the methodology about the different search steps. MeSH=Medical Subject Headings
RESULTS
According to this study review, numerous strategies have shown to have impact on reducing allogeneic blood consumption.
To facilitate the medical practitioner's course of action, the main strategies selected to avoid or reduce the transfusional practice were separated into three important pillars: I - optimize the erythrocyte mass and the coagulation status; II - minimize blood loss, and III - anemia tolerance.
To reach the aim of this review, additional helpful information obtained from this study has been structurally included in each of these pillars.
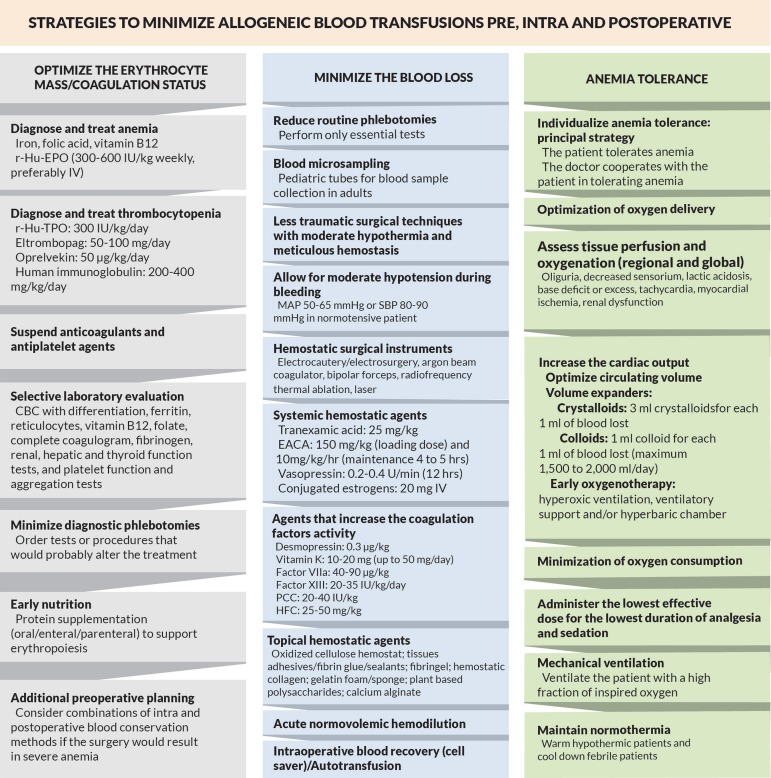
Figure 2 shows a flowchart containing important strategies in each pillar for a new nontransfusional approach during the pre, intra, and postoperative care.
Fig. 2.
Flowchart with strategies to minimize allogeneic blood transfusions and their adverse effects. CBC - Complete blood count; EACA - epsilon aminocaproic acid; Factor VIIa - Recombinant activated factor VII; HFC - Human fibrinogen concentrate; IV - Intravenous; MAP - Mean arterial pressure; PCC - Prothrombin complex concentrate; r-Hu-EPO - Recombinant human erythropoietin; r-Hu-TPO - Recombinant human thrombopoietin; SBP - Systolic blood pressure
I - Optimize the erythrocyte mass and the coagulation status
To reduce or avoid allogeneic blood transfusion is necessary to perform a preoperative assessment that includes a detection and treatment of anemia and thrombocytopenia.
Table 1 shows the main strategies to optimize the erythrocyte mass and the coagulation status. We briefly described each one of them below:
Table 1.
Main estrategies to optimize the erythrocyte mass and coagulation status.
| Research study | Main estrategies to optimize the erythrocyte mass and coagulation |
|---|---|
| Drews[17] | To identify anemia, thrombocytopenia and coagulopathy |
| Goodnough[18] | To treat iron deficiencies |
| Weltert et al.[20] | r-Hu-EPO in the preoperative period for anemic patients |
| Corwin et al.[21] | Dose of 40,000 UI/week reduces blood transfusions |
| Silverberg et al.[24] | Anabolic androgenic therapy can optimize the responses to r-Hu-EPO |
| Bussel et al.[29] | Eltrombopag stimulates trombocytopoiesis and reduces platelets transfusion |
| Wang et al.[30] | r-Hu-TPO stimulates trombocytopoiesis and reduces platelets transfusion |
r-Hu-EPO=Recombinant human erythropoietin, r-Hu-TPO=Recombinant human thrombopoietin
1 - History of anemia and abnormal bleeding
Assessment of congenital or acquired hemorrhagic disturbances.
2 - Identify the use of medications that could have adverse effects on anemia, thrombocytopenia and coagulopathy(17)
Acetylsalicylic acid (ASA), nonsteroidal anti-inflammatory drugs, anticoagulants, platelet aggregation inhibitors, beta-lactam antibiotics (such as penicillins, ticarcillin), beta blockers, calcium channel blockers, H2 blockers, furosemide, thiazide diuretics, alpha-methyldopa, quinidine, anticonvulsants, myelosuppressive agents, diet and herbal supplements can affect the coagulation or the platelet function.
3 - Physical exam
Search for manifestations of illnesses known to be associated with hemostatic dysfunction (hepatomegaly, splenomegaly, petechiae, purpura, ecchymosis, hemarthrosis, evidence of collagen vascular disorders, telangiectasia).
4 -Selective laboratory evaluation
Complete blood count with differentiation, reticulocytes, ferritin, vitamin B12, folate, complete coagulogram, platelet function and aggregation tests, fibrinogen concentration, hepatic, renal and thyroid function tests. If the preoperative laboratorial results are abnormal, then ideally the surgery should be postponed until the abnormalities are corrected or until more detailed preliminary studies can be obtained. In such cases, a hematologist evaluation is recommended. In the event of urgent surgeries, normalize the coagulation status with appropriate agents, as courses of actions well defined later in this article.
5 - Anemia treatment
a) Identify and treat hematinic deficiencies
Iron deficiency is the most treatable and frequent cause of anemia[18]. Prophylactic administration of hematinics (iron, folic acid, vitamin B12) should always be considered.
b) Treatment of iron deficiencies
Intravenous (IV) iron by saline infusion, for example, iron hydroxide saccharate (dosage of 3 to 5 mg/kg/day and the maximum of 200 mg/day) or carboxymaltose (dosage 15 mg/kg and the maximum of 1,000 mg in single infusion) can rapidly replenish iron reserves. Oral iron bioavailability can be increased by a concomitant administration of ascorbic acid. The simultaneous use of dairy products, egg yolks, coffee, tea, antacids, or fiber reduces the absorption of oral iron. Parenteral administration of iron is recommended in patients with oral iron intolerance, inadequate absorption, chronic or severe blood loss, or nonresponsiveness to treatment[19].
c) Recombinant human erythropoietin (r-Hu-EPO) therapy
This therapy is recommended preoperatively to increase the erythrocyte mass in patients who are anemic or who refuse the use of blood components or in patients with risk of anemia postoperatively[16,20]. Before the initiation and/or during treatment with r-Hu-EPO, correct deficiencies in iron, folic acid and vitamin B12.
d) Dosage and administration route r-Hu-EPO
The responsiveness to r-Hu-EPO depends on the dosage and varies from patient to patient. Excluding chronic renal insufficiency, r-Hu-EPO dosages varying from 150 to more than 600 IU/kg/week with various dosing intervals and schedules have been reported to accelerate the recovery of acute anemia[20]. In critically ill patients in intensive care, the subcutaneous administration of r-Hu-EPO 40,000 IU per week resulted in a significant increase in hemoglobin levels and consequently in a reduction of 19% of blood transfusion needs[21].
