Abstract
The term “immunogenic cell death” (ICD) is commonly employed to indicate a peculiar instance of regulated cell death (RCD) that engages the adaptive arm of the immune system. The inoculation of cancer cells undergoing ICD into immunocompetent animals elicits a specific immune response associated with the establishment of immunological memory. Only a few agents are intrinsically endowed with the ability to trigger ICD. These include a few chemotherapeutics that are routinely employed in the clinic, like doxorubicin, mitoxantrone, oxaliplatin, and cyclophosphamide, as well as some agents that have not yet been approved for use in humans. Accumulating clinical data indicate that the activation of adaptive immune responses against dying cancer cells is associated with improved disease outcome in patients affected by various neoplasms. Thus, novel therapeutic regimens that trigger ICD are urgently awaited. Here, we discuss current combinatorial approaches to convert otherwise non-immunogenic instances of RCD into bona fide ICD.
Keywords: ATP, autophagy, calreticulin, endoplasmic reticulum stress, HMGB1, type I interferon
Introduction
The expression “immunogenic cell death” (ICD) generally refers to a functionally peculiar case of regulated cell death (RCD) that – in immunocompetent hosts – is capable of activating an adaptive immune response against dead cell-associated antigens (1–5). Of note, ICD generally (but not obligatorily) manifests with apoptotic morphological features, and at least some of its manifestations depend on components of the apoptotic apparatus (6–8). Irrespective of these morphological and biochemical considerations, immunocompetent mice injected s.c. with cancer cells succumbing to bona fide ICD (in the absence of any adjuvant) develop a cellular immune response associated with the establishment of immunological memory that protects them from a subsequent challenge with living cells of the same type (1–3). Importantly, vaccination experiments of this type, involving murine cells and syngeneic mice, remain the gold-standard method to identify bona fide ICD, though several tests have been developed to detect some of its cellular manifestations (see below) (2, 3, 9, 10).
Only a few lethal stimuli are intrinsically endowed with the ability to trigger ICD (9, 11–14). These include some chemotherapeutic agents that are employed in the clinic, including (1) various anthracyclines (i.e., doxorubicin, epirubicin, and idarubicin), which are commonly used against a wide panel of malignant conditions (15–17); (2) mitoxantrone, an anthracenedione generally used for the treatment of acute myeloid leukemia, breast carcinoma, non-Hodgkin’s lymphoma, and prostate carcinoma (15, 16); (3) oxaliplatin, a platinum derivative approved for use in combination with 5-fluorouracil to treat advanced colorectal carcinoma (18, 19); (4) cyclophosphamide, an alkylating agent that is employed against various neoplastic and autoimmune conditions (20–23); and (5) bortezomib, a proteasomal inhibitor approved for the therapy of multiple myeloma and mantle cell lymphoma (24–26). Specific forms of irradiation as well as photodynamic therapy, both of which are habitually employed for the treatment of various neoplasms, have also been shown to trigger bona fide ICD (27–34). Finally, a bunch of hitherto experimental agents is intrinsically endowed with the capacity to initiate ICD, including (but not limited to) some oncolytic viruses (35–39), the microtubular inhibitor patupilone (40–42), and elevated hydrostatic pressures (43).
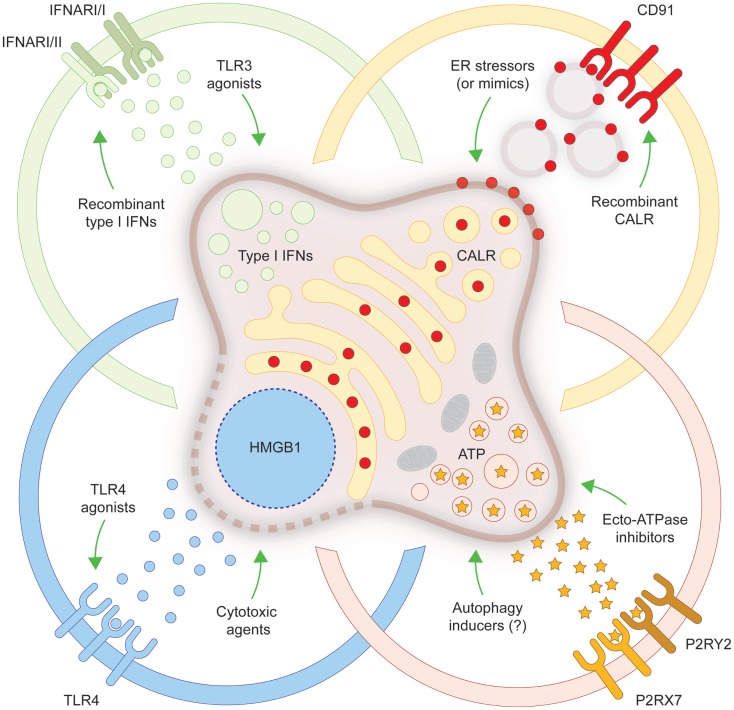
According to accepted models, ICD relies on the establishment of adaptive stress responses that promote the spatiotemporally coordinated emission of endogenous danger signals from dying cells (44, 45). The endogenous molecules that dispatch danger signals in response to stress are cumulatively known as “damage-associated molecular patterns” (DAMPs) and operate upon binding to receptors expressed by bystander cells, including cellular components of both the innate and adaptive immune system (2, 46–49). As it stands, four DAMPs have been shown to be required for RCD as induced by anthracyclines to be perceived as immunogenic, namely, (1) the exposure of the endoplasmic reticulum (ER) chaperone calreticulin (CALR) on the outer surface of the plasma membrane (16); (2) the secretion of ATP (50); (3) the production of type I interferon (IFN) (51); and (4) the release of the non-histone chromatin-binding protein high-mobility group box 1 (HMGB1) into the extracellular space (52). This said, it cannot be formally excluded that other hitherto undiscovered DAMPs are required for anthracycline-elicited RCD to promote an adaptive immune response. Along similar lines, not all these DAMPs may be required for RCD as induced by agents other than anthracyclines to be perceived as immunogenic (53–55).
In this context, i.e., anthracycline-induced ICD, CALR exposure obligatorily relies on the establishment of a pre-mortem ER stress response centered around the phosphorylation of eukaryotic translation initiation factor 2A, 65 kDa (EIF2A) (7, 56), ATP secretion requires the induction of autophagy (57), and type I IFN production stems from toll-like receptor 3 (TLR3) signaling (51). The molecular mechanisms underlying the ability of anthracyclines and other ICD inducers to promote HMGB1 release remain obscure (2, 3). Cumulatively, these DAMPs recruit antigen-presenting cells (APCs) to sites of active ICD and stimulate the uptake, processing, and presentation of dead cell-associated antigens, eventually resulting in the priming of an adaptive immune response (2, 3). In particular, CALR promotes antigen uptake by APCs by binding to low density lipoprotein receptor-related protein 1 (LRP1, best known as CD91) (58); ATP stimulates the recruitment of APCs and their activation upon binding to purinergic receptor P2Y, G-protein coupled, 2 (P2RY2) and purinergic receptor P2X, ligand-gated ion channel, 7 (P2RX7), respectively (50, 59, 60); type I IFNs exert immunostimulatory effects via IFN (alpha, beta, and omega) receptors (IFNARs) (51); and HMGB1 does so through TLR4 and advanced glycosylation end product-specific receptor (AGER, best known as RAGE) (52, 61).