Some patients require higher dosages of r-Hu-EPO to reach an adequate response. Some evidences suggest that r-Hu-EPO dosages in intervals of 24-72 hours (150-300 IU/kg) can be more effective than weekly single doses (600 IU/kg). If unable to determine or correct the cause r-Hu-EPO treatment resistance or hyporesponsiveness, consider the use of higher dosages[22].
In severe anemia, the concomitant use of IV iron can optimize the response to erythropoietic agents[18]. Aggressive anemia therapy must not be delayed until the hemoglobin levels fall and reach critically low levels. Erythropoietin of up to 200 IU/kg/day (1,400 IU/kg/week in divided dosages) has been shown to be safe and well tolerated by children[23]. Concomitant anabolic androgenic therapy can optimize the response to erythropoietic agents by increasing the sensibility of the progenitor erythroid cells[24].
The intravenous route allows for higher levels of erythropoietin concentration in the plasma. For severe acute anemia, critical illnesses, deficiency in subcutaneous absorption (due to edema or blood flow alterations) consider initial IV administration of r-Hu-EPO followed by subcutaneous dosages[25].
e) Other considerations about r-Hu-EPO
The treatment with r-Hu-EPO can produce a moderate and temporary dose-dependent increase on platelet reactivity[26]. A poor response to erythropoiesis-stimulating therapy includes iron deficiency as well as infectious, inflammatory or malignant processes, occult blood loss, hyperparathyroidism and hematologic diseases[27].
Erythropoietic agents have been associated with increased blood pressure levels in patients with chronic renal insufficiency or previous hypertension. Monitoring and controlling blood pressure levels are necessary. In patients with anemia and malignant neoplasm, the risks and benefits of r-Hu-EPO treatment should be assessed due to increased thromboembolic events, tumor progression and mortality risk with this patient population[28].
Darbepoetin (dose 0.45 µg/kg/week) and CERA (continuous erythropoietin receptor activator - dose 0.60 µg/kg every two weeks) are other erythropoiesis-stimulating agents with higher metabolic stability and longer half-life, currently not yet available in many countries.
6 - Thrombocytopenia treatment
The majority of thrombocytopenia cases resolves by suspending or avoiding the causative agents (cytotoxic drugs, cardiopulmonary bypass (CPB), excessive hemodilution), treating disorders that affect the bone marrow (severe infections, idiopathic or thrombotic thrombocytopenic purpura), splenectomy (hypersplenism). Regardless of the etiology, the following drugs can be separately used to stimulate thrombocytopoiesis: a) Eltrombopag: dose 50-100 mg/day; b) Recombinant human interleukin-11(oprelvekin): dose 50 µg/kg/day; c) Recombinant human thrombopoietin (r-Hu-TPO): dose 300 IU/kg/day; d) Human immunoglobulin: dose 200-400 mg/kg/day.
The literature shows that eltrombopag[29] and r-Hu-TPO[30] are effective in stimulating thrombocytopoiesis and consequently reducing platelet transfusions.
7 - Nutrition
Early enteral feeding as tolerated. Intravenous parenteral nutrition in patients who cannot be fed through the digestive system. Protein supplementation to support erythropoiesis.
8 - Additional preoperative planning
If high blood loss is expected postoperatively, potentially causing a severe case of anemia, consider a differential surgical approach or appropriate combinations of preoperative strategies to optimize perioperative hemoglobin levels, coagulation factors, and patient's condition. Combination of blood conservation methods during the intra and postoperative care can also be considered.
Preoperative autologous blood donation has not yet been well defined in relation to its real benefit and cost-effectiveness. Its use in cardiac surgery is very disputable.
II - Minimize blood loss
Cardiac surgery has been frequently associated with bleeding. The higher the blood loss, the greater the indication will be to use multiple modalities of blood preservation. The use of appropriate combinations of techniques has a synergistic effect in reducing surgical and nonsurgical bleeding (caused by coagulopathy). Excessive blood loss is linked to adverse effects[31].
Table 2 shows the main strategies to minimize surgical blood loss. We briefly described each one of them below:
Table 2.
Main strategies to minimize surgical blood loss.
| Research study | Main strategies to minimize surgical blood loss |
|---|---|
| Chu et al.[32] | To eliminate daily routine phlebotomies |
| Dech et al.[33] | To minimize blood volumes withdrawn for diagnostic test (use small tubes) |
| Lamy et al.[35] | To avoid or reduce as much as possible the time of CPB |
| Zangrillo et al.[36] | Mini-circuit systems in CPB reduce blood transfusions |
| Boodhwani et al.[37] | Ultrafiltration reduces hemorrhage and blood transfusions |
| Van der Linden et al.[39] | Moderate hypotermia (temperature during CPB between 30 and 32ºC) |
| Degoute[40] | Arterial blood pressure at the lowest possible level that mantains tissue perfusion (MAP of 50-65 mmHg) |
| Milas et al.[34] | Meticulous hemostasis (fast and extremaly careful control of hemorrhages) |
| Abrishami et al.[50] | Topics hemostatics agents to control local bleeding |
| Menkis et al.[16] | Pharmacological hemostasis with tranexamic acid or epsilon animocaproic acid |
| Carless et al.[42] | Desmopressin increases platelet adhesion and improves hemostasis |
| Lin et al.[45] | r-FVIIa to control hemorrhages associated with thrombocytopenia, disorders of platelet function and preexistent or drug-induced coagulopaties |
| Nienaber et al.[47] | PCC to rapidly restore the clotting factors and to control major bleeding |
| Rahe-Meyer et al.[48] | Human fibrogen concentrate to replace fibrinogen and to control major bleeding |
| Ferraris et al.[49] | Human recombinant factor XIII to control major bleedings, when others hemostatic agents have not produced satisfactory results |
| Davies et al.[53] | Acut normovolemic hemodilution is safe and cost-effective in reducing allogenic blood transfusion in surgeriess |
| Carless et al.[56] | Intraoperative blood recovery (cell saver) in surgeries for autologous blood conservation |
CPB=Cardiopulmonary bypass, MAP=Mean arterial pressure, PCC=Prothrombin complex concentrate, r-FVIIa=Recombinant activated factor VII
1 - Reducing diagnostic phlebotomies
Perform only essential tests. Eliminate daily multiple routine phlebotomies[32]. Only order tests or procedures that would probably alter the treatment.
2 - Minimizing blood volumes withdrawn for tests
Utilize pediatric tubes (small volume) to draw blood in adults[33]. Perform blood microsampling. Noninvasive monitoring (pulse oximetry, transcutaneous oximetry). Restrictive use of indwelling catheters.
3 - Reducing blood loss during invasive medical procedures
Minimize placements of central arterial or venous catheters, hemofiltration, dialysis, cardiac catheterization. Use caution with invasive procedures in patients receiving anticoagulants or platelet aggregation inhibitors.
4 - Intraoperative blood preservation and autologous blood management
a) Surgical techniques to minimize blood loss
Meticulous hemostasis. Fast and very careful control of bleeding[34]. Less traumatic surgical approach (minimally invasive surgery and/or consider an approach that avoids operating through known or suspected adhesions). Mechanical occlusion (ligature, vascular clips, staples, sutures).
Reducing surgical duration, especially the use of CPB. A multicenter study has shown evidence of significant reduction of blood transfusion needs and reoperation for bleeding in surgeries without CPB[35]. In a meta-analysis of randomized studies, the data shows that the use of mini-circuit systems in CPB has been effective in reducing the number of blood transfusions[36]. If possible, adapt the CPB circuit to accommodate a small prime of about 750 to 1,200 ml of crystalloids. The use of ultrafiltration in cardiac surgery, assessed in another meta-analysis, resulted in reduction of hemorrhage and consequently in significant reduction in blood transfusions[37]. The use of retrograde autologous priming during the CPB is safe and effective in reducing the degree of hemodilution, therefore, providing adequate oxygen delivery minimizing allogeneic blood consumption[38].
Moderate hypothermia. Temperature maintained between 30 and 32C during the CPB is associated with reduction of bleeding during the intra and postoperative period[39]. Re-infuse all the blood of the CPB circuit and if possible the blood collected from the drainage of the mediastinum during the first six hours postoperatively. Verify, during and immediately after the CPB, the activated clotting time (ACT) to avoid hyperheparinemia. Correct it with protamine sulfate (1 mg of protamine is able to inactivate in average 100 IU of sodium heparin). Divide complex procedures into stages.
b) Prophylactic preoperative angiographic embolization
If bleeding is suspected but the cause is unknown, employ the use of early selective angiography and embolization to quickly stop the blood loss.
c) Allow for moderate hypotension during bleeding
In uncontrolled bleedings, normalizing blood pressure could be harmful. In patients with dangerous acute hemorrhages, tolerating light to moderate hypotension is suggested, that is, arterial blood pressure at the lowest level possible to maintain tissue perfusion (mean arterial pressure of 50-65 mmHg)[40].
d) Blood pressure management
Slow and gradual return to normal pressure after the bleeding has been controlled. Postoperative moderate hypotension (systolic blood pressure of 80-90 mmHg in normotensive patient) is sufficient to maintain perfusion of vital organs and to avoid pressure peaks that could potentially cause late hemorrhaging[40].
e) Hemostatic surgical instruments: electrocautery/electrosurgery; argon beam coagulator; bipolar forceps; radiofrequency thermal ablation; laser.
f) Pharmacological hemostasis: systemic hemostatic agents
The surgical cardiac guidelines[16] recommend initially: tranexamic acid: dose 25 mg/kg of body weight. The maximum dose cannot exceed 50-100 mg/kg, due to its neurotoxicity effects; epsilon aminocaproic acid: loading dose of 150 mg/kg. The administration should be continued with an infusion of 10 mg/kg/hour, for four to five hours, with a maximum dose of 24 g (one gram per hour).
In case of excessive bleeding, other hemostatic agents can also be used: vasopressin: dose 0.2-0.4 U/min, until the bleeding is stopped, maintenance dose of 12 hours; conjugated estrogens: dose of 20 mg via IV, preferably. If necessary repeat the administration after 6 to 12 hours. Caution should be used with renal, hepatic and severe cardiomyopathy patients[41].
g) Pharmacological hemostasis: agents that increase the coagulation factors activity
Desmopressin acetate (DDAVP)
Dose 0.3 µg/kg of body weight. Used prophylactically for bleeding with CABG, particularly with patients using ASA or in cases with prolonged CPB time[16]. Desmopressin can increase the platelet adhesion and the levels of coagulation factors VIII and von Willebrand in the plasma[42]. In a meta-analysis of 38 randomized placebo-controlled studies, desmopressin was shown to significantly reduce intraoperative bleeding and transfusion of blood components without increasing the risks of thromboembolic complications[43]. Desmopressin can be used with epsilon aminocaproic and tranexamic acids without adverse effects. Due to risk of hypotension, monitoring patient is suggested.
Vitamin K (phytomenadione)
Dose in adults: 10-20 mg IV slow (maximum 50 mg/day) and 100 mg by mouth. Postoperative administration of parenteral vitamin K can be considered with hemorrhages[44].
Recombinant activated factor VII (r-FVIIa)
Dose 40-90 µg/kg of body weight. This dose can be repeated every 2 hours according to the type and severity of the hemorrhage. A single dose of 270 µg/kg can be used in cases of moderate hemorrhages. The use of r-FVIIa can be considered in clinical situations where the conventional approach to surgical and pharmacological hemostasis has failed and an uncontrolled hemorrhaging has increased the risk of serious outcomes and the risk of death[16]. The r-FVIIa has been associated with blood loss reduction in nonhemophiliac patients in numerous clinical situations including postoperative bleeding, thrombocytopenia, congenital or acquired disorders of platelet function, acquired bleeding predisposition and pre-existent or drug induced coagulopathies[45].
Replacement therapy of clotting factor VIII (concentrate)
Factor VIII (25 IU/kg of body weight) is available as a recombinant product and specific use.
Prothrombin complex concentrate (PCC)
Dose 20-40 IU/kg of body weight. PCC acts in the phases of initiation and amplification of coagulation. Rapidly restoring normal levels of clotting factors. Allows for progression of the prothrombin pathway[46]. The literature shows the efficacy of PCC is similar to the transfusion of fresh frozen plasma in controlling major bleeding and avoiding post trauma mortality[47].
Human fibrinogen concentrate (HFC)
Dose 25-50 mg/kg of body weight. The use of 1-2 g for small bleeding and 4-8 g for excessive bleeding is recommended. It is effective in controlling major bleeding during surgery, hence, avoiding or minimizing the use of plasma and/or platelet transfusions[48]. It is recommended a minimum fibrinogen concentration of 1.5-2.0 g/L in surgical patients. Conditions associated with hypofibrinogenemia: massive blood loss, massive transfusion, blood dilution with plasma substitutes, extensive tissue injury, disseminated intravascular coagulation, hemodialysis, surgery or injury of organs with pro-fibrinolytic potential, hepatic insufficiency and fibrinolytic therapy.
Human recombinant factor XIII
Dose 20-35 IU/kg/day until bleeding is stopped. Recommended for clot stabilization in post-surgical cardiac patients with excessive bleeding, when other hemostatic agents have not produced satisfactory results[49].
h) Pharmacological hemostasis: topical hemostatic agents
Efficient in controlling local bleeding, especially when surgical hemostasis has not been effective[49,50]: oxidized cellulose hemostat for wound compression; tissues adhesives/fibrin glue/sealants; fibrin or platelet gel; hemostatic collagen; gelatin sponge/foam; thrombin-soaked or topical thrombin tamponade; plant based polysaccharides; calcium alginate.
5 - Acute Normovolemic Hemodilution (ANH)
Hemodilution, besides being a low-cost strategy, can produce beneficial effects, such as less organ insufficiencies due to the increased release of oxygen in the microcirculatory level and less thrombolytic complications resulted from decreased platelet aggregation. In addition to conventional techniques of myocardial preservation, preoperative ANH promotes greater cardiac protection in patients undergoing CABG[51].