A detailed discussion of the molecular and cellular mechanisms involved in the detection of ICD-associated DAMPs goes beyond the scope of this review and can be found in Ref. (2, 3). However, it is important to note that the failure of cancer cells to emit one (or more) of these DAMPs completely compromises the immunogenicity of RCD (2, 3). Thus, at odds with their wild-type counterparts, Calr−/− murine CT26 colorectal cells exposed to anthracyclines are unable to vaccinate mice against a subsequent inoculation with malignant cells of the same type (16). The same holds true in several other situations in which adaptive responses cannot proceed normally, including the genetic inhibition of autophagy (e.g., upon the expression of short-hairpin RNAs targeting the essential autophagy proteins Atg5 or Atg7) or the unfolded protein response (e.g., upon the expression of a non-phosphorylatable variant of EIF2A) (7, 57, 62, 63).
Accumulating clinical evidence indicates that the (re-)activation of a proficient immune response against malignant cells is associated with improved disease outcome in patients affected by a wide panel of neoplasms (64–68), in particular when malignant lesions are highly infiltrated by immune effector cells prior to therapy (69). Considerable efforts are therefore being devoted to the development of clinically implementable strategies that (re-)instate anticancer immunosurveillance (70, 71). So far, the most successful of these approaches involves the administration of monoclonal antibodies (mAbs) that block immunosuppressive receptors expressed by activated T cells, such as cytotoxic T lymphocyte-associated protein 4 (CTLA4) and programed cell death 1 (PDCD1, best known as PD-1) (72, 73). Three distinct checkpoint blockers of this type, namely, the CTLA4-targeting mAb ipilimumab and the PD-1-targeting mAbs nivolumab and pembrolizumab, are approved by the US Food and Drug Administration and other regulatory agencies worldwide for use as standalone immunotherapeutic interventions in melanoma patients (74–77). In addition, the administration of checkpoint blockers has been shown to improve the clinical profile of various chemotherapeutic and immunotherapeutic agents (78). Along similar lines, various combinatorial immuno(chemo)therapeutic regimens are being investigated in clinical trials for their ability to mediate superior antineoplastic effects as compared to monotherapies based on their constituents (79, 80). In this framework, various attempts are being made to render immunogenic otherwise non-immunogenic instances of therapy-induced RCD, thereby converting them into bona fide ICD (79, 81–84). This can be due to molecular defects that prevent cancer cells from emitting DAMPs appropriately, as mentioned above, as well as to the intrinsic features of the therapeutic agent under consideration (Table 1). For instance, at odds with its derivative oxaliplatin, cisplatin is intrinsically unable to trigger ICD since it does not stimulate the exposure of CALR on the outer surface of the plasma membrane (18, 19, 85).
Table 1.
Immunogenicity of chemotherapy-induced regulated cell death (examples).
| Drug | CALR exposure | ATP secretion | Type I IFN production | HMGB1 release | aBona fide ICD inducer | Restoration of ICD | Reference |
|---|---|---|---|---|---|---|---|
| 5-Fluorouracil | Debated | No | n.d. | Yes | n.d. | RT | (16) |
| (86) | |||||||
| (87) | |||||||
| Bleomycin | Yes | Yes | Yes | Yes | Yes | n.a. | (88) |
| Bortezomib | Yes | n.d. | Yes | Yes | Yes | n.a. | (24) |
| (25) | |||||||
| (26) | |||||||
| (89) | |||||||
| Camptothecin | Debated | No | n.d. | Yes | No | n.d. | (16) |
| (87) | |||||||
| Carboplatin | Partial | Yes | n.d. | Partial | No | RT | (16) |
| (86) | |||||||
| Cisplatin | No | Yes | n.d. | Yes | No | Pyridoxine | (19) |
| Thapsigargin | (90) | ||||||
| Tunicamycin | (91) | ||||||
| ZnCl2 | (92) | ||||||
| (93) | |||||||
| (94) | |||||||
| Cyclophosphamide | Yes | Yes | Yes | Yes | Yes | n.a. | (20) |
| (21) | |||||||
| (95) | |||||||
| Digitoxin | Yes | Yes | n.d. | Partial | No | Cytotoxic agents | (81) |
| Digoxin | (83) | ||||||
| Docetaxel | Yes | No | n.d. | No | No | n.d. | (96) |
| (97) | |||||||
| Doxorubicin | Yes | Yes | Yes | Yes | Yes | n.a. | (15) |
| (16) | |||||||
| (17) | |||||||
| (51) | |||||||
| (98) | |||||||
| (99) | |||||||
| Epirubicin | Yes | Yes | n.d. | Yes | Yes | n.a. | (16) |
| (17) | |||||||
| Etoposide | No | Yes | n.d. | Yes | No | Calyculin A | (16) |
| Salubrinal | (17) | ||||||
| Tautomycin | (93) | ||||||
| PP1/GADD34-targeting peptides | (100) | ||||||
| 2-deoxyglucose | (101) | ||||||
| (102) | |||||||
| Gemcitabine | No | Partial | n.d. | Yes | No | PX-478 | (103) |
| Idarubicin | Yes | n.d. | n.d. | Yes | Yes | n.a. | (17) |
| (16) | |||||||
| (104) | |||||||
| Irinotecan | n.d. | n.d. | n.d. | Yes | n.d. | n.d. | (105) |
| Mafosfamide | Yes | n.d. | n.d. | Yes | Yes | n.d. | (20) |
| Melphalan | Debated | n.d. | n.d. | Yes | n.d. | n.d. | (106) |
| (107) | |||||||
| (108) | |||||||
| Mitomycin C | Debated | No | n.d. | Yes | No | n.d. | (16) |
| (87) | |||||||
| Mitoxantrone | Yes | Yes | Yes | Yes | Yes | n.a. | (7) |
| (16) | |||||||
| (17) | |||||||
| (51) | |||||||
| (57) | |||||||
| (93) | |||||||
| (109) | |||||||
| Oxaliplatin | Yes | Yes | Yes | n.d. | Yes | n.a. | (7) |
| (18) | |||||||
| (52) | |||||||
| (57) | |||||||
| (93) | |||||||
| (110) | |||||||
| Patupilone | Yesb | n.d. | Yes | Yesb | Yesb | n.a. | (41) |
| (42) | |||||||
| Temozolomide | No | Yes | n.d. | Yes | n.d. | Oncolytic virotherapy | (111) |
| Cyclophosphamide | (112) | ||||||
| Vemurafenib | Yes | n.d. | n.d. | Yes | n.d. | n.d. | (103) |
| (113) | |||||||
CALR, calreticulin; HMGB1, high-mobility group box 1; ICD, immunogenic cell death; IFN, interferon; n.a., not applicable; n.d., not determined; RT, radiation therapy.
aAs determined in gold-standard vaccination experiments.
bUnpublished observations from our group.