The amount of blood to be removed during hemodilution is determined by Gross's formula[52]: V = EBV x (Hcti - Hctf)/Hctav where V = volume of blood withdrawn, EBV is the estimated blood volume, Hcti is the ideal initial hematocrit, Hctf is the minimum hematocrit and Hctav is the average hematocrit [(Hcti + Hctf)/2]). General practical rule: for each unit of blood removed (450 ml), slowly infuse (to avoid hypervolemia) 1,000 ml of crystalloids. The ANH might be more effective if able to remove at least 1,000 ml of blood in the beginning of surgery.
The ANH, besides being cost-effective, has been safely used in avoiding or reducing homologous blood transfusions in adult[16,53] and pediatric[54] cardiac surgeries.
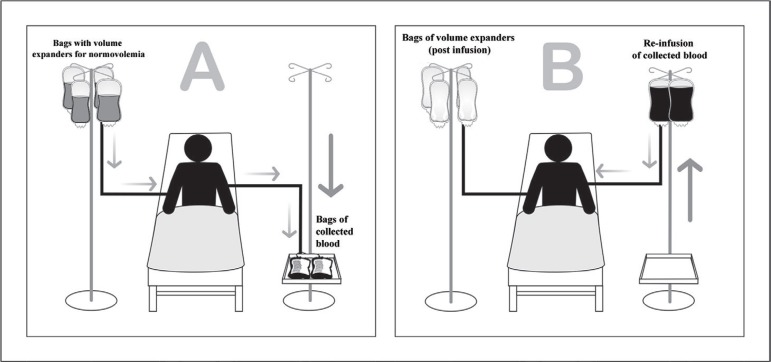
Figure 3 shows a schematic representation of an ANH.
Fig. 3.
Schematic representation of acute normovolemic hemodilution. (A) Bags of blood being removed immediately before the initiation of surgery, along with the infusion of volume expanders to maintain normovolemia. (B) Bags of blood being re-infused during and/or immediately after the surgery is completed.
6 - Intraoperative blood recovery (cell saver)/Autotransfusion
Intraoperative blood recovery is one of the most important strategies in cardiothoracic surgeries with the ability to reduce the total blood loss and homologous blood transfusion needs and it is recommended by the autologous blood conservation guidelines[16,49,55]. Since this strategy is easy to be applied, some authors defend its use in all patients undergoing cardiac surgery with CPB, regardless of the expected surgical blood loss[55]. This is an important strategy for serious and complex pediatric cardiac surgery[54].
In surgical cardiac and cancer patients with blood recovery needs, consider the use of leukocyte removal filters alone or in combination with irradiation.
In a recent systemic revision conducted by Cochrane Database of Systematic Reviews[56] the authors concluded that intraoperative blood recovery is efficient in reducing allogeneic blood transfusion needs in cardiac surgery.
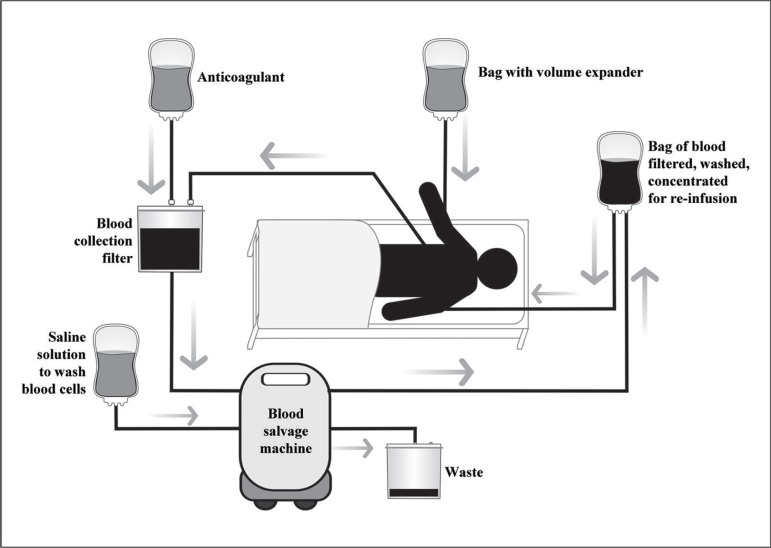
Figure 4 shows a schematic representation of an intraoperative blood recovery.
Fig. 4.
Schematic representation of intraoperative blood recovery. The collected blood is mixed with anticoagulant, filtered, washed, concentrated and returned to the patient.
7 - Additional Strategies
Another strategy to reduce surgical bleeding consists of continuous infusion of noradrenaline and restrictive hydration. The absolute risk of blood transfusions during hospitalizations had a 28% reduction. The authors also concluded that this procedure is safe, inexpensive and easy to accomplish[57].
III - Anemia tolerance
Individual patient anemia tolerance is one of the most important factors in deciding whether to transfuse or not. We do not have in the literature studies demonstrating a minimum hemoglobin trigger to recommend a safe blood transfusion. However, we have numerous randomized clinical trials demonstrating that a restrictive strategy of blood transfusion is safe and efficient in reducing allogeneic blood and does not increase the risk of complications or death in cardiac surgeries in adults[12] and children[58]. Through a recent meta-analysis and systematic review of the literature it was confirmed that a greater tolerance of anemia, a more restrictive approach to transfusion, is actually beneficial to critically ill patients[59].
Senay et al.[14] observed that a hematocrit of 17% during CABG is well tolerated and does not have an adverse impact on the outcome. Knowledge of this evidence would prevent many unnecessary allogeneic transfusions, since in most cases it is not the patient but the doctor who does not tolerate anemia. Frequently, transfusions are indicated based on laboratory results of hemoglobin and/or hematocrit. This practice is not recommended by Murphy et al.[60] when indications for blood transfusions for cardiac surgeries were considered due to the evidence that hematocrit is not the ideal clinical indicator of tissue oxygen delivery.
The literature shows that the human being is capable of tolerating extremely low levels of hemoglobin. Graffeo et al.[61] reports a case that, after a severe hemorrhagic event and disseminated intravascular coagulation, reached its nadir minimum hemoglobin level of 1.9 g/dL. In another case, after surgery of severe thoracic-lumbar scoliosis, a patient had also survived without the use of blood transfusion even after reaching a critical hemoglobin level of 1.4 g/dL[62]. In these two cases, anemia tolerance was a therapeutic option that resulted in economy of blood components for blood banks.
Table 3 shows the main strategies to improve anemia tolerance. They involve at least two fundamental therapies:
Table 3.
Main strategies to improve anemia tolerance.
| Research study | Main strategies to improve anemia tolerance |
|---|---|
| Senay et al.[14] | Patient tolerates anemia in the perioperative period |
| Graffeo et al.[61] | Patient tolerates anemia in the postoperative period |
| Salpeter et al.[59] | Restrictive conduct towards blood transfusion |
| Demetriades et al.[63] | To evaluate indexes of tissue perfusion/hypoxia |
| Murphy & Angelini[60] | Hematocrit is not the ideal indicator of tissue oxygen delivery |
| Wettstein et al.[65] | Careful and adequate fluid replacement to optimize the microvascular flow and oxygenation in severe anemia |
| Rasmussen et al.[66] | The infusion of crystalloid (Ringer's lactate) results in less blood loss than colloid |
| Perel et al.[67] | Correcting hipovolemia with crystalloids, instead of starch solutions, results in lower mortality risk |
| Araújo Azi et al. [62] | Mechanical ventilation (hyperoxic) assures tissue oxigenation in the presence of severe acute anemia |
| Marik[72] | Mantain normothermia in critically ill patients (warm hipothermic patients and cool down febrile patients) |
1 - Optimization of oxygen delivery
a) Assess tissue perfusion and oxygenation
Indices of global perfusion: markers of hypoperfusion include oliguria, decreased sensorium, lactic acidosis, base excess or deficit, and tachycardia.