Here, we discuss strategies to convert non-immunogenic instances of RCD into bona fide ICD. In particular, we will review approaches for (1) correcting the incapacity of some therapeutic agents to kill cancer cells while provoking the emission of one or more DAMP(s); or (2) complementing the missing DAMP(s) with exogenous interventions. On the contrary, we will not dwell on strategies that boost the immunogenicity of RCD by operating downstream of DAMP-sensing receptors.
Combinatorial Strategies to Restore CALR Exposure
Some anticancer therapeutics efficiently kill cancer cells (hence promoting the release of HMGB1) and stimulate the secretion of both ATP and type I IFNs, but selectively fail to promote CALR exposure. Most often, such a defect originates from the inability of these agents to trigger an ER stress response resulting in EIF2A phosphorylation (56, 114), and hence can be corrected by the co-administration of an ER stressors. As mentioned above, cisplatin is one of the antineoplastic agents that fail to trigger bona fide ICD as it does not drive a robust ER stress response (18, 19, 85). The ER-stressing agents that have been shown to correct this defect, hence rendering cisplatin-induced RCD immunogenic, include thapsigargin, an inhibitor of various members of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) (19, 114); tunicamycin, an inhibitor of N-glycosylation (19, 94, 114); pyridoxine, a cell-permeant precursor of bioactive vitamin B6 (90, 91, 115); and ZnCl2 (92). Similar results have been obtained by establishing an ER stress response through the enforced overexpression of reticulon 1 (RTN1), an ER protein involved in vesicular trafficking and secretion (116, 117). The latter approach is obviously incompatible with clinical applications. Nonetheless, these data reinforce the notion that the immunogenicity of cisplatin-induced RCD can be restored by various interventions that induce an ER stress (94).
Another strategy that successfully restores CALR exposure in cells succumbing to chemicals that per se do not enable this phenomenon consists in the co-administration of inhibitors of the EIF2A phosphatase composed of protein phosphatase 1, regulatory subunit 15A (PPP1R15A, best known as GADD34), and pyrophosphatase (inorganic) 1 (PPA1, best known as PP1), resulting in accrued EIF2A phosphorylation even in the absence of overt ER stress (16). Thus, whereas CT26 cells treated with etoposide (a topoisomerase II inhibitor currently approved for the treatment of various malignancies) (118, 119) do not expose CALR as they die, and hence fail to vaccinate mice against a subsequent challenge with neoplastic cells of the same type, they efficiently do so in the presence of tautomycin, calyculin A, and salubrinal (three distinct GADD34/PP1 inhibitors) (16). Similar results have been obtained with the small-interfering RNA (siRNA)-mediated downregulation of PP1 or GADD34 (16), as well as with short cell-permeant peptides that disrupt the physical interaction between these two proteins (102). Although siRNA- and peptide-based strategies may not be easily implemented in clinical settings, these results corroborate the specificity of tautomycin, calyculin A, and salubrinal, and lend further support to the notion that interventions that stimulate EIF2A phosphorylation efficiently promote CALR exposure even in the absence of overt ER stress (120).
At least theoretically, the co-administration of ER stressors or molecules that promote EIF2A phosphorylation can be harnessed to reconstitute the immunogenicity of RCD induced by all anticancer agents that per se do not stimulate CALR exposure on the cell surface but provoke ATP secretion, type I IFN production, and HMGB1 release. In addition, the inability of some anticancer agents to cause the translocation of CALR to the outer leaflet of the plasma membrane can be corrected, at least in some settings, by the co-administration of exogenous, recombinant CALR (7, 16, 106). CALR is indeed relatively “sticky” and its absorption on malignant cells succumbing to non-immunogenic RCD in vitro has been shown to fully restore the ability of these cells to vaccinate syngeneic mice against a subsequent neoplastic challenge (16). To the best of our knowledge, however, whether the systemic or intratumoral administration of recombinant CALR to tumor-bearing mice treated with non-immunogenic therapeutics is able to convert them into bona fide ICD inducers has not been tested yet. As compared to administration of small molecules that establish an ER stress response or promote EIF2A phosphorylation, the use of recombinant CALR appears advantageous in that (at least theoretically) it would complement the lack of CALR exposure in all scenarios, irrespective of the underlying molecular defects (including the downregulation or loss of CALR itself). However, such an approach may not be implementable in the clinic, owing to pharmacodynamic and pharmacokinetic issues (e.g., distribution of the recombinant protein, serum half-life, etc…) as well as economic considerations. Current efforts are therefore being focused on the identification of novel (and the refinement of existing) small molecule-based strategies to stimulate CALR exposure upon the establishment of an ER stress or the induction of EIF2A phosphorylation.
Combinatorial Strategies to Boost ATP Secretion
In some settings, anticancer agents kill malignant cells in an efficient fashion (which corresponds to a consistent release of HMGB1), while stimulating the exposure of CALR and the production of type I IFN, but this is not accompanied by the accumulation of extracellular ATP (57, 121), a defect that can stem from at least three different causes. First, some therapeutic agents are unable to stimulate (or even inhibit) autophagic responses, which are required for dying cells to secrete ATP in sufficient amount for signaling via P2RY2 and P2RX7 receptors (57). Second, some malignant cells bear genetic or epigenetic defects that affect the molecular machinery for autophagy (122, 123). These cells are intrinsically unable to preserve the intracellular ATP pool in the course of stress responses, resulting in limited ATP secretion during death (124). Third, some neoplastic cells express high levels of either ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1, best known as CD39) or 5′-nucleotidase, ecto (NT5E, best known as CD73), two membrane-bound nucleotidases that degrade extracellular ATP (125).
So far, one general strategy has been shown to restore extracellular ATP concentrations to levels that are compatible with the efficient recruitment and activation of APCs, namely, the pharmacological inhibition of CD39. Thus, CT26 cells lacking essential components of the autophagic machinery, such as Atg5, Atg7, or Beclin 1 (Becn1), secrete limited amounts of ATP as they succumb to anthracyclines, and hence are incapable of vaccinating syngeneic mice against a subsequent challenge with malignant cells of the same type (57). Such a functional defect can be corrected by the co-administration of ARL67156, a broad spectrum inhibitor of extracellular nucleotidases (57). Further confirming these findings, CT26 engineered to overexpress CD39 and exposed to anthracyclines are unable to protect syngeneic mice against a subsequent injection with neoplastic cells of the same type (57, 125). This defect can be corrected by the co-administration of ARL67156, along with the restoration of RCD-associated ATP secretion (57, 125). Taken together, these results indicate that inhibitors of extracellular nucleotidases may constitute a convenient manner to boost the immunogenicity of RCD instances that are normally not associated with ATP secretion.
Importantly, the pharmacological activation of autophagy does not suffice for cancer cells to become immunogenic (16, 57). Nonetheless, combining anticancer agents that per se are unable to trigger ATP secretion with molecules that upregulate the autophagic flux, such as inhibitors of mechanistic target of rapamycin (MTOR) complex I (MTORCI), may efficiently convert non-immunogenic RCD instances into bona fide ICD. This hypothesis awaits formal experimental confirmation. Indeed, while other inducers of autophagy such as the glycolytic inhibitor 2-deoxyglucose (126) have been shown to reinstate the immunogenicity of etoposide-elicited RCD, such an effect was ascribed to the restoration of CALR exposure (indeed, etoposide kills malignant cells while promoting ATP secretion) (100). Finally, it should be noted that the establishment of an ATP gradient around dying cells may not constitute a general requirement for the perception of RCD as immunogenic (127). Moreover, at least in some settings, autophagy may actually inhibit ICD by limiting the production of reactive oxygen species in the course of adaptive stress responses, hence counteracting the establishment of ER stress and consequent CALR exposure (54, 55). Thus, further work is required to precisely identify malignancies in which autophagy supports ICD. Only in these scenarios, the co-administration of autophagy inducers may constitute a proper approach to reinstate the immunogenicity of RCD.
Combinatorial Strategies to Promote Type I IFN Production
Whereas the role of type I IFN in the regulation of innate and adaptive immune responses is well known (128, 129), type I IFN signaling in malignant cells has been identified as a requirement for (anthracycline-induced) ICD only recently (51). Thus, cancer cells respond to various anthracyclines by activating a TLR3-elicited signal transduction cascade resulting in type I IFN release, autocrine/paracrine type I IFN signaling, and chemokine (C–X–C motif) ligand 10 (CXCL10) secretion, two phenomena that underlie their vaccinating potential. At odds with their wild-type counterparts, Tlr3−/− and Ifnar1−/− murine cancer cells exposed to anthracyclines fail to vaccinate syngeneic mice against a subsequent injection of living cells of the same type (51). It has already been demonstrated that the inability of Tlr3−/− cells to undergo ICD can be corrected by the co-administration of recombinant type I IFNs or recombinant CXCL10. Similarly, Ifnar1−/− cells succumbing to anthracyclines turn immunogenic in the presence of recombinant CXCL10 (but not type I IFNs) (51).
Various synthetic TLR3 agonists are available and some of them, including polyinosinic:polycytidylic acid (polyI:C) and its clinical grade analog polyI:polyC12U (also known as rintatolimod and Ampligen™), have been extensively tested as immunostimulants in cancer patients (130, 131). It is therefore tempting to speculate that the co-administration of TLR3 agonists may restore the ability of anticancer agents that per se do not promote type I IFN release to trigger bona fide ICD. This hypothesis awaits urgent experimental confirmation. For the considerations presented above, small molecules that trigger TLR3 signaling would indeed be more convenient as clinical tools to restore type I IFN signaling than recombinant type I IFN or CXCL10 themselves.
Combinatorial Strategies to Substitute for HMGB1 Release
HMGB1 release occurs upon (nuclear and) plasma membrane permeabilization, i.e., it constitutes a post-mortem event (5, 132). Thus, all antineoplastic agents that efficiently kill malignant cells (as opposed to molecules that exert cytostatic effects or induce cell senescence) (133) promote HMGB1 release, perhaps with different kinetics (5, 132). However, the expression levels of HMGB1 vary in different tumor types and evolve along with tumor progression, implying that some malignant cells may express HMGB1 to levels that are not compatible with the activation of TLR4 and RAGE in immune cells upon release (134, 135). Importantly, the immunogenicity of anthracycline-induced RCD is compromised in these cells, as well in cells artificially depleted of HMGB1 by means of specific siRNAs (135). Recent results indicate that this defect can be efficiently corrected by the exogenous supply of a synthetic TLR4 agonist, i.e., dendrophilin, at least in experimental models (135). Since dendrophilin has not yet entered clinical development (130, 131), it will be interesting to see whether TLR4 agonists that are already licensed by regulatory agencies for use in humans, such as the Bacillus Calmette–Guérin (BCG) (80) and monophosphoryl lipid A (MPL) (136), are also able to restore the immunogenicity of HMGB1-deficient cells succumbing to ICD.
In this context, it is worth noting that cancer cells exposing CALR, secreting ATP, producing type I IFNs but releasing limited amounts of HMGB1 as they respond to a lethal stimulus in a suboptimal manner fail to elicit adaptive immune responses (137). Upon inoculation into immunocompetent mice, these cells actually form tumors at the vaccination site (as a significant fraction of them is not dying) and the animals are unable to control a subsequent challenge with cell of the same type (3). We have observed this to occur in murine cancer cells treated with digoxin or digitoxin, two glycosides approved in many countries for the treatment of cardiac conditions (81). These molecules efficiently inhibit the human Na+/K+ ATPase, which explains their pharmacological properties and their ability to kill some neoplastic cells of human origin, but not its murine counterpart (83). Thus, cardiac glycosides per se are unable to trigger ICD, at least in the murine system. However, clinical data indicate that they may convert non-immunogenic RCD as elicited by a very large panel of chemotherapeutics into bona fide ICD (83). From another standpoint, any anticancer agent that efficiently kills malignant cells could be considered as a means to restore the immunogenicity of cells responding to cardiac glycosides. We have recently initiated a clinical trial to prospectively test this hypothesis in head and neck squamous carcinoma patients.
Concluding Remarks
In spite of old beliefs, cancer cells continuously interact with the immune system: first, as they are generated by healthy cells upon malignant transformation; second, as they evolve and acquire additional neoplastic features; and third, when they are challenged with therapeutic interventions. During the last decade, such a conceptual revolution, i.e., considering tumors as entities that can be detected and destroyed by the immune system, has paved the way toward the development of novel therapeutic agents conceived to re(instate) anticancer immunity, and some of these interventions have already been licensed for use in humans by international regulatory agencies. In addition, it has become clear that many therapeutics that had been used for decades in the clinic are efficient (for the most part) because they engage the host immune system against malignant cells. ICD is one of the several mechanisms through which cytotoxic chemotherapeutics, targeted anticancer agents as well as some forms of radiotherapy can elicit tumor-targeting immune responses. Identifying novel ICD inducers as well as measures that convert non-immunogenic RCD into bona fide ICD is of primordial importance. Promising preclinical results and preliminary clinical findings suggest, indeed, that agents that promote CALR exposure, ATP secretion, type I IFN production, HMGB1 release or stimulate the downstream signal transduction pathway may considerably improve the clinical profile of conventional therapeutic regimens (Figure 1). A systematic investigation of the ability of currently available anticancer agents to elicit the abovementioned ICD-associated processes in human cancer cells of distinct histological origin is urgently awaited. These data may pave the way to the clinical implementation of combinatorial immuno(chemo)regimens that efficiently promote ICD and hence mediate complete tumor regression in a high proportion of patients.