Indices of regional perfusion: assess the markers of organic functions: myocardial ischemia (abnormalities of ST segment), renal dysfunction (decreased urine output and/or increased levels of urea/creatinine) and central nervous system dysfunction (altered mental status). Observe the evidence in combination with the indices of tissue perfusion/hypoxia[63].
b) Increase the cardiac output
Optimize the circulating volume
Know the patient's cardiac capacity. The mean arterial pressure, cardiac rhythm, respiratory pattern, urinary output and fluid balance should be assessed. Fluid replacement should not be based only on blood pressure or heart rate. It should be individualized, based on physiological parameters that include continuous reevaluation of tissue perfusion and oxygenation as well as hemodynamic function.
If unsure on the volume status or cardiac output of the anemic patient, perform an appropriate assessment of the patient's clinical condition, for example: evidence of volume, echodopplercardiogram, etc.
Variations both in the systolic arterial pressure and in the pulse pressure with the ventilatory cycle can indicate volume depletion in the patient on mechanical ventilation. Even during relative hypotension, the microcirculatory blood flow and oxygenation do not always depend on the arterial blood pressure[64].
Volume expanders without blood
Crystalloids: the infusion should be three milliliters of crystalloids for each one milliliter of blood lost with an infusion rate of 60-80 ml/kg/hour (preferably Ringer's lactate). The Advanced Trauma Life Support (ATLS) recommends in the case of trauma patient with a 700 ml of blood loss, the patient should receive 2,100 ml of crystalloids. Other solutions may be used: 1) normal saline solution, 2) balanced electrolyte solutions or 3) hypertonic saline solution.
Colloids (starch and gelatin solutions): dose of one milliliter of colloid for each one milliliter of blood lost, with the maximum recommended dose of 1,500 to 2,000 ml per day.
Careful and adequate fluid replacement. In the hypovolemic patient, the strategy for volume replacement (duration, rate of administration and the amount) can be more important than the choice of the solution. There is risk of causing more bleeding with excessive hemodilution and by increasing the blood pressure excessively. Careful administration of the volume, with low levels of hemoglobin, can optimize the microvascular flow and oxygenation, as well as increase anemia tolerance[65]. In moderate amounts, crystalloids are not associated with significant side effects, particularly in hemostasis. Crystalloid infusion (Ringer's lactate) has resulted in less blood loss than colloids (Hydroxyethyl Starch) in recent investigation[66].
Correct hypovolemia, if possible, always with crystalloid. In a systematic revision conducted by Cochrane Database of Systematic Reviews[67] the authors have observed evidence of greater mortality risk with the starch solutions.
c) Early oxygenotherapy/Oxygen supplementation
Anemia tolerance can be increased ventilating the patient with a high fraction of inspired oxygen (FiO2). While normovolemia is being maintained, hyperoxic ventilation (100% of oxygen) can be considered a lifesaving therapy in the presence of gross hemorrhage associated with severe acute anemia with the risk of death[62,68]. Ventilation with 100% oxygen results in rapid increase of the arterial oxygen content, assures tissue oxygenation even with very low hemoglobin and shows to be an important strategy in reducing allogeneic transfusion[69].
For patients with insufficient response to other strategies to improve oxygenation (for example: correction of volume in circulation, vasoactive agents, inotropes), the recommendation is to use sedation and ventilatory support (continuous positive airway pressure [CPAP], intermittent positive pressure [BIPAP]). The faster the tissue oxygen deficiency is detected and corrected, the more probability of better results. Once hypoxemia presents greater immediate risks than oxygen toxicity or hypercapnia, it might be worth the risks involved in supranormal fractions of inspired oxygen to sustain the life of the patient with severe anemia[70].
The use of hyperbaric oxygen therapy to reach a high arterial tension (PaO2) has the potential to save the life of the patient with severe anemia[71].
d) Artificial oxygen carriers and polymerized hemoglobin solutions
Perfluorocarbon and MP4OX (oxygenated polyethylene glycol-modified hemoglobin) still need evidences guaranteeing safety and efficiency, as well as cost-effectiveness.
2 - Minimization of oxygen consumption
a) Appropriate analgesia
b) Sedation and muscle relaxants
It is recommended to administer the lowest effective dose for the lowest duration of analgesia and sedation. Consider neuromuscular blockade. Decrease oxygen consumption by reducing the metabolic rate and preventing shivering, agitation and anxiety.
c) Mechanical ventilation
It can be recommended in severe anemia cases to improve the supply and to reduce the consumption of oxygen by the tissues.
d) Temperature Control
Maintain normothermia (actively warm hypothermic patients and cool down febrile patients)[72]. The use of therapeutic cooling in patients with severe anemia in intensive therapy can reduce the need of tissue oxygenation, decrease the metabolic rate and offer cerebral protection in subgroups of patients[73].
DISCUSSION
Blood transfusion is in essence a transplant of allogeneic cells, consisting of the infusion of foreign antigens in great quantities in the recipient's circulation, resulting in multiple inflammatory and immunological reactions[74]. This is one of the main explanations to the adverse outcomes of this medical practice.
Centers worldwide are seeking to institute protocols to limit the use of blood and this has become a criterion for high-quality hospital care surveyed by certifying agencies of quality assurance, such as the Joint Commission International[75]. Numerous pathways can be utilized as part of these blood conservation protocols. It is important to raise awareness of the main therapeutic possibilities to transfusions in order to economize the already scarce hemocomponent supply of blood banks. When there is multiprofessional purpose and involvement in managing and conserving autologous blood, complex cardiac surgeries such as cardiac retransplantation can be performed without the use of allogeneic blood[54].
By applying one or more strategies as described in Tables 1, 2 and 3, allogeneic blood consumption can be decreased. A simple course of action, but fundamentally important, is the diagnosis and treatment of anemia with iron, folic acid and vitamin B12 replacement and, when necessary, r-Hu-EPO. The principal strategies to minimize blood loss involve eliminating multiple daily routine phlebotomies, avoiding or reducing the time of the CPB, meticulous hemostasis, pharmacological hemostasis with tranexamic acid or epsilon aminocaproic acid, use of medications that increase the activity of coagulation factors (desmopressin, r-FVIIa, PCC, Factor XIII), in addition to ANH and intraoperative blood recovery (cell saver). Another important strategy to limit blood consumption is to adopt a restrictive transfusion practice, with a plan of action to improve tissue oxygenation of the anemic patient. These and other strategies are described in Figure 2. The more alternative therapies used, the higher the possibility to treat a patient without the need of transfusions[76]. The benefits are not restricted to the economic sphere, but also to the gravity and incidence of complications, in particular to mortality, related to allogeneic blood transfusions.