Figure 1.
Strategies to convert non-immunogenic RCD into bona fide ICD. Upon inoculation into immunocompetent syngeneic hosts, cancer cells responding to a panel of lethal stimuli trigger an adaptive immune response against dead cell-associated antigens. Such an immunogenic variant of regulated cell death (RCD), commonly known as immunogenic cell death (ICD), relies on the exposure of calreticulin (CALR) on the cell surface, on the secretion of ATP, on the production of type I interferons (IFNs) and on the release of high-mobility group box 1 (HMGB1, which accompanies cell death). When any of these damage-associated molecular patterns cannot be emitted (in the appropriate spatiotemporal order), dying cancer cells cannot be perceived anymore as immunogenic by the host immune system. Several strategies have been conceived to correct these defects, hence converting non-immunogenic RCD into bona fide ICD. ER, endoplasmic reticulum; IFNAR, interferon (alpha, beta, and omega) receptor; P2RX7, purinergic receptor P2X, ligand gated ion channel, 7; P2RY2, purinergic receptor P2Y, G-protein coupled, 2; TLR, toll-like receptor.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The Guest Associate Editor, Patrizia Agostinis, declares that despite having co-authored a few manuscripts with the authors Lorenzo Galluzzi, Oliver Kepp and Guido Kroemer in the past 2 years, there has been no conflict of interest during the review and handling of this manuscript.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labelisée); Agence National de la Recherche (ANR); Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
Abbreviations
APC, antigen-presenting cell; CALR, calreticulin; CTLA4, cytotoxic T lymphocyte-associated protein 4; CXCL10, chemokine (C–X–C motif) ligand 10; DAMP, damage-associated molecular pattern; EIF2A, eukaryotic translation initiation factor 2A, 65 kDa; ER, endoplasmic reticulum; HMGB1, high-mobility group box 1; ICD, immunogenic cell death; IFN, interferon; IFNAR, interferon (alpha, beta, and omega) receptor; mAb, monoclonal antibody; P2RX7, purinergic receptor P2X, ligand gated ion channel, 7; P2RY2, purinergic receptor P2Y, G-protein coupled, 2; RCD, regulated cell death; siRNA, small-interfering RNA; TLR, toll-like receptor.
References
- 1.Cirone M, Di Renzo L, Lotti LV, Conte V, Trivedi P, Santarelli R, et al. Activation of dendritic cells by tumor cell death. Oncoimmunology (2012) 1:1218–9. 10.4161/onci.20428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer (2012) 12:860–75. 10.1038/nrc3380 [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol (2013) 31:51–72. 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 4.Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol (2014) 35:24–32. 10.1016/j.semcdb.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ (2015) 22:58–73. 10.1038/cdd.2014.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ (2009) 16:3–11. 10.1038/cdd.2008.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J (2009) 28:578–90. 10.1038/emboj.2009.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ (2012) 19:107–20. 10.1038/cdd.2011.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology (2014) 3:e955691. 10.4161/21624011.2014.955691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, et al. Screening of novel immunogenic cell death inducers within the NCI mechanistic diversity set. Oncoimmunology (2014) 3:e28473. 10.4161/onci.28473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev (2013) 24:319–33. 10.1016/j.cytogfr.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 12.Vacchelli E, Senovilla L, Eggermont A, Fridman WH, Galon J, Zitvogel L, et al. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology (2013) 2:e23510. 10.4161/onci.23510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kepp O, Senovilla L, Kroemer G. Immunogenic cell death inducers as anticancer agents. Oncotarget (2014) 5:5190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vacchelli E, Aranda F, Eggermont A, Galon J, Sautes-Fridman C, Cremer I, et al. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology (2014) 3:e27878. 10.4161/onci.27878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med (2005) 202:1691–701. 10.1084/jem.20050915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med (2007) 13:54–61. 10.1038/nm1523 [DOI] [PubMed] [Google Scholar]
- 17.Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res (2011) 71:4821–33. 10.1158/0008-5472.CAN-11-0950 [DOI] [PubMed] [Google Scholar]
- 18.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene (2010) 29:482–91. 10.1038/onc.2009.356 [DOI] [PubMed] [Google Scholar]
- 19.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene (2011) 30:1147–58. 10.1038/onc.2010.500 [DOI] [PubMed] [Google Scholar]
- 20.Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res (2011) 71:768–78. 10.1158/0008-5472.CAN-10-2788 [DOI] [PubMed] [Google Scholar]
- 21.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol (2011) 33:369–83. 10.1007/s00281-011-0245-0 [DOI] [PubMed] [Google Scholar]
- 22.Stoetzer OJ, Fersching DM, Salat C, Steinkohl O, Gabka CJ, Hamann U, et al. Circulating immunogenic cell death biomarkers HMGB1 and RAGE in breast cancer patients during neoadjuvant chemotherapy. Tumour Biol (2013) 34:81–90. 10.1007/s13277-012-0513-1 [DOI] [PubMed] [Google Scholar]
- 23.Ziccheddu G, Proietti E, Moschella F. The Janus face of cyclophosphamide: a sterile inflammatory response that potentiates cancer immunotherapy. Oncoimmunology (2013) 2:e25789. 10.4161/onci.25789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demaria S, Santori FR, Ng B, Liebes L, Formenti SC, Vukmanovic S. Select forms of tumor cell apoptosis induce dendritic cell maturation. J Leukoc Biol (2005) 77:361–8. 10.1189/jlb.0804478 [DOI] [PubMed] [Google Scholar]
- 25.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood (2007) 109:4839–45. 10.1182/blood-2006-10-054221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cirone M, Di Renzo L, Lotti LV, Conte V, Trivedi P, Santarelli R, et al. Primary effusion lymphoma cell death induced by bortezomib and AG 490 activates dendritic cells through CD91. PLoS One (2012) 7:e31732. 10.1371/journal.pone.0031732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res (2005) 65:1018–26. [PubMed] [Google Scholar]
- 28.Korbelik M, Zhang W, Merchant S. Involvement of damage-associated molecular patterns in tumor response to photodynamic therapy: surface expression of calreticulin and high-mobility group box-1 release. Cancer Immunol Immunother (2011) 60:1431–7. 10.1007/s00262-011-1047-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg AD, Krysko DV, Vandenabeele P, Agostinis P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol Immunother (2012) 61:215–21. 10.1007/s00262-011-1184-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galluzzi L, Kepp O, Kroemer G. Immunogenic cell death in radiation therapy. Oncoimmunology (2013) 2:e26536. 10.4161/onci.26536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vacchelli E, Vitale I, Tartour E, Eggermont A, Sautes-Fridman C, Galon J, et al. Trial watch: anticancer radioimmunotherapy. Oncoimmunology (2013) 2:e25595. 10.4161/onci.25595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloy N, Pol J, Manic G, Vitale I, Eggermont A, Galon J, et al. Trial watch: radioimmunotherapy for oncological indications. Oncoimmunology (2014) 3:e954929. 10.4161/21624011.2014.954929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg AD, Agostinis P. ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem Photobiol Sci (2014) 13:474–87. 10.1039/c3pp50333j [DOI] [PubMed] [Google Scholar]
- 34.Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol (2015) 25:11–7. 10.1016/j.semradonc.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 35.Donnelly OG, Errington-Mais F, Steele L, Hadac E, Jennings V, Scott K, et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther (2013) 20:7–15. 10.1038/gt.2011.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, et al. Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology (2013) 2:e24612. 10.4161/onci.25238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Workenhe ST, Mossman KL. Rewiring cancer cell death to enhance oncolytic viro-immunotherapy. Oncoimmunology (2013) 2:e27138. 10.4161/onci.27138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Workenhe ST, Pol JG, Lichty BD, Cummings DT, Mossman KL. Combining oncolytic HSV-1 with immunogenic cell death-inducing drug mitoxantrone breaks cancer immune tolerance and improves therapeutic efficacy. Cancer Immunol Res (2013) 1:309–19. 10.1158/2326-6066.CIR-13-0059-T [DOI] [PubMed] [Google Scholar]
- 39.Pol J, Bloy N, Obrist F, Eggermont A, Galon J, Cremer I, et al. Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology (2014) 3:e28694. 10.4161/onci.28185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann J, Vitale I, Buchmann B, Galluzzi L, Schwede W, Senovilla L, et al. Improved cellular pharmacokinetics and pharmacodynamics underlie the wide anticancer activity of sagopilone. Cancer Res (2008) 68:5301–8. 10.1158/0008-5472.CAN-08-0237 [DOI] [PubMed] [Google Scholar]
- 41.Pellicciotta I, Yang CP, Goldberg GL, Shahabi S. Epothilone B enhances Class I HLA and HLA-A2 surface molecule expression in ovarian cancer cells. Gynecol Oncol (2011) 122:625–31. 10.1016/j.ygyno.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 42.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science (2012) 337:1678–84. 10.1126/science.1224922 [DOI] [PubMed] [Google Scholar]
- 43.Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, Partlova S, et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer (2014) 135:1165–77. 10.1002/ijc.28766 [DOI] [PubMed] [Google Scholar]
- 44.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell (2010) 140:798–804. 10.1016/j.cell.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 45.Galluzzi L, Bravo-San Pedro JM, Kroemer G. Organelle-specific initiation of cell death. Nat Cell Biol (2014) 16:728–36. 10.1038/ncb3005 [DOI] [PubMed] [Google Scholar]
- 46.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta (2010) 1805:53–71. 10.1016/j.bbcan.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 47.Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol (2012) 13:780–8. 10.1038/nrm3479 [DOI] [PubMed] [Google Scholar]
- 48.Garg AD, Dudek AM, Agostinis P. Cancer immunogenicity, danger signals, and DAMPs: what, when, and how? Biofactors (2013) 39:355–67. 10.1002/biof.1125 [DOI] [PubMed] [Google Scholar]
- 49.Krysko O, Love Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death Dis (2013) 4:e631. 10.1038/cddis.2013.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med (2009) 15:1170–8. 10.1038/nm.2028 [DOI] [PubMed] [Google Scholar]
- 51.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med (2014) 20:1301–9. 10.1038/nm.3708 [DOI] [PubMed] [Google Scholar]
- 52.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med (2007) 13:1050–9. 10.1038/nm1622 [DOI] [PubMed] [Google Scholar]
- 53.Galluzzi L, Kepp O, Kroemer G. Enlightening the impact of immunogenic cell death in photodynamic cancer therapy. EMBO J (2012) 31:1055–7. 10.1038/emboj.2012.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garg AD, Dudek AM, Agostinis P. Autophagy-dependent suppression of cancer immunogenicity and effector mechanisms of innate and adaptive immunity. Oncoimmunology (2013) 2:e26260. 10.4161/onci.26260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garg AD, Dudek AM, Ferreira GB, Verfaillie T, Vandenabeele P, Krysko DV, et al. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy (2013) 9:1292–307. 10.4161/auto.25399 [DOI] [PubMed] [Google Scholar]
- 56.Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, et al. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev (2013) 24:311–8. 10.1016/j.cytogfr.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 57.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science (2011) 334:1573–7. 10.1126/science.1208347 [DOI] [PubMed] [Google Scholar]
- 58.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J (2012) 31:1062–79. 10.1038/emboj.2011.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature (2009) 461:282–6. 10.1038/nature08296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity (2013) 38:729–41. 10.1016/j.immuni.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 61.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature (2002) 418:191–5. 10.1038/nature00858 [DOI] [PubMed] [Google Scholar]
- 62.Ma Y, Adjemian S, Yang H, Catani JP, Hannani D, Martins I, et al. ATP-dependent recruitment, survival and differentiation of dendritic cell precursors in the tumor bed after anticancer chemotherapy. Oncoimmunology (2013) 2:e24568. 10.4161/onci.24568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michaud M, Xie X, Bravo-San Pedro JM, Zitvogel L, White E, Kroemer G. An autophagy-dependent anticancer immune response determines the efficacy of melanoma chemotherapy. Oncoimmunology (2014) 3:e944047. 10.4161/21624011.2014.944047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res (2011) 71:5601–5. 10.1158/0008-5472.CAN-11-1316 [DOI] [PubMed] [Google Scholar]
- 65.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer (2012) 12:298–306. 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 66.Senovilla L, Vacchelli E, Galon J, Adjemian S, Eggermont A, Fridman WH, et al. Trial watch: prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology (2012) 1:1323–43. 10.4161/onci.22009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity (2013) 39:782–95. 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 68.Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res (2014) 20:1891–9. 10.1158/1078-0432.CCR-13-2830 [DOI] [PubMed] [Google Scholar]