This literature review compiles multiple therapeutic options to reduce the number of transfused patients as well as the amount of blood and its components administered to each patient. Nonetheless, before implementing these methods, it is fundamental to have a desire to change the transfusional attitude, especially in relation to anemia tolerance. Some of the methods are simple and safe and can be easily implemented; others require a carefully orchestrated process. These strategies are not mutually exclusive and the reality of each procedure can guide which plan of action is better and more feasible. A medical center that establishes an autologous blood conservation process can reduce in up to 70% of the number of blood component units infused as well as the number of hemotransfused patients[76]. We encourage a new concept in the transfusional medicine in that, with the least amount of allogeneic blood use in cardiac surgeries, can result in a reduction of the total morbimortality of patients, as well as easing the demand on blood banks. New studies are recommended to evaluate the safety and efficacy of allogeneic blood transfusions.
One of the main limitations of utilizing some of these options as alternatives to blood transfusions are related to the cost and availability of a determined strategy, such as the intraoperative cell salvage machine, certain medications (r-Hu-TPO, eltrombopag, Factor VIIa, Factor XIII, PCC, HFC) and the learning curve of more refined surgical techniques to avoid bleeding. The retrograde autologous priming technique is also a limited option, due to potential complications of hemodynamic instability resulted from hypovolemia. Another limitation in applying some of these strategies will occur with emergency surgeries. The assessment of the risk-benefit binomial is an important factor to consider in relation to the transfusional and nontransfusional practice. When medical decisions are made by multidisciplinary teams, the treatment potential damage is reduced whether for certain patient subgroups or even for the general population.
CONCLUSION
There are multiple clinical and surgical strategies with evidence of optimizing the erythrocyte mass and the coagulation status, minimizing blood loss and improving anemia tolerance. Treating anemia and thrombocytopenia, suspending anticoagulants and antiplatelet agents, reducing routine phlebotomies, utilizing less traumatic surgical techniques with moderate hypothermia and hypotension, meticulous hemostasis, use of topical and systemic hemostatic agents, acute normovolemic hemodilution, intraoperative blood recovery, anemia tolerance (supplemental oxygen and normothermia), as well as numerous other therapeutic options have proved to be effective strategies in reducing allogeneic blood transfusions. In order to decrease the consumption of blood components, diminish morbidity and mortality, and reduce hospital costs, these treatment strategies should be incorporated into medical practice worldwide.
| Authors' roles & responsibilities | |
|---|---|
| AAS | Conception and design of the study, analysis and/or interpretation of data, writing of the manuscript or critical review of its content, final approval of the manuscript |
| JPS | Writing of the manuscript or critical review of its content, analysis and/or interpretation of data, final approval of the manuscript |
| LFS | Writing of the manuscript or critical review of its content, final approval of the manuscript |
| AGS | Writing of the manuscript or critical review of its content, analysis and/or interpretation of data, final approval of the manuscript |
| RFP | Writing of the manuscript or critical review of its content, analysis and/or interpretation of data, final approval of the manuscript |
| JFB | Final approval of the manuscript |
Footnotes
No financial support.
This study was carried out at Real e Benemérita Associação Portuguesa de Beneficência de São Paulo, São Paulo, SP, Brazil.
REFERENCES
- 1.Bennett-Guerrero E, Zhao Y, O'Brien SM, Ferguson TB, Jr, Peterson ED, Gammie JS, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304(14):1568–1575. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- 2.Sojka BN, Sojka P. The blood donation experience: self-reported motives and obstacles for donating blood. Vox Sang. 2008;94(1):56–63. doi: 10.1111/j.1423-0410.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 3.Novaretti MCZ. Importância dos carreadores de oxigênio livre de células. Rev Bras Hematol Hemoterapia. 2007;29(4):394–405. [Google Scholar]
- 4.Mackenzie CF, Shander A. What to do if no blood is available but the patient is bleeding? South Afr J Anaesth Analg. 2008;14(1):39–43. [Google Scholar]
- 5.Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34(6):1608–1616. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 6.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 7.Dorneles CC, Bodanese LC, Guaragna JCVC, Macagnan FE, Coelho JC, Borges AP, et al. O impacto da hemotransfusão na morbimortalidade pós-operatória de cirurgias cardíacas. Rev Bras Cir Cardiovasc. 2011;26(2):222–229. doi: 10.1590/s0102-76382011000200012. [DOI] [PubMed] [Google Scholar]
- 8.Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74(4):1180–1186. doi: 10.1016/s0003-4975(02)03766-9. [DOI] [PubMed] [Google Scholar]
- 9.Santos AA, Sousa AG, Thomé HO, Machado RL, Piotto RF. Impact on early and late mortality after blood transfusion in coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2013;28(1):1–9. doi: 10.5935/1678-9741.20130003. [DOI] [PubMed] [Google Scholar]
- 10.Santos AA, Sousa AG, Piotto RF, Pedroso JC. Mortality risk is dose-dependent on the number of packed red blood cell transfused after coronary artery bypass graft. Rev Bras Cir Cardiovasc. 2013;28(4):509–517. doi: 10.5935/1678-9741.20130083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–765. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 13.Souza DD, Braile DM. Avaliação de nova técnica de hemoconcentração e da necessidade de transfusão de hemoderivados em pacientes submetidos à cirurgia cardíaca com circulação extracorpórea. Rev Bras Cir Cardiovasc. 2004;19(3):287–294. [Google Scholar]
- 14.Senay S, Toraman F, Karabulut H, Alhan C. Is it the patient or the physician who cannot tolerate anemia?: A prospective analysis in 1854 non-transfused coronary artery surgery patients. Perfusion. 2009;24(6):373–380. doi: 10.1177/0267659109358118. [DOI] [PubMed] [Google Scholar]
- 15.Society of Thoracic Surgeons Blood Conservation Guideline Task Force.Ferraris VA.Ferraris SP.Saha SP.Hessel EA 2nd.Haan CK.Royston BD Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists clinical practice guidelines. Ann Thorac Surg. 2007;83(5) Suppl:S27–S86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 16.Menkis AH, Martin J, Cheng DC, Fitzgerald DC, Freedman JJ, Gao C, et al. Drug, devices, technologies, and techniques for blood management in minimally invasive and conventional cardiothoracic surgery: a consensus statement from the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) 2011. Innovations (Phila) 2012;7(4):229–241. doi: 10.1097/IMI.0b013e3182747699. [DOI] [PubMed] [Google Scholar]