- 69.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature (2014) 515:568–71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov (2012) 11:215–33. 10.1038/nrd3626 [DOI] [PubMed] [Google Scholar]
- 71.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity (2013) 39:74–88. 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 72.Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Zitvogel L, Kroemer G, et al. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology (2013) 2:e22789. 10.4161/onci.25238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aranda F, Vacchelli E, Eggermont A, Galon J, Fridman WH, Zitvogel L, et al. Trial watch: immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology (2014) 3:e27297. 10.4161/onci.27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robert C, Thomas L, Bondarenko I, O’day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med (2011) 364:2517–26. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 75.Eggermont AM, Robert C. Melanoma: smart therapeutic strategies in immuno-oncology. Nat Rev Clin Oncol (2014) 11:181–2. 10.1038/nrclinonc.2014.36 [DOI] [PubMed] [Google Scholar]
- 76.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet (2014) 384:1109–17. 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- 77.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med (2015) 372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 78.Galluzzi L, Kroemer G, Eggermont A. Novel immune checkpoint blocker approved for the treatment of advanced melanoma. Oncoimmunology (2014) 3:e967147. 10.1016/j.clindermatol.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vacchelli E, Prada N, Kepp O, Galluzzi L. Current trends of anticancer immunochemotherapy. Oncoimmunology (2013) 2:e25396. 10.4161/onci.25396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buque A, Senovilla L, Baracco EE, et al. Classification of current anticancer immunotherapies. Oncotarget (2014) 5:12472–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med (2012) 4:143ra199. 10.1126/scitranslmed.3003807 [DOI] [PubMed] [Google Scholar]
- 82.Kono K, Mimura K. Immunogenic tumor cell death induced by chemoradiotherapy in a clinical setting. Oncoimmunology (2013) 2:e22197. 10.4161/onci.22197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menger L, Vacchelli E, Kepp O, Eggermont A, Tartour E, Zitvogel L, et al. Trial watch: cardiac glycosides and cancer therapy. Oncoimmunology (2013) 2:e23082. 10.4161/onci.23082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palombo F, Focaccetti C, Barnaba V. Therapeutic implications of immunogenic cell death in human cancer. Front Immunol (2014) 4:503. 10.3389/fimmu.2013.00503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene (2012) 31:1869–83. 10.1038/onc.2011.384 [DOI] [PubMed] [Google Scholar]
- 86.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology (2014) 3:e28518. 10.4161/onci.28518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamamura Y, Tsuchikawa T, Miyauchi K, Takeuchi S, Wada M, Kuwatani T, et al. The key role of calreticulin in immunomodulation induced by chemotherapeutic agents. Int J Clin Oncol (2015) 20:386–94. 10.1007/s10147-014-0719-x [DOI] [PubMed] [Google Scholar]
- 88.Bugaut H, Bruchard M, Berger H, Derangere V, Odoul L, Euvrard R, et al. Bleomycin exerts ambivalent antitumor immune effect by triggering both immunogenic cell death and proliferation of regulatory T cells. PLoS One (2013) 8:e65181. 10.1371/journal.pone.0065181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang CL, Hsu YT, Wu CC, Yang YC, Wang C, Wu TC, et al. Immune mechanism of the antitumor effects generated by bortezomib. J Immunol (2012) 189:3209–20. 10.4049/jimmunol.1103826 [DOI] [PubMed] [Google Scholar]
- 90.Aranda F, Bloy N, Galluzzi L, Kroemer G, Senovilla L. Vitamin B6 improves the immunogenicity of cisplatin-induced cell death. Oncoimmunology (2014) 3:e955685. 10.4161/21624011.2014.955685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aranda F, Bloy N, Pesquet J, Petit B, Chaba K, Sauvat A, et al. Immune-dependent antineoplastic effects of cisplatin plus pyridoxine in non-small-cell lung cancer. Oncogene (2014). 10.1038/onc.2014.234 [DOI] [PubMed] [Google Scholar]
- 92.Cirone M, Garufi A, Di Renzo L, Granato M, Faggioni A, D’orazi G. Zinc supplementation is required for the cytotoxic and immunogenic effects of chemotherapy in chemoresistant p53-functionally deficient cells. Oncoimmunology (2013) 2:e26198. 10.4161/onci.26198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle (2009) 8:3723–8. 10.4161/cc.8.22.10026 [DOI] [PubMed] [Google Scholar]
- 94.Mihailidou C, Chatzistamou I, Papavassiliou A, Kiaris H. Improvement of chemotherapeutic drug efficacy by endoplasmic reticulum stress. Endocr Relat Cancer (2015) 22(2):229–38. 10.1530/ERC-15-0019 [DOI] [PubMed] [Google Scholar]
- 95.Chen X, Yang Y, Zhou Q, Weiss JM, Howard OZ, McPherson JM, et al. Effective chemoimmunotherapy with anti-TGFbeta antibody and cyclophosphamide in a mouse model of breast cancer. PLoS One (2014) 9:e85398. 10.1371/journal.pone.0085398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, Ferrone S, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer (2013) 133:624–36. 10.1002/ijc.28070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang W, Qin S, Zhao L. Docetaxel enhances CD3+ CD56+ cytokine-induced killer cells-mediated killing through inducing tumor cells phenotype modulation. Biomed Pharmacother (2015) 69:18–23. 10.1016/j.biopha.2014.10.026 [DOI] [PubMed] [Google Scholar]
- 98.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med (2011) 208:491–503. 10.1084/jem.20100269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zappasodi R, Pupa SM, Ghedini GC, Bongarzone I, Magni M, Cabras AD, et al. Improved clinical outcome in indolent B-cell lymphoma patients vaccinated with autologous tumor cells experiencing immunogenic death. Cancer Res (2010) 70:9062–72. 10.1158/0008-5472.CAN-10-1825 [DOI] [PubMed] [Google Scholar]
- 100.Beneteau M, Zunino B, Jacquin MA, Meynet O, Chiche J, Pradelli LA, et al. Combination of glycolysis inhibition with chemotherapy results in an antitumor immune response. Proc Natl Acad Sci U S A (2012) 109:20071–6. 10.1073/pnas.1206360109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boyd-Tressler A, Penuela S, Laird DW, Dubyak GR. Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism. J Biol Chem (2014) 289:27246–63. 10.1074/jbc.M114.590240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kepp O, Galluzzi L, Giordanetto F, Tesniere A, Vitale I, Martins I, et al. Disruption of the PP1/GADD34 complex induces calreticulin exposure. Cell Cycle (2009) 8:3971–7. 10.4161/cc.8.23.10191 [DOI] [PubMed] [Google Scholar]