- 17.Drews RE. Critical issues in hematology:: anemia, thrombocytopenia, coagulopathy, and blood product transfusions in critically ill patients. Clin Chest Med. 2003;24(4):607–622. doi: 10.1016/s0272-5231(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 18.Goodnough LT. Iron deficiency syndromes and iron-restricted erythropoiesis (CME) Transfusion. 2012;52(7):1584–1592. doi: 10.1111/j.1537-2995.2011.03495.x. [DOI] [PubMed] [Google Scholar]
- 19.Swain RA, Kaplan B, Montgomery E. Iron deficiency anemia: When is parenteral therapy warranted? Postgrad Med. 1996;100(5):181–182. doi: 10.3810/pgm.1996.11.116. [DOI] [PubMed] [Google Scholar]
- 20.Weltert L, D'Alessandro S, Nardella S, Girola F, Bellisario A, Maselli D, et al. Preoperative very short-term, high-dose erythropoietin administration diminishes blood transfusion rate in off-pump coronary artery bypass: a randomized blind controlled study. J Thorac Cardiovasc Surg. 2010;139(3):621–626. doi: 10.1016/j.jtcvs.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Shapiro MJ, et al. EPO Critical Care Trials Group: Efficacy of recombinant human erythropoietin in critically ill patients: a randomized controlled trial. JAMA. 2002;288(22):2827–2835. doi: 10.1001/jama.288.22.2827. [DOI] [PubMed] [Google Scholar]
- 22.Eckardt KU. Anaemia of critical illness:: implications for understanding and treating rHuEPO resistance. Nephrol Dial Transplant. 2002;17(Suppl 5):48–55. doi: 10.1093/ndt/17.suppl_5.48. [DOI] [PubMed] [Google Scholar]
- 23.Ohls RK, Harcum J, Schibler KR, Christensen RD. The effect of erythropoietin on the transfusion requirements of preterm infants weighing 750 grams or less: a randomized, double-blind, placebo-controlled study. J Pediatr. 1997;131(5):661–665. doi: 10.1016/s0022-3476(97)70089-1. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg D, Wexler D, Blum M, Schwartz D, Iaina A. The use of androgens in anaemia resistant to erythropoietin and i.v. iron in patients with heart and renal failure. Nephrol Dial Transplant. 2004;19(4):1021–1021. doi: 10.1093/ndt/gfh006. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman JS, Reda DJ, Fye CL, Goldfarb DS, Henderson WG, Kleinman JG, et al. Subcutaneous compared with intravenous epoetin in patients receiving hemodialysis: Department of Veterans Affairs Cooperative Study Group on Erythropoietin in Hemodialysis Patients. N Engl J Med. 1998;339(9):578–583. doi: 10.1056/NEJM199808273390902. [DOI] [PubMed] [Google Scholar]
- 26.Stohlawetz PJ, Dzirlo L, Hergovich N, Lackner E, Mensik C, Eichler HG, et al. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood. 2000;95(9):2983–2989. [PubMed] [Google Scholar]
- 27.Drüeke T. Hyporesponsiveness to recombinant human erythropoietin. Nephrol Dial Transplant. 2001;16(7):25–28. doi: 10.1093/ndt/16.suppl_7.25. [DOI] [PubMed] [Google Scholar]
- 28.Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98(10):708–714. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- 29.Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9664):641–648. doi: 10.1016/S0140-6736(09)60402-5. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Wang Z, Wu L, Zhang J, Wang J, Yan L. Recombinant human thrombopoietin is an effective treatment for thrombocytopenia in hemophagocytic lymphohistiocytosis. Ann Hematol. 2013;92(12):1695–1699. doi: 10.1007/s00277-013-1819-9. [DOI] [PubMed] [Google Scholar]
- 31.Whitlock R, Crowther MA, Ng HJ. Bleeding in cardiac surgery: its prevention and treatment--an evidence-based review. Crit Care Clin. 2005;21(3):589–610. doi: 10.1016/j.ccc.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Chu UB, Clevenger FW, Imami ER, Lampard SD, Frykberg ER, Tepas 3rd JJ. The impact of selective laboratory evaluation on utilization of laboratory resources and patient care in a level-I trauma center. Am J Surg. 1996;172(5):558–562. doi: 10.1016/S0002-9610(96)00234-6. [DOI] [PubMed] [Google Scholar]
- 33.Dech ZF, Szaflarski NL. Nursing strategies to minimize blood loss associated with phlebotomy. AACN Clin Issues. 1996;7(2):277–287. doi: 10.1097/00044067-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Milas BL, Jobes DR, Gorman RC. Management of bleeding and coagulopathy after heart surgery. Semin Thorac Cardiovasc Surg. 2000;12(4):326–336. doi: 10.1053/stcs.2000.20511. [DOI] [PubMed] [Google Scholar]
- 35.Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, et al. CORONARY Investigators.: Off-pump or on-pump coronary-artery bypass grafting at 30 days . N Engl J Med. 2012;366(16):1489–1497. doi: 10.1056/NEJMoa1200388. [DOI] [PubMed] [Google Scholar]
- 36.Zangrillo A, Garozzo FA, Biondi-Zoccai G, Pappalardo F, Monaco F, et al. Miniaturized cardiopulmonary bypass improves short-term outcome in cardiac surgery: a meta-analysis of randomized controlled studies. J Thorac Cardiovasc Surg. 2010;139(5):1162–1169. doi: 10.1016/j.jtcvs.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 37.Boodhwani M, Williams K, Babaev A, Gill G, Saleem N, Rubens FD. Ultrafiltration reduces blood transfusions following cardiac surgery: a meta-analysis. Eur J Cardiothorac Surg. 2006;30(6):892–897. doi: 10.1016/j.ejcts.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Rosengart TK, DeBois W, O'Hara M, Helm R, Gomez M, Lang SJ, et al. Retrograde autologous priming for cardiopulmonary bypass: a safe and effective means of decreasing hemodilution and transfusion requirements. J Thorac Cardiovasc Surg. 1998;115(2):426–438. doi: 10.1016/S0022-5223(98)70287-9. [DOI] [PubMed] [Google Scholar]
- 39.Van der Linden P, De Hert S, Daper A, Trenchant A, Jacobs D, De Boelpaepe C, et al. A standardized multidisciplinary approach reduces the use of allogeneic blood products in patients undergoing cardiac surgery. Can J Anesth. 2001;48(9):894–901. doi: 10.1007/BF03017357. [DOI] [PubMed] [Google Scholar]
- 40.Degoute CS. Controlled hypotension: a guide to drug choice. Drugs. 2007;67(7):1053–1076. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- 41.Frenette L, Cox J, McArdle P, Eckhoff D, Bynon S. Conjugated estrogen reduces transfusion and coagulation factor requirements in orthotopic liver transplantation. Anesth Analg. 1998;86(6):1183–1186. doi: 10.1097/00000539-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Carless PA, Stokes BJ, Moxey AJ, Henry DA. Desmopressin use for minimizing perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2008;(1):CD001884–CD001884. [Google Scholar]
- 43.Crescenzi G, Landoni G, Biondi-Zoccai G, Pappalardo F, Nuzzi M, Bignami E, et al. Desmopressin reduces transfusion needs after surgery: a meta-analysis of randomized clinical trials. Anesthesiology. 2008;109(6):1063–1076. doi: 10.1097/ALN.0b013e31818db18b. [DOI] [PubMed] [Google Scholar]
- 44.Prasad GV, Abidi SM, McCauley J, Johnston JR. Vitamin K deficiency with hemorrhage after kidney and combined kidney-pancreas transplantation. Am J Kidney Dis. 1999;33(5):963–965. doi: 10.1016/s0272-6386(99)70433-6. [DOI] [PubMed] [Google Scholar]
- 45.Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2011;(2):CD005011–CD005011. doi: 10.1002/14651858.CD005011.pub3. [DOI] [PubMed] [Google Scholar]
- 46.Monroe DM, Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol. 2006;26(1):41–48. doi: 10.1161/01.ATV.0000193624.28251.83. [DOI] [PubMed] [Google Scholar]