- 103.Zhao T, Ren H, Jia L, Chen J, Xin W, Yan F, et al. Inhibition of HIF-1alpha by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma. Oncotarget (2015) 6:2250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wemeau M, Kepp O, Tesniere A, Panaretakis T, Flament C, De Botton S, et al. Calreticulin exposure on malignant blasts predicts a cellular anticancer immune response in patients with acute myeloid leukemia. Cell Death Dis (2010) 1:e104. 10.1038/cddis.2010.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frey B, Stache C, Rubner Y, Werthmoller N, Schulz K, Sieber R, et al. Combined treatment of human colorectal tumor cell lines with chemotherapeutic agents and ionizing irradiation can in vitro induce tumor cell death forms with immunogenic potential. J Immunotoxicol (2012) 9:301–13. 10.3109/1547691X.2012.693547 [DOI] [PubMed] [Google Scholar]
- 106.Dudek-Peric AM, Ferreira GB, Muchowicz A, Wouters J, Prada N, Martin S, et al. Antitumor immunity triggered by melphalan is potentiated by melanoma cell surface-associated calreticulin. Cancer Res (2015). 10.1158/0008-5472.CAN-14-2089 [DOI] [PubMed] [Google Scholar]
- 107.Lu X, Ding ZC, Cao Y, Liu C, Habtetsion T, Yu M, et al. Alkylating agent melphalan augments the efficacy of adoptive immunotherapy using tumor-specific CD4+ T cells. J Immunol (2015) 194:2011–21. 10.4049/jimmunol.1401894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou P, Teruya-Feldstein J, Lu P, Fleisher M, Olshen A, Comenzo RL. Calreticulin expression in the clonal plasma cells of patients with systemic light-chain (AL-) amyloidosis is associated with response to high-dose melphalan. Blood (2008) 111:549–57. 10.1182/blood-2007-11-125526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ (2008) 15:1499–509. 10.1038/cdd.2008.67 [DOI] [PubMed] [Google Scholar]
- 110.Gou HF, Huang J, Shi HS, Chen XC, Wang YS. Chemo-immunotherapy with oxaliplatin and interleukin-7 inhibits colon cancer metastasis in mice. PLoS One (2014) 9:e85789. 10.1371/journal.pone.0085789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liikanen I, Ahtiainen L, Hirvinen ML, Bramante S, Cerullo V, Nokisalmi P, et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther (2013) 21:1212–23. 10.1038/mt.2013.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rubner Y, Muth C, Strnad A, Derer A, Sieber R, Buslei R, et al. Fractionated radiotherapy is the main stimulus for the induction of cell death and of Hsp70 release of p53 mutated glioblastoma cell lines. Radiat Oncol (2014) 9:89. 10.1186/1748-717X-9-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin S, Dudek-Peric AM, Maes H, Garg AD, Gabrysiak M, Demirsoy S, et al. Concurrent MEK and autophagy inhibition is required to restore cell death associated danger-signalling in vemurafenib-resistant melanoma cells. Biochem Pharmacol (2015) 93:290–304. 10.1016/j.bcp.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 114.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol (2012) 13:89–102. 10.1038/nrm3270 [DOI] [PubMed] [Google Scholar]
- 115.Galluzzi L, Vitale I, Senovilla L, Olaussen KA, Pinna G, Eisenberg T, et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep (2012) 2:257–69. 10.1016/j.celrep.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 116.Steiner P, Kulangara K, Sarria JC, Glauser L, Regazzi R, Hirling H. Reticulon 1-C/neuroendocrine-specific protein-C interacts with SNARE proteins. J Neurochem (2004) 89:569–80. 10.1111/j.1471-4159.2004.02345.x [DOI] [PubMed] [Google Scholar]
- 117.Michaud M, Sukkurwala AQ, Di Sano F, Zitvogel L, Kepp O, Kroemer G. Synthetic induction of immunogenic cell death by genetic stimulation of endoplasmic reticulum stress. Oncoimmunology (2014) 3:e28276. 10.4161/onci.28276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dubois SG, Grier HE. Chemotherapy: the role of ifosfamide and etoposide in Ewing sarcoma. Nat Rev Clin Oncol (2009) 6:251–3. 10.1038/nrclinonc.2009.25 [DOI] [PubMed] [Google Scholar]
- 119.Johnson FM, Glisson BS. Chemotherapy: irinotecan or etoposide as front-line therapy for SCLC? Nat Rev Clin Oncol (2009) 6:562–3. 10.1038/nrclinonc.2009.141 [DOI] [PubMed] [Google Scholar]
- 120.Kepp O, Semeraro M, Pedro JM, Bloy N, Buque A, Huang X, et al. eIF2alpha phosphorylation as a biomarker of immunogenic cell death. Semin Cancer Biol (2015). 10.1016/j.semcancer.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 121.Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity (2013) 39:211–27. 10.1016/j.immuni.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 122.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science (2011) 333:1109–12. 10.1126/science.1201940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, et al. Autophagy in malignant transformation and cancer progression. EMBO J (2015) 34(7):856–80. 10.15252/embj.201490784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ (2014) 21:79–91. 10.1038/cdd.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Michaud M, Sukkurwala AQ, Martins I, Shen S, Zitvogel L, Kroemer G. Subversion of the chemotherapy-induced anticancer immune response by the ecto-ATPase CD39. Oncoimmunology (2012) 1:393–5. 10.4161/onci.19070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov (2013) 12:829–46. 10.1038/nrd4191 [DOI] [PubMed] [Google Scholar]
- 127.Koks CA, Garg AD, Ehrhardt M, Riva M, Vandenberk L, Boon L, et al. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer (2015) 136:E313–25. 10.1002/ijc.29202 [DOI] [PubMed] [Google Scholar]
- 128.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol (2014) 14:36–49. 10.1038/nri3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McNab F, Mayer-Barber K, Sher A, Wack A, O’garra A. Type I interferons in infectious disease. Nat Rev Immunol (2015) 15:87–103. 10.1038/nri3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, et al. Trial watch: toll-like receptor agonists for cancer therapy. Oncoimmunology (2013) 2:e25238. 10.4161/onci.25238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aranda F, Vacchelli E, Obrist F, Eggermont A, Galon J, Sautes-Fridman C, et al. Trial watch: toll-like receptor agonists in oncological indications. Oncoimmunology (2014) 3:e29179. 10.4161/onci.29179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol (2005) 5:331–42. 10.1038/nri1594 [DOI] [PubMed] [Google Scholar]
- 133.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell (2013) 153:1194–217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pang X, Zhang Y, Wei H, Zhang J, Luo Q, Huang C, et al. Expression and effects of high-mobility group box 1 in cervical cancer. Int J Mol Sci (2014) 15:8699–712. 10.3390/ijms15058699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ (2014) 21:69–78. 10.1038/cdd.2013.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Srivastava AK, Dinc G, Sharma RK, Yolcu ES, Zhao H, Shirwan H. SA-4-1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines. Cancer Res (2014) 74:6441–51. 10.1158/0008-5472.CAN-14-1768-A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev (2011) 30:61–9. 10.1007/s10555-011-9273-4 [DOI] [PubMed] [Google Scholar]