- 47.Nienaber U, Innerhofer P, Westermann I, Schöchl H, Attal R, Breitkopf R, et al. The impact of fresh frozen plasma vs coagulation factor concentrates on morbidity and mortality in trauma-associated haemorrhage and massive transfusion. Injury. 2011;42(7):697–701. doi: 10.1016/j.injury.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 48.Rahe-Meyer N, Hanke A, Schmidt DS, Hagl C, Pichlmaier M. Fibrinogen concentrate reduces intraoperative bleeding when used as first-line hemostatic therapy during major aortic replacement surgery: results from a randomized, placebo-controlled trial. J Thorac Cardiovasc Surg. 2013;145(3) Suppl:S178–S185. doi: 10.1016/j.jtcvs.2012.12.083. [DOI] [PubMed] [Google Scholar]
- 49.Society of Thoracic Surgeons Blood Conservation Guideline Task Force.Ferraris VA.Brown JR.Despotis GJ.Hammon JW.Reece TB. Saha SPSociety of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion.International Consortium for Evidence Based Perfusion 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 50.Abrishami A, Chung F, Wong J. Topical application of antifibrinolytic drugs for on-pump cardiac surgery: a systematic review and meta-analysis. Can J Anaesth. 2009;56(3):202–212. doi: 10.1007/s12630-008-9038-x. [DOI] [PubMed] [Google Scholar]
- 51.Licker M, Ellenberger C, Sierra J, Kalangos A, Diaper J, Morel D. Cardioprotective effects of acute normovolemic hemodilution in patients undergoing coronary artery bypass surgery. Chest. 2005;128(2):838–847. doi: 10.1378/chest.128.2.838. [DOI] [PubMed] [Google Scholar]
- 52.Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58(3):277–280. doi: 10.1097/00000542-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 53.Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C. Cost-effectiveness of cell salvage and alternative methods of minimizing perioperative allogeneic blood transfusion: : a systematic review and economic model. Health Tech Assess. 2006;10(44):iii–iiv. ix–ix, 1-210. doi: 10.3310/hta10440. [DOI] [PubMed] [Google Scholar]
- 54.Santos AA, Silva JP, Fonseca L, Baumgratz JF. Retransplante cardíaco em criança sem o uso de hemoderivados. Rev Bras Cir Cardiovasc. 2012;27(2):327–330. doi: 10.5935/1678-9741.20120051. [DOI] [PubMed] [Google Scholar]
- 55.Dunning J, Versteegh M, Fabbri A, Pavie A, Kolh P, Lockowandt U, et al. EACTS Audit and Guidelines Committee.: Guideline on antiplatelet and anticoagulation management in cardiac surgery. Eur J Cardiothorac Surg. 2008;34(1):73–92. doi: 10.1016/j.ejcts.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 56.Carless PA, Henry DA, Moxey AJ, O'Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010;(4):CD001888–CD001888. doi: 10.1002/14651858.CD001888.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wuethrich PY, Studer UE, Thalmann GN, Burkhard FC. Intraoperative continuous norepinephrine infusion combined with restrictive deferred hydration significantly reduces the need for blood transfusion in patients undergoing open radical cystectomy: results of a prospective randomised trial. Eur Urol. 2014;66(2):352–360. doi: 10.1016/j.eururo.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 58.Lacroix J, Hebert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, TRIPICU Investigators, et al. Canadian Critical Care Trials GroupPediatric Acute Lung Injury and Sepsis Investigators Network.: Transfusion strategies for patients in pediatric intensive care units . N Engl J Med. 2007;356(16):1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 59.Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med. 2014;127(2):124–131. doi: 10.1016/j.amjmed.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 60.Murphy GJ, Angelini GD. Indications for blood transfusion in cardiac surgery. Ann Thorac Surg. 2006;82(6):2323–2334. doi: 10.1016/j.athoracsur.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 61.Graffeo C, Dishong W. Severe blood loss anemia in a Jehovah's Witness treated with adjunctive hyperbaric oxygen therapy. Am J Emerg Med. 2013;31(4):756–756. doi: 10.1016/j.ajem.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Araújo Azi LM, Lopes FM, Garcia LV. Postoperative management of severe acute anemia in a Jehovah's Witness. Transfusion. 2014;54(4):1153–1157. doi: 10.1111/trf.12424. [DOI] [PubMed] [Google Scholar]
- 63.Demetriades D, Chan LS, Bhasin P, Berne TV, Ramicone E, Huicochea F, et al. Relative bradycardia in patients with traumatic hypotension. J Trauma. 1998;45(3):534–539. doi: 10.1097/00005373-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 64.LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28(8):2729–2732. doi: 10.1097/00003246-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Wettstein R, Tsai AG, Erni D, Lukyanov AN, Torchilin VP, Intaglietta M, et al. Improving microcirculation is more effective than substitution of red blood cells to correct metabolic disorder in experimental hemorrhagic shock. Shock. 2004;21(3):235–240. doi: 10.1097/01.shk.0000114301.36496.ea. [DOI] [PubMed] [Google Scholar]
- 66.Rasmussen KC, Johansson PI, Højskov M, Kridina K, Kistorp T, Thind P, et al. Hydroxyethyl starch reduces coagulation competence and increases blood loss during major surgery: results from a randomized controlled trial. Ann Surg. 2014;259(2):249–254. doi: 10.1097/SLA.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 67.Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013;2:CD000567–CD000567. doi: 10.1002/14651858.CD000567.pub6. [DOI] [PubMed] [Google Scholar]
- 68.Meier J, Kemming GI, Kisch-Wedel H, Wölkhammer S, Habler OP. Hyperoxic ventilation reduces 6-hour mortality at the critical hemoglobin concentration. Anesthesiology. 2004;100(1):70–76. doi: 10.1097/00000542-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Habler O, Kleen M, Kemming G, Zwissler B. Hyperoxia in extreme hemodilution. Eur Surg Res. 2002;34(1-2):181–187. doi: 10.1159/000048907. [DOI] [PubMed] [Google Scholar]
- 70.Neff TA, Stocker R, Wight E, Spahn DR. Extreme intraoperative blood loss and hemodilution in a Jehovah's Witness: new aspects in postoperative management. Anesthesiology. 1999;91(6):1949–1951. doi: 10.1097/00000542-199912000-00051. [DOI] [PubMed] [Google Scholar]
- 71.Van Meter KW. A systematic review of the application of hyperbaric oxygen in the treatment of severe anemia: an evidence-based approach. Undersea Hyperb Med. 2005;32(1):61–83. [PubMed] [Google Scholar]
- 72.Marik PE. Fever in the ICU. Chest. 2000;117(3):855–869. doi: 10.1378/chest.117.3.855. [DOI] [PubMed] [Google Scholar]
- 73.Akingbola OA, Custer JR, Bunchman TE, Sedman AB. Management of severe anemia without transfusion in a pediatric Jehovah's Witness patient. Crit Care Med. 1994;22(3):524–528. doi: 10.1097/00003246-199403000-00025. [DOI] [PubMed] [Google Scholar]
- 74.Flohé S, Kobbe P, Nast-Kolb D. Immunological reactions secondary to blood transfusion. Injury. 2007;38(12):1405–1408. doi: 10.1016/j.injury.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 75.Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(4):S112–S118. doi: 10.3949/ccjm.76.s4.18. [DOI] [PubMed] [Google Scholar]
- 76.Tinmouth A, Macdougall L, Fergusson D, Amin M, Graham ID, Hebert PC, et al. Reducing the amount of blood transfused: a systematic review of behavioral interventions to change physicians' transfusion practices. Arch Intern Med. 2005;165(8):845–852. doi: 10.1001/archinte.165.8.845. [DOI] [PubMed] [Google Scholar]